Abstract
Objective
To evaluate the anti-inflammatory and analgesic activities of leaf extract of Melanthera scandens (M. scandens).
Methods
The crude leaf extract (39–111 mg/kg) of M. scandens was investigated for anti-inflammatory and analgesic activities using various experimental models. The anti-inflammatory activity was investigated using carragenin, egg-albumin induced oedema models, while acetic acid, formalin-induced paw licking and thermal-induced pain models were used to evaluate the antinociceptive property.
Results
The extract caused a significant (P<0.05 – 0.001) dose-dependent reduction of inflammation and pains induced by different agents used.
Conclusions
The leaf extract possesses anti-inflammatory and analgesic effects which may be mediated through the phytochemical constituents of the plant.
Keywords: Melanthera scandens, Anti-inflammatory, Analgesic
1. Introduction
Melanthera scandens (M. scandens) (Schumach & Thonn.) Roberty (Asteraceae) is a perennial herb up to 1 m to 4 m high, branches quadrangular and scabrid. It distributes geographically in East, West and South Africa[1]. It is known as ‘ayara edemerong’ by the Ibibios of Akwa Ibom State of Nigeria. The leaves are used traditionally to treat various ailments such as stomach ulcer and sores in Gosomtwi-Atwimakwanwoma district of Ghana[2]. In Nigeria, the leaves are used to treat dysmenorrhoea diabetes and malaria[3]. It is also used by the Bete people of Issia district of Cote d'Ivoire to treat malaria[4],[5]. The antioxidant[6], in vitro antiplasmodial[4],[5] and antidiabetic[7], activities have been reported. Triterpenoid saponins have been reported in the leaves[8]. There is no information on anti-inflammatory and analgesic properties of this plant, hence we reported in this study the anti-inflammatory and analgesic activities of M. scandens in rodent.
2. Materials and methods
2.1. Plant materials
The leaves of M. scandens were collected from a bush in Ukap in Ikono Local Government Area of Akwa Ibom State and were identified and authenticated by Dr. Magaret Bassey, a taxonomist in the Department of Botany and Ecological Studies, University of Uyo, Uyo. A voucher specimen of the plant was deposited in the Hebarium of Department of Botany and Ecological Studies, University of Uyo, Uyo.
2.2. Extraction
The plant parts (leaves) were washed and shade-dried for two weeks. The dried leaves were further chopped into small pieces and reduced to powder. The powdered leaf (1.5 kg) was macerated in 97% ethanol for 72 h to give the crude ethanolic extract. The liquid filtrate was concentrated and evaporated to dryness in vacuo at 40 °C using rotary evaporator. The yield of crude ethanolic extract was 3.98%. The dried crude extract was stored in a refrigerator at 4 °C until use for the proposed experiment.
2.3. Phytochemical screening
Phytochemical screening of the crude extract was carried out employing standard procedures[9], to reveal the presence of chemical constituents such as alkaloids, flavonoids, tannins, terpenes, saponins, anthraquinones, reducing sugars, cardiac glycosides and others.
2.4. Animals
Swiss albino mice (17–24 g) and rats (120–145 g) of both sexes were used for these experiments. They were obtained from University of Uyo Animal House. The animals were housed in standard cages and were maintained on a standard pelleted feed (Guinea feed) and water ad libitum.
2.5. Determination of median lethal dose (LD50)
The LD50 of the extract was determined using albino mice. The extract was administered intraperitoneally (i.p.) and the method of Miller and Tainter[10] was adopted. This involved the administration of different doses of the extract (100–1000 mg/kg) to groups of six mice each. The animals were observed for physical manifestation of signs of toxicity. The number of deaths in each group within 24 h was recorded.
2.6. Evaluation of anti-inflammatory activity of the extract
2.6.1. Carrageenin-induced mice hind paw oedema
Increase in the mice hind paw linear circumference induced by plantar injection of the phlogistic agent was used as the measure of acute inflammation[11]. Adult albino mice of either sex were used after 24 h fast and deprived of water only during experiment. Inflammation of the hind paw was induced by injection of 0.1 mL of freshly prepared carrageenin suspension in normal saline into the sub plantar surface of the hind paw. The linear circumference of the injected paw was measured before and 0.5, 1, 2, 3, 4 and 5 h after administration of phlogistic agent. For routine drug testing, the increase in paw circumference 0.5, 1, 2, 3, 4 and 5 h after administration of phlogistic agent was adopted as the parameter for measuring inflammation[11],[12]. Edema (inflammation) was assessed as difference in paw circumference between the control and 0.5, 1, 2, 3, 4 and 5 h after administration of phlogistic agent[13]. The extract (37, 74 and 111 mg/kg i.p.) was administered to various groups of mice, 1 h before inducing inflammation. Control mice received carrageenin while reference group received aspirin (ASA) (100 mg/kg). The average (mean) oedema was assessed by measuring with vernier calipers.
