Abstract
Objective
To assess the effects of extraction methods on antioxidant activities of selected Indian medicinal flora.
Methods
Different parts of plants were extracted by hydroalcoholic and decoction methods using water and various concentrations of methanol (ME) viz. 75%, 50% and 25% ME. The antioxidant activity of all the different extracts was evaluated using two different antioxidant assays viz. 2,2-diphenyl-1-picryl hydrazyl (DPPH) free radical scavenging assay and superoxide anion radical scavenging assay. Total phenol and flavonoid content was also estimated.
Results
The results showed that the extracting solvent significantly altered the antioxidant property estimations of screened plants. High correlations between phenolic compositions and antioxidant activities of extracts were observed. High levels of antioxidant activities were detected in Manilkara zapota (M. zapota) as compared with other screened plants.
Conclusions
The results obtained appear to confirm the effect of different methods on extraction of antioxidants and antioxidant property of M. zapota.
Keywords: Antioxidant, Hydroalcoholic extraction, Total phenol content, DPPH, Superoxide, Manilkara zapota
1. Introduction
Oxidative stress depicts the existence of products called free radicals and reactive oxygen species (ROS), which are formed under normal physiological conditions but become deleterious when not being eliminated by the endogenous systems. In fact, oxidative stress results from an imbalance between the generation of ROS and endogenous antioxidant systems. ROS are major sources of primary catalysts that initiate oxidation in vivo and in vitro and create oxidative stress which results in numerous diseases and disorders[1] such as cancer[2], cardiovascular disease[3], neural disorders[4], Alzheimer's disease[5], mild congnitive impairment[6], Parkinson's disease[7], alcohol induced liver disease[8], ulcerative colitis[9], ageing[10], and atherosclerosis[11].
Oxygen derived free radicals such as superoxide anions, hydroxyl radicals and hydrogen peroxide are cytotoxic and give rise to tissue injuries[12]. Excessive amount of ROS is harmful because they initiate bimolecular oxidation which leads to cell death and creates oxidative stress. In addition, oxidative stress causes inadvertent enzyme activation and oxidative damage to cellular system[13]. Free radical is a chemical compound which contains an unpaired electron spinning on the peripheral layer around the nucleus. The family of free radicals generated from the oxygen is called ROS which can cause damage to other molecules by extracting electrons from them in order to attain stability. ROS are ions, atoms or molecules that have the ability to oxidize reduced molecules. ROS are various forms of activated oxygen, which include free radicals such as superoxide anion radicals (O2−) and hydroxyl radicals (OH−), as well as non-free radicals (H2O2) and singlet oxygen[14]. In the body, free radicals are derived from two sources: endogenous sources, e.g. nutrient metabolism, ageing process, etc and exogenous sources e.g. tobacco smoke, ionizing radiation, air pollution, organic solvents, pesticides, etc[15]. The mechanism of antioxidant protection becomes unbalanced in human body, antioxidant supplement may be used to help reduce oxidative damage.
Many medicinal plants contain large amount of antioxidants such as polyphenols, which can play an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen or decomposing peroxides[16]. Phenolic compounds in plants provide an array of natural source of antioxidants for use in foods and nutraceutical[17]. Polyphenols are antioxidants with redox properties which allow them to act as reducing agents, hydrogen donor and singlet oxygen quenchers[18]. The interest in polyphenol antioxidant has increased remarkably over the last decade because of their protective effects against different diseases including cardiovascular, inflammatory disease as well as cancers[19]. Natural antioxidants tend to be safer and also possess anti-viral, anti-inflammatory, anti-cancer, antimutagenic, anti-tumour, and hepatoprotective properties. The source of natural antioxidants may be all or any part of plants such as fruits, vegetables, nuts, seeds, leaves, roots, barks, peels, plant, etc[20]–[23].
