Abstract
Objective
To evaluate the safety of shilajit by 91 days repeated administration in different dose levels in rats.
Methods
In this study the albino rats were divided into four groups. Group I received vehicle and group II, III and IV received 500, 2 500 and 5 000 mg/kg of shilajit, respectively. Finally animals were sacrificed and subjected to histopathology and iron was estimated by flame atomic absorption spectroscopy and graphite furnace.
Results
The result showed that there were no significant changes in iron level of treated groups when compared with control except liver (5 000 mg/kg) and histological slides of all organs revealed normal except negligible changes in liver and intestine with the highest dose of shilajit. The weight of all organs was normal when compared with control.
Conclusions
The result suggests that black shilajit, an Ayurvedic formulation, is safe for long term use as a dietary supplement for a number of disorders like iron deficiency anaemia.
Keywords: Shilajit, Graphite furnace, Histopathology, Flame atomic absorption spectroscopy
1. Introduction
Shilajit is considered as one of the wonder medicines of Ayurveda. Neither a plant nor animal substance, it is a mineral pitch that oozes from the rocks of the Himalayas, as they become warm in the summer months. Shilajit (asphaltum) is widely used in the preparation of Ayurvedic medicines and is regarded as one of the most important ingredients in Ayurvedic system of medicine. It is used as an adaptogen[1]. Shilajit, also known as shilajit, mumijo, and momia, is used in the Ayurveda, the traditional Indian system of medicine[2]. The complement system is involved in many disease syndromes that have been traditionally treated with extracts of shilajit and other humic substances containing high levels of Fe (e.g., arthritis and asthma)[3],[4] and they are also used for anti-oxidant, hypoglycemic and iron deficiency anemia[5],[6]. In the Charak Samhita, shilajit is described as a product of four minerals i.e. gold, silver, copper and iron, whereas in the Susruta Samhita it included two more minerals, i.e. lead and zinc in its composition[7]. Iron has long been considered important for the body. Lauha bhasma (calcined iron) has been used in ancient Indian medicine. Iron was used for weakness, which is common in anaemia. In 1713 iron was shown to be present in blood[8]. The most obvious clinical result of iron deficiency is anaemia, and the only indication for therapy with iron is to provide material for haemoglobin synthesis[9]. The bioavailability of iron in the colon was increased by prebiotic and synbiotic diets[10], it is mainly stored in liver and spleen in the form of ferritin which is used to meet the daily requirements not provided by the diet. Nearly all cells contain ferritin. The ferritin will release the Fe+++ to circulating blood to meet their deficiency, and aggregated ferritin was referred to as haemosiderin[9]. Iron deficiency is the most common cause of anemia, or a low red blood cell count. Lack of iron slows production of the iron-containing hemoglobin, which limits the rate of red blood cell production. Similarly, iron deficiency causes a reduced concentration of myoglobin in the muscles, commonly leading to weakness and poor physical endurance. The reduced oxygen-carrying capacity of the blood that occurs with iron-deficiency anemia further aggravates myoglobin deficiency-related symptoms[11]. The decrease of iron leads to Trypanosoma cruzi infection in host[12], causes defects in the cognition and learning processes in humans[13], produces significant alterations in the metabolism of dopamine and other neurotransmitter in brain[14] and reduces γ-aminobutyric acid (GABA) and glutamic acid content of the brain. On rehabilitation with the iron-supplemented diet for 1 week, these decreased enzyme activities in brain attained the corresponding control values[15]. The iron preparation used for disorders in an over dose can cause toxicity to some vital organs. The Astanga Hrdayam reported that there are six types of shilajit but it is found that the shilajit coming out of iron is the best[16] and also there was no scientific proof for long term safety of black shilajit. So the present study was aimed to evaluate the safety of shilajit by 91 days repeated administration in different dose levels.