2.6.2. Egg albumin induced inflammation
Inflammation was induced in mice by the injection of egg albumin (0.1 mL, 1% in normal saline) into the sub plantar tissue of the right hind paw[14]. The linear circumference of the injected paw was measured before and 0.5, 1, 2, 3, 4 and 5 h after the administration of the phlogistic agent. The leaf extract (37, 74 and 111 mg/kg i.p.) and ASA (100 mg/kg orally) were administered to 24 h fasted mice 1 h before the induction of inflammation. Control group received 10 mL/kg of distilled water orally. Edema (inflammation) was assessed as the difference in paw circumference between the control and 0.5, 1, 2, 3, 4 and 5h after the administration of the phlogistic agent[13]. The average (mean) edema was assessed by measuring with vernier calipers.
2.7. Evaluation of analgesic potential of the extract
2.7.1. Acetic acid induced writhing in mice
The abdominal constrictions resulting from intraperitoneal (i.p.) injection of 3% acetic acid consisting of the contraction of abdominal muscles together with the stretching of hindlimbs, were carried out according to the procedure described by Nwafor et al[12]. The animals were divided into 5 groups of 6 mice per group. Group 1 served as negative control and received 10 mL/kg of normal saline, while groups 2, 3 and 4 were pre-treated with 37, 74 and 111 mg/kg doses of M. scandens extract intraperitoneally, and group 5 received 100 mg/kg of acetylsalicylic acid. After 30 min, 0.2 mL of 2% acetic acid was administered intraperitoneally (i.p.). The number of writhing movements was counted for 30 min. Antinociception (analgesia) was expressed as the reduction of the number of abdominal constrictions between control animals and mice pretreated with extracts.
2.7.2. Formalin induced hind paw licking in mice
The procedure was essentially similar to that described by Okokon and Nwafor[14]. The animals were used to analyze the first phase of formalin-induced licking and 20 µL of 2.5% formalin solution (0.9% formaldehyde) made up in phosphate buffer solution (PBS concentration: NaCl 137 mM, KCl 2.7 mM and phosphate buffer, 10 mM) was injected subcutaneously under the surface of the right hind paw. The amount of time spent licking the injected paw was timed and considered as indication of pain. The first of the nociceptive response normally peaks 5 min after injection and the second phase 15–30 min after formalin injection, representing the neurogenic and inflammatory pain responses, respectively[15]. Adult albino mice (23–27 g) of either sex randomized into five groups of 6 mice each were used for the experiment. The mice were fasted for 24 h before being used but allowed access to water. The animals in group 1 (negative control) received 10 mL/kg of normal saline, groups 2–4 received 37, 74 and 111 mg/kg doses of the extract, while group 5 received 100 mg/kg of acetylsalicylic acid 30 min before being challenged with buffered formalin. The responses were measured for 5 min after formalin injection (first phase) and 15–30 min after formalin injection (second phase).
2.7.3. Thermally induced pain in mice
The effect of extract on hot plate induced pain was investigated in adult mice. The hot plate was used to measure the response latencies according to the method reported by Mbagwu et al[16]. In these experiments, the hot plate was maintained at (45±1) °C, each animal was placed into a glass beaker of 50 cm diameter on the heated surface, and the time(s) between placement and shaking or licking of the paws or jumping was recorded as the index of response latency. An automatic 30-sec cut-off was used to prevent tissue damage. The animals were randomly divided into 5 groups of 6 mice each and fasted for 24 h but allowed access to water. Group 1 served as negative control and received 10 mL/kg of normal saline. Groups 2, 3 and 4 were pre-treated intraperitoneally with 37, 74 and 111 mg/kg doses of M. scandens extract, respectively, while group 5 animals received 100 mg/kg of acetylsalicylic acid intraperitoneally, 30 min prior to the placement on the hot plate.
2.8. Statistical analysis
Data obtained from this work were analyzed statistically using Students' t-test and ANOVA (one- or two-way) followed by a post test (Tukey-Kramer multiple comparison test). Differences between means will be considered significant at 1% and 5% level of significance i.e. P≤0.01 and 0.05.