A great number of in vitro methods have been developed to measure the efficiency of natural antioxidants either as pure compounds or as plant extracts. There is no single, widely acceptable assay method for evaluating antioxidant capacity applicable to different compounds and different plant extracts, but the most commonly used methods for measuring antioxidant activity are those that involve the generation of free radical species which are then neutralized by antioxidant compounds[24]. However, it is essential to use more than one method to evaluate antioxidant capacity of plant materials because of the complex nature of phytochemicals[25],[26]. Considering the above, in the present study, six plants which are traditionally used in treating various diseases and disorders are selected to evaluate their antioxidant potential.
2. Materials and methods
2.1. Chemicals
2,2-diphenyl-1-picrylhydrazyl (DPPH), nitroblue tetrazolium (NBT), phenazine methosulphate (PMS), nicotinamide adenine dinucleotide reduced (NADH), gallic acid, ascorbic acid, quercetin, Folin-Ciocalteu reagent, aluminium chloride, potassium acetate, Tris-HCl, were obtained from Hi-Media, Mumbai, India; petroleum ether, methanol, etc were obtained from Merck, India.
2.2. Plant materials
The dry powder of all the plant parts was purchased locally, in the month of August, 2010, Gujarat, India.
2.3. Plant description
Six plants belonging to different families were used in the present study. The description of the plants with their therapeutic uses[27] was given below.
2.3.1. Azadirachta indica (A. indica) A. juss.
A. indica belongs to the family of Meliaceae with a vernacular name called “Limbdo”. Its bark is often used for uses such as tonic, antiperiodic, refrigerant, anthelmintic, naturant, pectoral astringent. It is often used in vomiting, burning sensation near the heart, fatigue, fever, thirst, bad taste in the mouth, cough, cures alters, inflammations, earache, rheumatism, symphilitic sores, boils, and blood impurities.
2.3.2. Hemidesmus indicus (H. indicus) (L.) R. Br.
H. indicus belongs to the family of Asclepiadaceae with vernacular names called “Anantmul, Upalsari”. Its roots are often used for uses such as bitter, sweet, cooling, antipyretic, astringent, aromatic, refrigerant, emollient, depurative, aphrodisiac, carminative, appetizer, anthelmantic, diuretic, tonic. Its stem is used for bitter, diaphorestic, diuretic, laxative. Its root is used in leprosy, leucoderma, itching, skin diseases, fevers, foul odour from the body, loss of appetite, asthma, bronchitis, diseases of blood, leucoderma, dysentery and diarrhea, thirst, burning sensation, piles, rat bite poisoning, eye troubles, epileptic, fits, in children, wasting diseases, useful in heminarnia, pain in joints, syphilis, leucoderma, sarsaparilla, in anorexia, fever, skin diseases, as remedy for heat or inflammations of the urinary passages, applied to swelling, vitiated condition of pitta, burning sensation, leucoderma, leprosy, pruritus, asthma, bronchitis, helminthiasis, diarrhea, dysentery, haemorrhoids, strangury, leacorrhoea, syphilis, abscess, arthralgia, fever and general dedility.
2.3.3. Manilkara zapota (M. zapota) L.
M. zapota belongs to the family of Sapotaceae with a vernacular name called “Chiku”. The part of leaves are often used. The seeds are aperients, diuretic, tonic and febrifuge. Bark is antibiotic, astringent and febrifuge. Fruits are edible, sweet with rich fine flavour. Chicle from bark is used in dental surgery. Bark is used as tonic and the decoction is given in diarrhea and peludism.
2.3.4. Psorelea corylifolia (P. corylifolia) L.
P. corylifolia belongs to the family of Fabaceae with vernacular names called “Babchi, Bavachi”. Its seeds are often used. The seeds are bitter, acrid, anthalmintic, laxative, stomachic, stimulant, aphrodisiac, diuretic, rubefacient. They are useful in leucoderma, ulcers, scabies, leprosy and vitiated conditions of ‘pitta’ mucomembranous disorders and dermatitis. It is a good hair tonic.