2. Materials and methods
2.1. Acute toxicity
Twenty four (12 male and 12 female) albino mice, weighing approximately (18–25 g) were used for the study. The acute toxicity study was carried out as per guidelines for Ayurveda and Siddha drugs. Healthy mice of either sex were selected and grouped into four comprising six animals in each group. The animals were fasted for four hours prior to the study with free access to water. The test drug was suspended in the suitable medium (reverse osmosis water) and given in graded doses up to maximum dose level. One group received vehicle only, which served as untreated control. The animals were observed for any toxic manifestations and mortality for 72 hours. Thereafter, animals were observed for 14 days.
2.2. Chronic toxicity study/ 91 days study
The Wistar rats of 4–6 weeks were selected for the present study. Body weights of the rats for chronic toxicity study were taken after the initial stabilization period. They were ranging between (130–140 g) for males and (120–130 g) for females. A total of twenty four Wistar rats (12 males + 12 females) were randomized and equally divided into four groups comprising six animals in each group. The animals in all the groups were provided with pellet diet depending upon the body weights and their food consumption profile. The animals of group II, III and IV were administered with shilajit at the respective doses of 500, 2 500 and 5 000 mg/kg once daily for 91 days, whereas animals in group I received vehicle (reverse osmosis water). After 91 days administration, blood was collected from retro orbital and serum was separated. Finally animals were sacrificed, and different organs were isolated and weighed. The isolated organs were subjected to histopathology studies and the iron was estimated by flame atomic absorption spectrometry (AAS) and the serum iron by graphite furnace. The experimental protocol was reviewed and approved by Institutional Animal Ethical Committee (No: 23/SASTRA/IAEC/RPP).
2.3. Metal analysis
2.3.1. Sample collection
The samples were cleaned and dried under shade. The dried samples were then grinded and powdered in an agate pestle and mortar. Samples were labeled and stored in pre-cleaned polyethylene bottles for further analysis.
2.3.2. Reagents and apparatus
All the reagents such as HNO3, and H2O2 were purchased from MERCK. Millipore water was used for all analytical work and all the digestion vessel, polyethylene bottles (sample container), micro pipette tips and others were washed with 10% HCl, rinsed with de-ionized water before preparing standards, reagents and samples.
2.3.3. Digestion of samples (sample preparation)
A Multiwave 3000 micro oven system (from Anton paar, USA) with 16 position teflon vessels with capping was being used for digestion process. The digestion vessels were provided with a controlled pressure, temperature and release valve. Before using, all teflon vessels were soaked with 10% HNO3. The system was initially programmed by giving gradual rise of 20%, 40%, and 50% power for 5, 15 and 20 minutes, respectively for the due warming up. The powder samples were used without any further treatment for sample preparation. 0.25 g of sample was weighed into the teflon vessels followed by digestion mixture of HNO3, and H2O2 in the ratio of 3:1, according to the nature of samples which were being applied.
The resulting solution after microwave digestion was filtered through Whatman No. 40 filter paper (if necessary) and diluted to 25 mL with Millipore water. A sample blank containing only acid mixture was prepared at the same time. The method of standard addition was generally adapted to calibrate the instrument before going for the observation of the samples.
2.3.4. Calibration of instruments
More than three working standard solution of elements to be determined were prepared, covering the concentration range as recommended by the manufacturer of the instrument for the elements to be determined. Before the analysis of samples, the instruments were calibrated with prepared working standard solution. The calibration curves were obtained for concentration and absorbance data were statistically analyzed. Calibration of the instrument was repeated periodically during operations and blanks were carried with each set of 10 samples or aspirating any one of the prepared working standard for every 10 samples to check the instrument drift and to validate analytical procedures and performance. Reagent blank readings were taken and necessary correction was made during the calculation of concentration of various elements.
Standard Certified Reference (SRM) of National Institute of Standard and Technology (NIST) was used for day-to-day for the evaluation of methods of analysis or test and for long-term quality assurance of measurements.