3. Results
3.1. Phytochemical screening
The phytochemical screening of the ethanolic extract of the leaves of M. scandens revealed the presence of cardiac glycosides, tannins, saponins, terpenes and flavonoids.
3.2. Acute toxicty
The LD50 was calculated to be (370.00±23.33) mg/kg. The physical signs of toxicity included excitation, paw licking, increased respiratory rate, decreased motor activity, gasping and coma which was followed by death.
3.3. Anti-inflammatory activity
3.3.1. Carragenin-induced oedema in mice
The effect of ethanolic leaf extract of M. scandens on carragenin-induced oedema was shown in Table 1. The extract exerted a dose-dependent anti-inflammatory effect. This effect was significant (P<0.05 – 0.001) when compared with control and comparable to that of the standard drug i.e. ASA (100 mg/kg).
Table 1. Effect of M. scandens leaf extract on carrageenin-induced oedema and egg albumin induced oedema in mice (mean±SEM) (n=6).
| Anti-inflammatory models | Treatment (mg/kg) | Time intervals (h) |
||||||
| 0 | 0.5 | 1 | 2 | 3 | 4 | 5 | ||
| Carrageenin induced oedema | Control (A) | 0.28±0.01 | 0.36±0.01 | 0.36±0.01 | 0.35±0.01 | 0.34±0.01 | 0.33±0.01 | 0.31±0.01 |
| M. scandens extract (37) | 0.25±0.01 | 0.35±0.01 | 0.34±0.01a | 0.31±0.01b | 0.30±0.01b | 0.28±0.01b | 0.27±0.01b | |
| M. scandens extract (74) | 0.26±0.01 | 0.36±0.01 | 0.33±0.01a | 0.30±0.01b | 0.29±0.01b | 0.28±0.01b | 0.26±0.01b | |
| M. scandens extract (111) | 0.24±0.01 | 0.33±0.01 | 0.30±0.01b | 0.29±0.01b | 0.26±0.01b | 0.25±0.01b | 0.25±0.01b | |
| ASA (100) | 0.25±0.01 | 0.35±0.01 | 0.34±0.01a | 0.32±0.01b | 0.30±0.01b | 0.28±0.01b | 0.26±0.01b | |
| Egg albumin induced oedema | Control (B) | 0.25±0.01 | 0.33±0.01 | 0.35±0.01 | 0.34±0.01 | 0.33±0.01 | 0.32±0.01 | 0.31±0.01 |
| M. scandens extract (37) | 0.26±0.01 | 0.34±0.01 | 0.34±0.01 | 0.32±0.01 | 0.31±0.01a | 0.29±0.01a | 0.28±0.01a | |
| M. scandens extract (74) | 0.26±0.01 | 0.35±0.01 | 0.33±0.01 | 0.32±0.01a | 0.29±0.01a | 0.28±0.01b | 0.27±0.01b | |
| M. scandens extract (111) | 0.24±0.01 | 0.35±0.01 | 0.33±0.01 | 0.31±0.01a | 0.28±0.01b | 0.27±0.01b | 0.25±0.01b | |
| ASA (100) | 0.25±0.01 | 0.31±0.01a | 0.28±0.01a | 0.27±0.01b | 0.26±0.01b | 0.26±0.01b | 0.25±0.01b | |
a: P<0.05, b: P<0.001 when compared with control; A: carrageenin; B: 10 mL/kg distilled water.
3.3.2. Egg albumin induced oedema
Administration of leaf extract of M. scandens on egg albumin induced oedema in mice caused a significant (P<0.05 – 0.001) dose-dependent anti-inflammatory effect against oedema caused by egg albumin.The effect was comparable to that of standard drug i.e. ASA (100 mg/kg) (Table 1).
3.4. Effect of ethanolic crude extract of leaves of M. scandens on acetic acid-induced writhing in mice
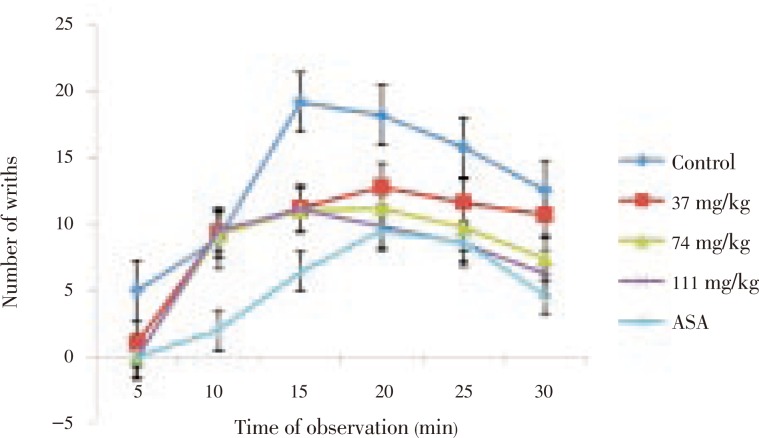
The extract (37–111 mg/kg) demonstrated a dose-dependent reduction in acetic acid-induced writhing in mice. The reductions were statistically significant (P<0.001) relative to control and comparable to that of the standard drug, ASA, at the highest dose, 111 mg/kg (Figure 1).