2.3.5. Rubia cordifolia (R. cordifolia) L.
R. cordifolia belongs to the family of Rubiaceae with a vernacular name called “Majith”. Its root is often used.Roots are sweet, bitter, astringent, thermogenic, anti-inflammatory, anodyne, anti-setic, digestive, carminative, constipating, anti-dysenteric, anthelmintic, depurative, vulnerary, emmenagogue, diuretic, galactapurifier, alterant, ophthalmic, febrifuge, rejuvenating and tonic. They are useful in vitiated conditions of kapha pitta, rheumatoid arthritis, neuralgia, cephalgia, dyspepsia, flatulence, colic, diarrhea, dysentery, helminthiasis, leprosy, skin diseases, leucoderma, pruritus, wounds, ulcers, amenorhoea, dysmenorrhoea, strangury, ophthalamopathy, intermittent, fever, pharyngitis, cough, diabetes, discolouration of the skin and the mucous tissues, otopathy, urethrorrhea, haemorrhoids, jaundice, hepatopathy, splenopathy, arthralgia, leucorrhoea, pectotoral diseases and general debility.
2.3.6. Tinospora cordifolia (T. cordifolia) (Willd.) Miers ex Hook. F. Thoms.
T. cordifolia belongs to the family of Menispermaceae with a vernacular name called “Gulvel”. Its stem is often used.The stem is bitter, astringent, sweet, thermogenicanodyne, anthelmintic, alterant, antiperiodic, antispasmodic, anti-inflammatory, antipyretic, antiemetic, digestive, carminative, appetister, stomachic, constipating, cardiotonic, depurative, haematinic, expectorant, aphrodisiac, rejuvenating, galactapurifier and tonic. It is useful in vitiated condition of vata, burning sensation, hyperdipsia, helmenthiasis, dyspepsia, flatulence, stomachalgia, intermittent fevers, chronic fevers, inflammations, gout, vomiting, cardiac debility, skin diseases, leprosy, erysipelas, anaemia, cough, asthma, general debility, jaundice, seminal weakness, uropathy and splenopathy.
2.4. Extraction
Extraction was done by two different methods as described below.
2.4.1. Hydroalcoholic method
The dried powder of plant parts was individually extracted by hydroalcoholic cold percolation method[28]. 10 g of dried powder was taken in 100 mL of petroleum ether in a conical flask, plugged with cotton wool and then kept on a rotary shaker at 120 rpm for 24 h. After 24 h, it was filtered through eight layers of muslin cloth, centrifuged at 5 000 rpm for 15 min and the supernatant was collected and air dried under reduced pressure to obtain the dried residue. Petroleum ether was evaporated from the powder. This dry powder was then taken individually in 100 mL of each solvent i.e. methanol (ME), 75% ME, 50% ME, 25% ME and water and was kept on a rotary shaker at 120 rpm for 24 h. Then the procedure followed was same as above, and the residues were weighed to obtain the extractive yield of all the extracts and were stored in air tight bottles at 4 °C.
2.4.2. Decoction method
For the decoction method[29], 5 g of dried powder was extracted with 100 mL of deionized water at 100 °C for 30 min in a water bath. It was filtered with eight layers of muslin cloth and centrifuged at 5 000 rpm for 10 min. The supernatant was collected and the solvent was evaporated to dryness. The residue was weighed to obtain the extractive yield, and it was stored in air tight bottle at 4 °C.
2.5. Quantitative phytochemical analysis
2.5.1. Determination of total phenol content
The amount of total phenol content, in different solvent extracts, was determined by Folin-Ciocalteu reagent method[30]. 0.5 mL of extract and 0.1 mL (0.5 N) Folin-Ciocalteu reagent were mixed and the mixture was incubated at room temperature for 15 min. Then 2.5 mL saturated sodium carbonate solution was added and further incubated for 30 min at room temperature and the absorbance was measured at 760 nm. Gallic acid was used as a positive control. Total phenol values are expressed in terms of gallic acid equivalent (mg/g of extracted compounds).
2.5.2. Determination of flavonoid content
The amount of flavonoid content, in different solvent extracts, was determination by aluminium chloride colorimetric method[31]. The reaction mixture (3.0 mL) consisting of 1.0 mL sample (1 mg/mL), 1.0 mL methanol, 0.5 mL (1.2%) aluminium chloride and 0.5 mL (120 mM) potassium acetate, was incubated at room temperature for 30 min. The absorbance of all samples was measured at 415 nm. Quercetin was used as positive control. The flavonoid content is expressed in terms of quercetin equivalent (mg/g of extracted compound).