2.3.5. Fe analysis by flame AAS/graphite furnace
After calibrating the instrument with prepared working standard, the digests liquid sample's solution was subjected to analysis of Fe in organs by flame AAS and Fe in serum by graphite furnace with specific instrumental conditions as given by instruments manufacturer. The solution was introduced into flame, and the reading was recored, using the mean of the three reading and the quantified concentration of the metals in the given samples against the standard calibration curve obtained from concentration vs. absorbance of the prepared known concentration on the day of the analysis.
2.4. Statistical analysis
The statistical analysis was performed by one-way analysis of variance (ANOVA) by Dunnett's test. A different level at P<0.05 was considered to be statistically significant.
3. Results
3.1. Acute toxicity
The test drug and vehicle were administered to mice as per the guidelines, under close observation for 4 hours and followed by daily observation for 14 days. There was neither behavioral changes nor mortality and morbidity in all the groups of animals. So, the test drug was proved suitable for further evaluation.
3.2. Cage side observation
There was no abnormality in the feces color, consistency and amount, hair coat, respiration character and rate convulsions, biting, static limb position, abnormal gait, ataxic gait, head position and rearing activity during the experimental period.
3.3. Metal estimation
The estimation of metal concentration was given in Table 1, which showed no significant difference of iron content in all groups of animals, and in all the organs except liver. Metal estimation (iron) values of rats treated with shilajit (group IV) showed significant difference in liver when compared with group I, but no significant changes were observed in group II and group III.
Table 1. Estimated iron content of shilajit in various organs and serum (mean±SEM) (n=6).
| Groups | Heart | Liver | Spleen | Kidney | Serum |
| Group I | 5.66±0.32 | 8.36±0.29 | 6.23±0.29 | 5.58±0.32 | 256.60±4.17 |
| Group II | 6.79±0.69 | 9.08±0.46 | 5.86±0.95 | 5.41±0.23 | 327.17±8.65 |
| Group III | 5.89±0.34 | 9.44±0.38 | 6.10±0.40 | 5.66±0.32 | 342.35±6.33 |
| Group IV | 6.21±0.55 | 9.87±0.17* | 6.94±0.39 | 6.26±0.25 | 367.70±3.45 |
*: P<0.05 comparing with the control group.
3.4. Organ weights
There was no adverse effect of shilajit on any vital organs of the rats, which received the drug for 91 days. There was no indication of atrophy or hypertrophy of any organ of rats in all treated groups. It was represented in Table 2.
Table 2. Effect of shilajit on organ weights (g) in rats (mean±SEM) (n=6).
| Groups | Heart | Lungs | Liver | Kidney | Spleen | Stomach | Intestine | Testes | Ovaries |
| Group I | 0.50±0.04a | 0.73±0.05a | 5.95±0.45a | 1.18±0.13a | 0.18±0.02a | 1.32±0.06a | 11.53±0.61a | 2.43±0.17a | 0.06±0.01a |
| Group II | 0.65±0.08a | 1.00±0.13a | 6.18±0.84a | 1.32±0.20a | 0.30±0.08a | 1.28±0.19a | 12.72±1.55a | 2.30±0.10a | 0.05±0.01a |
| Group III | 0.77±0.10a | 0.98±0.10a | 6.00±0.43a | 1.45±0.16a | 0.30±0.04a | 1.57±0.17a | 12.50±0.68a | 1.93±0.22a | 0.08±0.01a |
| Group IV | 0.87±0.13a | 0.98±0.07a | 6.27±0.73a | 1.28±0.10a | 0.27±0.02a | 1.20±0.09a | 12.33±0.85a | 2.23±0.13a | 0.06±0.01a |
Means bearing same superscripts do not differ significantly (P<0.05).
3.5. Histopathological studies
The histological changes in the main organs were shown in Figure 1–11. On histological studies, the highest dose of test drug caused changes in liver and intestine showing congestion and degeneration of hepotocytes, and fusion of villi (Figure 4, 8) and all other organs revealed normal. In group I, group II and group III, all the organs were normal and no specific abnormalities were seen. When compared with control, the highest dose showed microscopic changes.