Figure 1. Effect of M. scandens leaf extract on acetic acid induced writhing in mice.
3.5. Effect of ethanolic leaves extract of M. scandens on formalin-induced hind paw licking in mice
The extract exhibited a dose-dependent effect on formalin-induced hind paw licking in mice. This inhibition was significant relative to the control (P<0.001) and comparable to that of the standard drug, ASA, at the highest dose, 111 mg/kg (Figure 2).
Figure 2. Effect of M. scandens leaf extract on formalin-induced hind paw licking in mice.
3.6. Effect of ethanolic crude extract of leaves of M. scandens on thermally-induced pain in mice
The extract exhibited a dose-dependent effect on thermally-induced pain in mice. This inhibition was statistically significant (P<0.001) relative to the control (Table 2).
Table 2. Effect of M. scandens leaf extract on hot plate test (mean±SEM) (n=6).
| Groups | Dose (mg/kg) | Reaction time (sec) | Inhibition (%) |
| Control | – | 3.34±0.25 | |
| M. scandens | 37 | 4.43±0.28a | 32.63 |
| 74 | 5.31±0.15a | 58.98 | |
| 111 | 7.68±0.20b | 129.94 | |
| ASA | 100 | 16.22±0.17b | 385.62 |
a: P<0.05, b: P<0.01 when compared with control.
4. Discussion
M. scandens leaves are used traditionally to treat various ailments such as stomach ulcer and sores[2], dysmenorrhoea[17], malaria and its symptoms[4],[5],[18]. These prompted the need to evaluate the anti-inflammatory and analgesic potentials of the crude leaves extract of M. scandens.
In this work, median lethal dose (LD50) was determined to be (370.00±23.33) mg/kg and the extract was relatively safe[19].
In the carragenin-induced oedema, the extract (37–111 mg/kg) was observed to have exerted significant effect at the early stage of inflammation (1–2 h) indicating effect probably on histamine, serotonin and kinnins that are involved in the early stage of carragenin induced oedema[20],[21]. The extract further reduced later stage of the oedema maybe due to its ability to inhibit prostaglandin which is known to mediate the second phase of carragenin induced inflammation[22]. However, ASA (100 mg/kg) a prototype NSAID, a cyclooxygenase inhibitor whose mechanism of action involves inhibition of prostaglandin, inhibited significantly (P<0.01 - 0.001) paw swelling due to carragenin injection.
The extract also inhibited egg albumin-induced oedema demonstrating that it can inhibit inflammation by blocking the release of histamine and 5-HT, two mediators that are released by egg albumin[15]. However, ASA, a cyclooxygenase inhibitor reduced significantly oedema produced by egg albumin.
Flavonoids are reported to be involved in antiinflammatory activity of plants[23]. These have been found to be present in the extract.
The extract significantly reduced acetic acid-induced writhing, formalin-induced hind paw licking as well as delayed the reaction time of animals (mice) to thermally induced pain. Acetic acid causes inflammatory pain by inducing capillary permeability and in part through local peritoneal receptors from peritoneal fluid concentration of PGE2 and PGF2α[24]. This test alone can not specify the involvement of either central or peripheral activity[25]. Thus, formalin tests as well as hot-plate test are usually carried out in addition to the above to distinguish between peripheral and central pain. Centrally acting drugs inhibit both abdominal constriction test and hot plate tests[26], while the peripherally acting drugs inhibit only the abdominal constriction[27].
Formalin exhibits neurogenic and inflammatory pains[28] and measures both centrally and peripherally mediated activities that are characteristics of biphasic pain response. The injection of formalin has been reported to cause an immediate and intense increase in the spontaneous activity of C fiber afferent and evoke a distinct quantifiable behavior indicative of pain demonstrated in paw licking by the animals[29]–[31].