2.6. Antioxidant assays
2.6.1. DPPH free radical scavenging assay
The free radical scavenging activity of different solvent extracts was measured by using DPPH with the modified method of Mc Cune and Johns[32]. The reaction mixture (3.0 mL) consisting of 1.0 mL DPPH (0.3 mM), 1.0 mL extract (different concentrations) and 1.0 mL methanol, was incubated for 10 min, in dark, after which the absorbance was measured at 517 nm. Ascorbic acid was used as positive control. Percentage of inhibition was calculated using the following formula:
% Inhibition = [1 − (A/B)] × 100
Where, B is the absorbance of the blank (DPPH plus methanol) and A is absorbance of the sample (DPPH, methanol, plus sample).
2.6.2. Superoxide anion radical scavenging assay
The superoxide anion radical scavenging activity of different solvent extracts was measured by the method as described by Robak and Gryglewski[33]. Superoxide anion radicals are generated by oxidation of NADH and assayed by the reduction of NBT. The reaction mixture (3.0 mL) consisted of 0.5 mL Tris-HCl buffer (16 mM, pH 8), 0.5 mL NBT (0.3 mM), 0.5 mL NADH (0.936 mM), 0.5 mL PMS (0.12 mM) and 1.0 mL of different concentrations of different solvent extracts. The superoxide radical generating reaction was started by the addition of PMS solution to the mixture. The reaction mixture was incubated at 25 °C for 5 min and then the absorbance was measured at 560 nm against a blank sample. Gallic acid was used as a positive control. The percentage inhibition was calculated as described above.
3. Results
3.1. Extractive yield
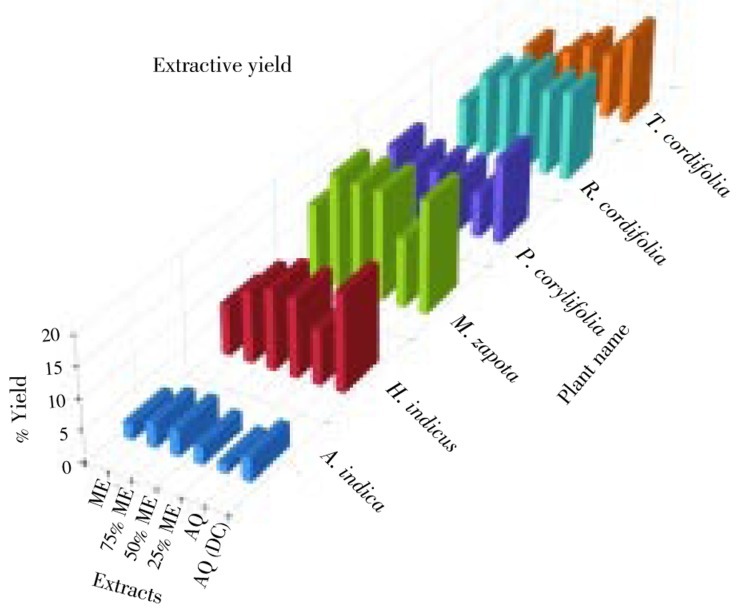
The dry powder of the six plants was extracted by hydroalcoholic cold percolation method and decoction extraction method. The extractive yield of all the six plants in different solvent was given in Figure 1. In all six plants, the extractive yield was higher by decoction method than hydroalcoholic method. Different plant extracts can be ranked from high to low in the following order: M. zapota > R. cordifolia > H. indicus > T. cordifolia > P. corylifolia > A. indica in different extracts. Amongst all the six plants, the maximum yield was in M. zapota.
Figure 1. Percentage extractive yield of different extracts of screened plants.
AQ: Aqueous extract; DC: Decoction.