Figure 1. Photomicrograph of heart (control).
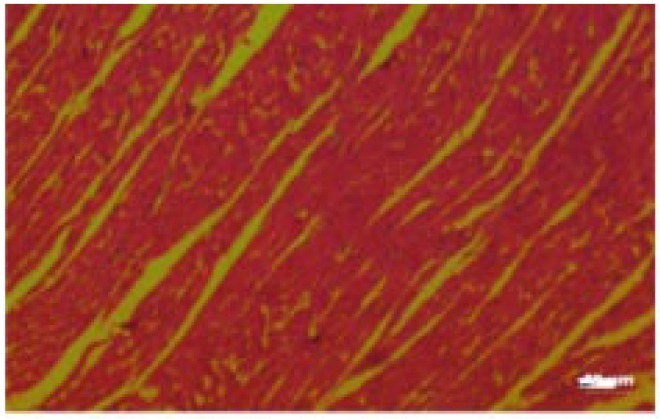
Figure 11. Photomicrograph of ovary (control).
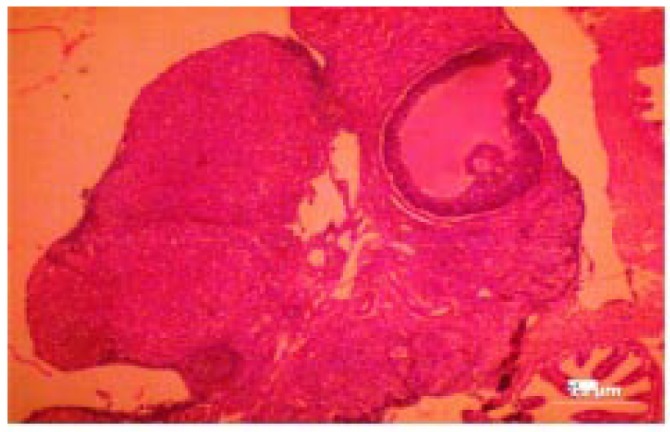
Figure 4. Photomicrograph of liver-treated with shilajit (the highest dose) showing congestion and degeneration of hepatocytes.
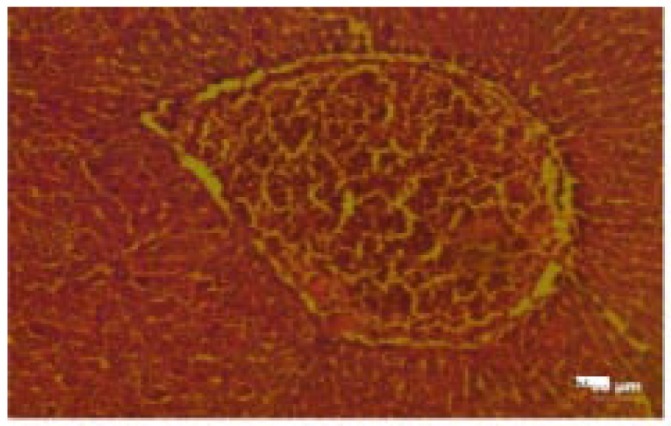
Figure 8. Photomicrograph of intestine-treated with shilagit (the highest dose) showing fusion of villi.
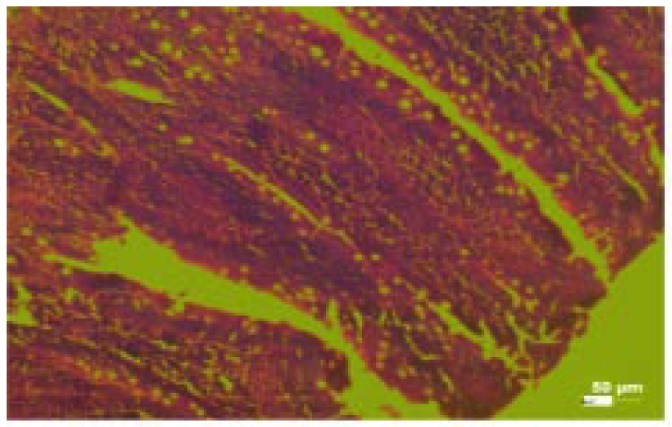
Figure 2. Photomicrograph of lungs (control).