The study also shows that the extract significantly delayed the reaction time of thermally-induced (hot plate) test. This model is selective for centrally acting analgesics and indicates narcotic involvement[32] with opiod receptors.
The antinociceptive activities exerted by this extract may be attributed to the presence of secondary metabolites like saponins, flavonoids, tannins, and terpenes. Flavonoids also have anti-inflammatory effects through its inhibition of the cyclooxygenase pathway[33]. That the extract inhibited neurogenic and non-neurogenic pains as well as narcotic pains may in part explain the mechanisms of its action and these effects are due to the presence of phytochemical components in the extract.
In conclusion, the results of this study demonstrated that M. scandens possesses anti-inflammatory and analgesic properties. Further investigation is being advocated especially in elucidating cellular mechanisms and establishing structural components of the active ingredients with a view of standardizing them.
Acknowledgments
The authors are grateful to Mr Enefiok Ukpong of Pharmacology and Toxicology Department for his technical assistance.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Burkill HM. The useful plants of west tropical Africa. 2nd ed. London: Royal Botanic Garden; 1985. p. 127. [Google Scholar]
- 2.Agyare C, Asase A, Lechtenberg M, Niehues M, Deters A, Hensi A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Gosomtwi-Atwima-Kwanwoma area, Ghana. J Ethnopharmacol. 2009;125:393–403. doi: 10.1016/j.jep.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Ajibesin KK, Ekpo BA, Bala DN, Essien EE, Adesanya SA. Ethnobotanical survey of Akwa Ibom State of Nigeria. J Ethnopharmacol. 2008;115:387–408. doi: 10.1016/j.jep.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Guede NZ, N'guessan K, Dibie TE, Grellier P. Ethnopharmacological study of plants used to treat malaria in traditional medicine by Bete populations of Issia (Cote d'voire) J Pharm Sci Res. 2010;2(4):216–227. [Google Scholar]
- 5.Guede ZN, Mambu L, Guede-Guina F, Bodo B, Grellier P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J Ethnopharmacol. 2005;98:281–285. doi: 10.1016/j.jep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Adeseguna SA, Alabia SA, Olabanja PA, Alexander H, Coker B. Evaluation of antioxidant potential of Melanthera scandens. J Acupunct Meridian Stud. 2010;3(4):267–271. doi: 10.1016/S2005-2901(10)60047-7. [DOI] [PubMed] [Google Scholar]
- 7.Offong E. Antidiabetic and hypolipidemic activities of Melanthera scadens (M.Sc. dissertation) Uyo, Nigeria: University of Uyo; 2011. [Google Scholar]
- 8.Penders A, Delaude C. Triterpenoids saponins from Melanthera scadens. Phytochemistry. 1994;37(3):821–825. doi: 10.1016/s0031-9422(00)90364-9. [DOI] [PubMed] [Google Scholar]
- 9.Trease GE, Evans WC. Pharmacognosy. 13th ed. London: Bailliere Tindal; 1996. pp. 683–684. [Google Scholar]
- 10.Miller LC, Tainter ML. Estimation of ED50 or LD50 values and their error using logarithmic-probit graph paper. Proc Soc Exp Biol Med. 1944;57:261–264. [Google Scholar]
- 11.Winter CA, Risley EA, Nuss GW. Carrageenin-induced oedema in hind paw of the rats as an assay of anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 12.Nwafor PA, Nwajiobi N, Uko IE, Obot JS. Analgesic and anti-inflammatory activities of an ethanol extract of Smilax krausiana leaf in mice. Afr J Biomed Res. 2010;13:141–148. [Google Scholar]
- 13.Sanchez-Mateo CC, Bonkanka CX, Hernandez-Perez M, Rabanal RM. Evaluation of the analgesic and topical antiinflammatory effects of Hypericum reflexum L. fil. J Ethnopharmacol. 2006;107:1–6. doi: 10.1016/j.jep.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 14.Okokon JE, Nwafor PA. Antiinflammatory, analgesic and antipyretic activities of ethanolic root extract of Croton zambesicus. Pak J Pharm sci. 2010;23(4):383–390. [PubMed] [Google Scholar]