3.2. Total phenol and flavonoid content
Total phenol content and flavonoid content of screened plants was shown in Figure 2. In all the plants in all the extracts, total phenol content was considerably more than flavonoid content except in P. corylifolia. The flavonoid content of pure ME extract was considerably more than total phenol content in P. corylifolia (Figure 2). Maximum total phenol content was present in M. zapota. Except A. indica, in all the other plants, the phenol content was more in aqueous extract by decoction method than cold percolation method. When phenol content of pure solvent extracts of the six plants was compared, generally phenol content was more in ME than both aqueous extracts except in P. coylifolia. Therefore, in hydroalcoholic extracts, the phenol content decreased to some extent or remained same. Maximum phenol content was in pure ME extract of M. zapota (Figure 2). Hence, it can be stated that ME was able to extract more phenolic compounds as compared with water (aqueous).
Figure 2. Total phenol and flavonoid content of different extracts of screened plants.
AQ: Aqueous extract; DC: Decoction.
3.3. Antioxidant activity
3.3.1. DPPH free radical scavenging activity
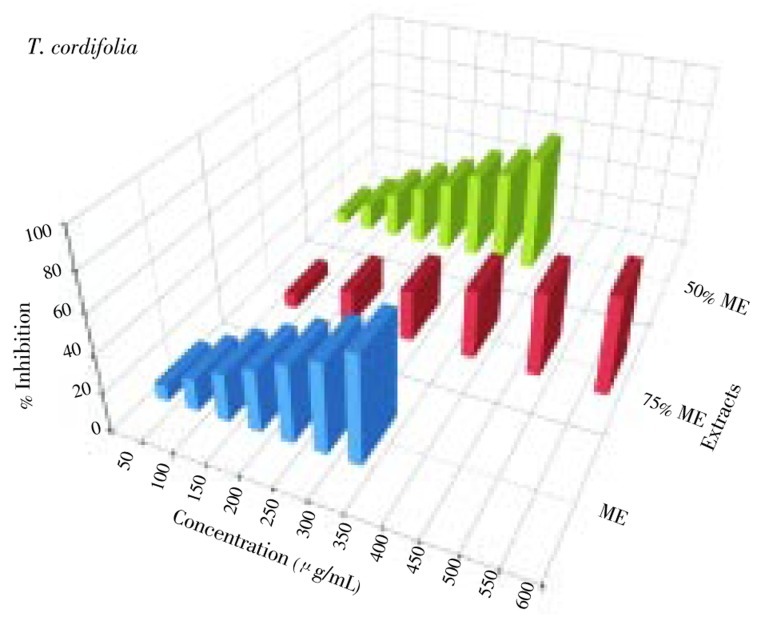
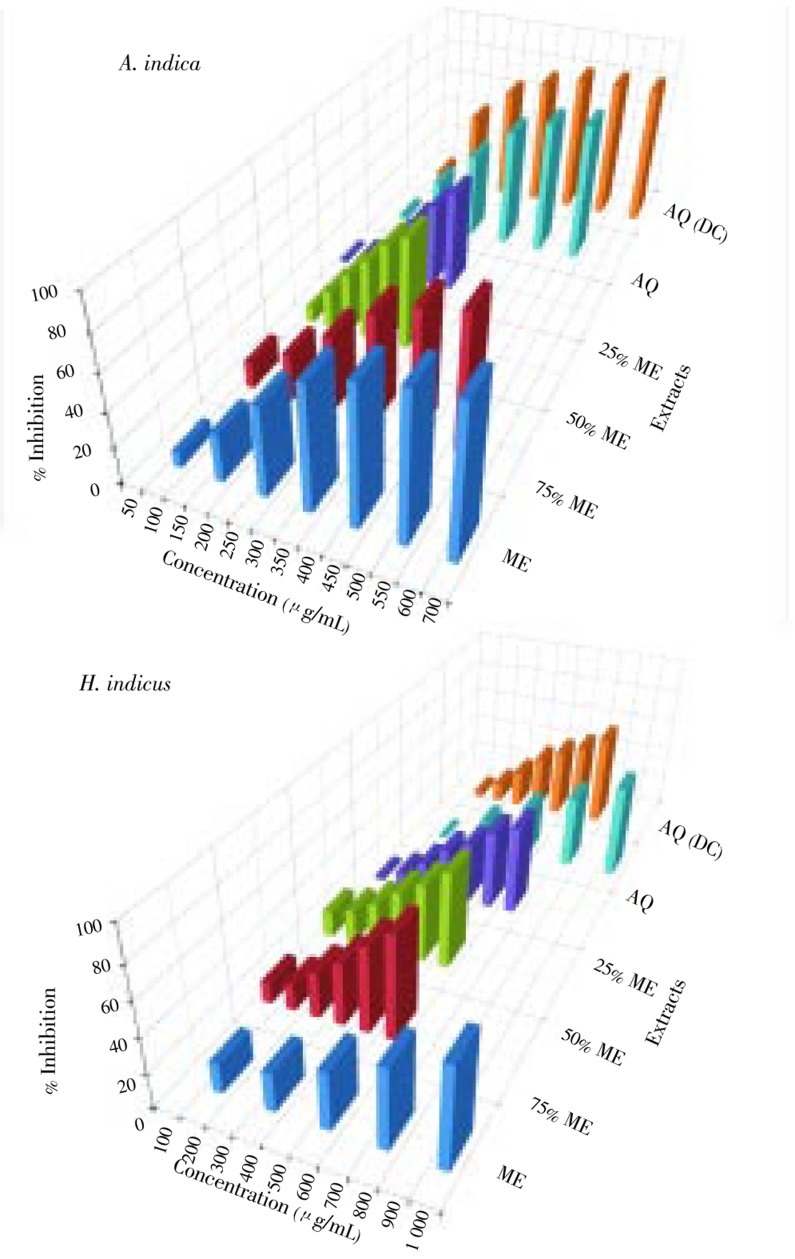
The DPPH free radical scavenging activity of screened plants was shown in Figures 3–5. Six different extracts of six plants were evaluated for their DPPH free radical scavenging activity. Out of 36 extracts investigated, 13 extracts showed IC50 value of more than 1 000 µg/mL (Table 1) while the remaining 23 showed varied levels of DPPH free radical scavenging activity (Table 1). IC50 values ranged from 30 to 620 µg/mL (Table 1). Ascorbic acid was used