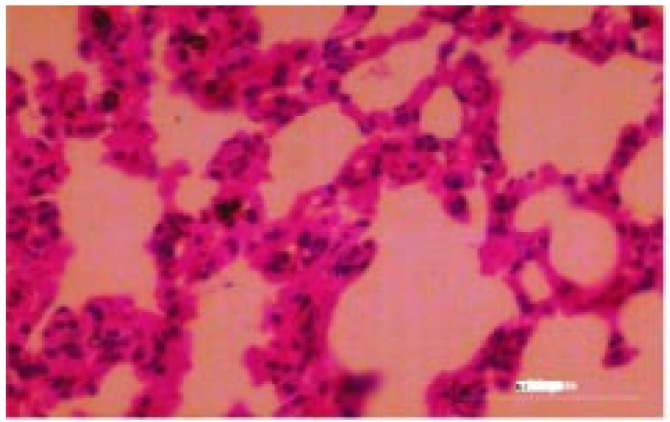
Figure 3. Photomicrograph of liver (control).
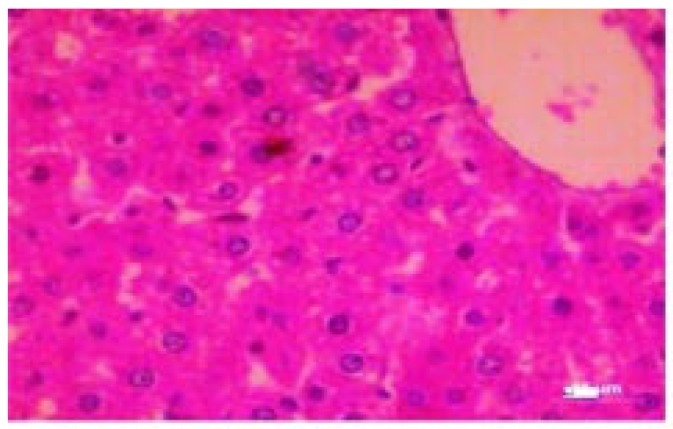
Figure 5. Photomicrograph of spleen (control).
Figure 6. Photomicrograph of stomach (control).
Figure 7. Photomicrograph of intestine (control).
Figure 9. Photomcrograph of kidney (control).
Figure 10. Photomicrograph of testis (control).
4. Discussion
The overload iron can cause undesirable side effect or toxicity. The excess iron is distributed throughout the body and slowly accumulates in several organs, including heart, liver, spleen, and skin. Ferritin is a unique iron storage protein containing 24 storage proteins. When excess dietary iron is absorbed, the body produces more ferritin. Ferritin is greatly abundant in the heart and liver, therefore there is a large amount in these organs, and iron rushes to these organs for storage[17],[18]. Iron storage is the most common cause of death among patients with hemochromatosis, sickle cell disease, thalassemia, and heart disease[19].
The shilajit contains iron and other minerals, and is widely used for various disorders in long term therapy like anaemia and diabetes. So it may cause toxicity, but the present study data suggest that long term use of shilajit did not show any toxicity and the iron content of shilajit almost has the same level in all the dose level and histopathological studies show the normal histology of all the organs except the intestine and liver, which did not have significant change in the histological architecture. Hence, it can be considered being normal. Again the organ weight shows no significant in all organs when compared to control and there was no mortality observed in all treated groups up to the end of study, which will further support that the shilajit was safe for long term use.