- 15.Nwafor PA, Jacks TW, Ekanem AU. Analgesic and anti-inflammatory effects of methanolic extract of Pausinystalia mecroceras stem bark in rodents. J Pharmacol. 2007;3:86–90. [Google Scholar]
- 16.Mbagwu HO, Anene RA, Adeyemi OO. Analgesic, antipyretic and antiinflammatory properties of Mezoneuron benthamianum Baill (Caesalpiniaceae) Nig Q J Hosp Med. 2007;17(1):35–41. doi: 10.4314/nqjhm.v17i1.12540. [DOI] [PubMed] [Google Scholar]
- 17.Okokon JE, Ettebong EO, Udobang JA, Obot J. Antiplasmodial and antiulcer activities of Melanthera scandens. Asian Pac J Trop Biomed. 2012;2:16–20. doi: 10.1016/S2221-1691(11)60182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajibesin KK, Ekpo BA, Bala DN, Essien EE, Adesanya SA. Ethnobotanical survey of Akwa Ibom State of Nigeria. J Ethnopharmacol. 2008;115:387–408. doi: 10.1016/j.jep.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Homburger F. In vivo testing in the study of toxicity and safety evaluation. In: Marquis JK, editor. A guide to general toxicology. 2nd ed. New York: Karger; 1989. [Google Scholar]
- 20.Georgewill OA, Georgewill UO, Nwankwoala RNP. Antiinflammatory effects of Morninga oleifera lam extract in rats. Asian Pac J Trop Med. 2010;3(2):133–135. [Google Scholar]
- 21.Georgewill OA, Georgewill UO. Evaluation of the antiinflammatory activity of extract of Vernonia amygdalina. Asian Pac J Trop Med. 2010;3(2):150–151. [Google Scholar]
- 22.Vane T, Booting R. Inflammation and mechanism of action of anti-inflammatory drugs. J Fed Am Soc Exp Biol. 1987;1:89–96. [PubMed] [Google Scholar]
- 23.Parmer NS, Ghosh MN. Anti-inflammatory activity of gossypin a biflavonoid isolated from Hibiscus vitifolicus Linn. Indian J Pharmacol. 1978;10:277–293. [Google Scholar]
- 24.Bentley GA, Newton SH, Starr J. Studies on the antinociceptive action of agonist drugs and their interaction with opoid mechanisms. Br J Pharm. 1983;79:125–134. doi: 10.1111/j.1476-5381.1983.tb10504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan TE, Tsai HY, Tian-Shang W. Antiinflammatory and analgesic activities from the root of Angelica pubescens. Planta Med. 1995;61:2–8. doi: 10.1055/s-2006-957987. [DOI] [PubMed] [Google Scholar]
- 26.Hosseinzadeh H, Younnesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petals extract in mice. BMC Pharmacol. 2002;2:7–14. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amanlou M, Dadkhah F, Salehnia A, Farsam H, Dehpour AR. Antiinflammatory and antinociceptive effects of hydrochlororic extract of Satureja khuzistanica Jamzad extract. J Pharm Pharm Sci. 2005;8:102–106. [PubMed] [Google Scholar]
- 28.Vaz ZR, Cechinel V, Yunes RA, Calixto JB. Antinociceptive action of 2-(4-bromobenzoyl)-3-methyl-4-6-dimethoxy bezofuran, a novel xanthoxyline derivative of chemical and thermal models of nociception in mice. J Pharm Exp Ther. 1996;278:304–312. [PubMed] [Google Scholar]
- 29.Heapy CG, Jamieson A, Russell NJW. Afferent C-fiber and A-delta activity in models of inflammation. Br J Pharmacol. 1987;90:164. [Google Scholar]
- 30.Karthikeyan M, Deepa MK. Anti-inflammatory activity of Premna corymbosa (Burm.f.) Rottl. & Willd. leaves extracts in Wistar albino rats. Asian Pac J Trop Med. 2011;4(7):510–513. doi: 10.1016/S1995-7645(11)60136-3. [DOI] [PubMed] [Google Scholar]
- 31.Ijeoma UF, Aderonke SO, Ogbonna O, Augustina MA, Ifeyinwa CN. Antinociceptive and anti-inflammatory activities of crude extracts of Ipomoea involucrata leaves in mice and rats. Asian Pac J Trop Med. 2011;4(2):121–124. doi: 10.1016/S1995-7645(11)60050-3. [DOI] [PubMed] [Google Scholar]
- 32.Turner RA. Screening methods in pharmacology. New York: Academic Press; 1995. pp. 85–106. [Google Scholar]
- 33.Liang YC, Huang YT, Tsau SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclo-oxygenase and inducible nitric oxide synthase by apigenia and related flavonoid in mouse macrophages. Carcinogenesis. 1999;20(10):1945–1952. doi: 10.1093/carcin/20.10.1945. [DOI] [PubMed] [Google Scholar]