as a standard and its IC50 value was 11.4 µg/mL (Table 1). Amongst all the extracts, the lowest IC50 value was of 75% ME extract of M. zapota (30 µg/mL) and the highest IC50 value was of 75% ME extract of P. corylifolia (495 µg/mL). Amongst all extracts of all the plant studied, ME, 75% ME and 50% ME showed better DPPH free radical scavenging activity than 25% ME, and both aqueous extracts. Amongst all the plants M. zapota showed the best DPPH free radical scavenging activity.
Figure 3. DPPH free radical scavenging activity of different extracts of A. indica and H. indicus.
AQ: Aqueous extract; DC: Decoction.
Figure 5. DPPH free radical scavenging activity of different extracts of T. cordifolia.
Table 1. IC50 values of DPPH free radical (DPPH) scavenging and superoxide anion radical (SO) scavenging activities.
| Assay | Plant | IC50 values (µg/mL) |
|||||
| Hydroalcoholic |
Decoction aqueous extract | ||||||
| ME | 75% ME | 50% ME | 25% ME | Aqueous extract | |||
| DPPH | A. indica | 34 | 33 | 50 | 121 | 214 | 125 |
| H. indicus | 33 | 53 | 79 | 212 | 480 | 328 | |
| M. zapota | 34 | 30 | 49 | 67 | 120 | 74 | |
| P. corylifolia | 375 | 620 | – | – | – | – | |
| R. cordifolia | – | – | – | – | – | – | |
| T. cordifolia | 315 | 560 | 325 | – | – | – | |
| Standard (ascorbic acid) | 11.4 | ||||||
| SO | A. indica | 360 | 350 | 275 | 305 | 360 | 225 |
| H. indicus | 840 | 490 | 510 | 690 | 920 | 700 | |
| M. zapota | 210 | 225 | 220 | 305 | 360 | 305 | |
| P. corylifolia | – | – | – | – | – | – | |
| R. cordifolia | – | – | – | – | – | – | |
| T. cordifolia | – | – | – | – | – | – | |
| Standard (gallic acid) | 185 | ||||||
Figure 4. DPPH free radical scavenging activity of different extracts of M. zapota and P. corylifolia.