Footnotes
Foundation Project: This work was financially supported by Shanmugha Arts, Science, Technology and Research Academy University (grant No. 23/SASTRA/IAEC/RPP).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Agarwal SP, Khanna R, Karmarkar R, Anwer MK, Khar RK. Shilajit: a review. Phytother Res. 2007;21:401–405. doi: 10.1002/ptr.2100. [DOI] [PubMed] [Google Scholar]
- 2.Winston D, Steven M. Adaptogens: herbs for strength, stamina, and stress relief. Vermont: Healing Arts Press Rochester; 2007. pp. 202–204. [Google Scholar]
- 3.Mizuno M. A review of current knowledge of the complement system and the therapeutic opportunities in inflammatory arthritis. Curr Med Chem. 2006;13:1707–1717. doi: 10.2174/092986706777441959. [DOI] [PubMed] [Google Scholar]
- 4.Wills-Karp M. Complement activation pathways: a bridge between innate and adaptive immune responses in asthma. Proc Am Thorac Soc. 2007;4:247–251. doi: 10.1513/pats.200704-046AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh R, Kadam PP, Kadam VJ. Antioxidant and hypolipidemic activity of Kumbhajatu in hypercholesterolemic rats. Int J Ayurveda Res. 2010;1(3):159–162. doi: 10.4103/0974-7788.72487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velmurugan C, Vivek B, Shekar SB, Sudha SP, Sundaram T. Shilajit in management of iron deficiency anaemia. J Pharm Biomed Sci. 2010;1(1):1–2. [Google Scholar]
- 7.Ghosal S, Singh SK, Kumar Y, Srivatsava R. Antiulcerogenic activity of fulvic acids and 4-metoxy-6-carbomethyl biphenyl isolated from shilajit. Phytother Res. 1988;2:187–191. [Google Scholar]
- 8.Tripathi KD. Essentials of medical pharmacology. 6th ed. New Delhi: Jaypee Brothers Medical Publishers; 2008. p. 581. [Google Scholar]
- 9.Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 5th ed. Elsevier Publication; 2007. p. 348. [Google Scholar]
- 10.Perez CD. Effect of probiotic, prebiotic and symbiotic follow-up infant formulas on iron bio availability in rats. Food Sci Technol Int. 2007;13:69–77. [Google Scholar]
- 11.Kraemer WJ. Exercise physiology: integrating theory and application. 1st ed. New york: Lipincott; 2011. [Google Scholar]
- 12.Marilda JA, Maria LP, Helen RM, Vanja MV, Marta DL, Maria TB, et al. Treatment with the iron chelator desferrioxamine reduces parasitemia and mortality in experimentally infected mice. Exp Parasitol. 2007;117:43–50. doi: 10.1016/j.exppara.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Carter CR, Joseph L, Matthew J, Armony-Sivan R, Neil C, Angelilli ML, et al. Iron deficiency anemia and cognitive function in infancy. Pediatrics. 2010;126(2):427–434. doi: 10.1542/peds.2009-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr. 2011;141:740–746. doi: 10.3945/jn.110.131169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yehuda S, Mostofsky D. Iron deficiency and overload: from basic biology to clinical medicine. New York: Humana Press; 2009. p. 151. [Google Scholar]
- 16.Murthy KRS. Astanga hrdayam. 5th ed. Varnasi: Krishnadas Academy; 2001. [Google Scholar]
- 17.Mozzarelli A, Bettati S. Chemistry and biochemistry of oxygen therapeutics: from transfusion to artificial blood. 1st ed. United Kingdom: John Wiley & Sonsltd; 2011. p. 403. [Google Scholar]
- 18.Prasad MNV. Trace elements as contaminants and nutrients: consequences in ecosystems and and human health. United Kingdom: Wiley; 2008. pp. 485–486. [Google Scholar]
- 19.Nelson JE, Mugford VR, Kilcourse E, Wang RS, Kowdley KV. Relationship between gene expression of duodenal iron transporters and iron stores in hemochromatosis subjects. Am J Physiol Gastrointest Liver Physiol. 2010;298:57–62. doi: 10.1152/ajpgi.00175.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