AQ: Aqueous extract; DC: Decoction.
3.3.2. Superoxide anion radical scavenging activity
The superoxide anion radical scavenging activity of screened plants was shown in Figure 6 and 7. Six different extracts of six plants were evaluated for their superoxide anion radical scavenging activity. Out of 36 extracts investigated, 18 extracts showed IC50 value of more than 1 000 µg/mL (Table 1) while the remaining 18 showed varied levels of superoxide anion radical scavenging activity (Table 1). IC50 values ranged from 210 to 920 µg/mL (Table 1). Gallic acid was used as a standard and its IC50 value was 185 µg/mL (Table 1). Among all the extracts, the lowest IC50 value was of ME extract of M. zapota and the highest IC50 value was of aqueous extract of H. indicus (Table 1). Amongst all different extracts of all the plant studied, ME, 75% ME and 50% ME showed better superoxide anion radical scavenging activity than 25% ME and both aqueous extracts. Amongst all the plant studied, different extracts of M. zapota showed the best superoxide anion radical scavenging activity, in some case comparable with standard gallic acid.
Figure 6. Superoxide anion radical scavenging activity of different extracts of A. indica and H. indicus.
AQ: Aqueous extract; DC: Decoction.
Figure 7. Superoxide anion radical scavenging activity of different extracts of M. zapota.
AQ: Aqueous extract; DC: Decoction.
4. Discussion
The extractive yield depends on solvents, time and temperature of extraction as well as the chemical nature of sample. Under the same time and temperature conditions, the solvent used and the chemical property of sample are the most important factors[34]. The traditional healers or practitioners make use of water primarily as a solvent but there are many reports where organic solvents showed better activity as compared with aqueous extracts[35],[36]. In the present study, when aqueous extracts of all the six plants were compared, the extractive yield was maximum in aqueous extract by decoction method than by cold percolation method. This may be because the phytoconstituents present in these plants are extracted better on application of heat. Similar results were obtained in some plants of Zingiberaceae as reported by Chandarana et al[37]. On the other hand, when aqueous extracts by cold percolation method and pure methanol extracts are compared, a different result was obtained. Three plants had more yield in pure methanol while the other three plants had more yield in aqueous extract, which implies that there is no universal criteria for extraction. It varies from plant to plant again may be because of the nature of secondary metabolites present in them and also their proportion. When hydroalcoholic extracts of the six plants are considered, the yield again either increased or decreased than pure solvents.
Phenolics are secondary plant metabolites that are ubiquitously present in plant products[38]. Plant phenolics are biosynthesized following different routes, the shikimic acid pathway being the most biosynthetic route involved[39]. Plant phenolics have been reported to have several biological activities including antioxidant activity[40]. Many of the phenolics have been shown to contain high levels of antioxidant activities[41]. Among the six plants investigated in the present work, M. zapota had considerably greater total phenol content, and methanol was able to extract more phenolic compounds than aqueous, which is also supported by other researchers[42]–[44]. It is important to examine the correlation between the content of total phenols and the antioxidant potential because some authors have reported that there is no correlation between the content of phenolic compound and the radical scavenging capacity[22],[45]. The results obtained in this study do not support these claims.
Because multiple reaction characteristics and mechanisms are likely to be involved, no single assay will accurately reflect all antioxidants in a mixed or complex system. Thus, to fully elucidate a full profile of antioxidant capacity of different extracts, different antioxidant capacity assays were used in this study. DPPH, superoxide anion radical scavenging activity assays are most commonly accepted ones which have been used in the present investigation[46],[47]. DPPH has been used extensively as a free radical to evaluate reducing substances and is a useful reagent for investigating the free radical scavenging activities of compounds[48]. The use of the DPPH radical provides an easy, rapid and convenient method to evaluate the antioxidant and radical scavengers[49],[50]. The DPPH radical scavenging activity of extract has been attributed to the ability of these extracts in pairing with the old electron of DPPH radical[51]. The antioxidants are able to reduce the stable DPPH radical to yellow-colored and the antioxidant power is indicated by the degree of discoloration which could be determined by measuring of a decrease in the absorbance at 517 nm[52]. Because of the ease and convenience of this reaction, it has now widespread use in the free radical scavenging activity assessment[40]. The lowest IC50 value was in M. zapota irrespective of the solvent used. Incidentally this plant in different methanol concentrations, i.e. ME, 75% ME and 50% ME had maximum phenol content thus supporting the general view that phenol content is a good indicator of antioxidant activity.
Scavenging of superoxide anion radical is important for protection against early events in oxidative damage[53]. Superoxide anion is the most common free radical in vivo and is generated in a variety of biological system and the concentration of superoxide anion increases, under condition of oxidative stress[54]. In the PMS-NADH-NBT system, superoxide anions were derived from dissolved oxygen by the PMS-NADH coupling reaction, which then reduced to NBT. The decreased absorbance at 500 nm with antioxidants indicates consumption of the superoxide anion in the reaction mixture[33]. The lowest IC50 value was in M. zapota in ME, 75% ME and 50% ME except in aqueous extract by decoction method in which A. indica had the lowest IC50 value. Incidentally these three extracts of M. zapota had the highest phenolic content which again suggests that there is a direct correlation between phenol content and antioxidant activity. This is in accordance with results of others[23],[55],[56].
Antioxidant properties, especially radical scavenging activities, are very important due to the deleterious role of free radicals in foods and in biological systems[57]. The difference in the antioxidant activity of the different hydroalcoholic extracts may be ascribed to the difference in the total phenolic compositions. As far as the free radical scavenging efficiency was concerned, the antioxidant activity of the different hydroalcoholic extracts analyses was found to have a strong positive correlation with their total phenolic content. Results from this study also provide a better understanding for the selection of an appropriate solvent and extraction method especially for M. zapota so that its full potential can be utilized. It could also be useful for further investigations.
This study indicated that different types of extraction method and solvent had great influence on the antioxidant property of obtained extracts. The highest total phenol content and the best antioxidant activity i.e. the lowest IC50 value in DPPH as well as in superoxide anion radical scavenging activity was shown by M. zapota. Therefore, it can be stated that there was a direct correlation observed between total phenolic content and antioxidant activity. Methanolic extracts (ME, 75% ME and 50% ME) showed better antioxidant activity and more phenol content as compared with aqueous extracts. Hence, it can be concluded that M. zapota is the best source of natural antioxidants. Organic solvent extraction (either 100% or 75%) is better than aqueous extraction by either method i.e. cold percolation or decoction method. The specific components that confer the antioxidant properties on the M. zapota extracts are currently unknown. A further study is underway to identify and characterize chemicals contributing to antioxidant properties of M. zapota extracts.
Acknowledgments
The authors thank Prof. Singh SP, Head, Department of Biosciences, Saurashtra University, Rajkot, Gujarat, India for providing excellent research facilities. One of the author, Mr. Mital Kaneria is also thankful to University Grants Commission, New Delhi, India for providing financial support as Junior Research Fellow.
Footnotes
Foundation Project: This work was financially supported by University Grants Commission, New Delhi, India [grant No. F.4-3/2006(BSR)/5-121/2007(BSR)].
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Rackova L, Oblozinsky M, Kostalova D, Kettmann V, Bezakova L. Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids. J Inflamm. 2007;4:15–21. doi: 10.1186/1476-9255-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinnula VL, Crapo JD. Superoxide dismutases in malignant cells and human tumors. Free Radic Biol Med. 2004;36:718–744. doi: 10.1016/j.freeradbiomed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Leopold JA, Loscalzo J. Oxidative risk for a therombotic cardiovascular diseases. Free Radic Biol Med. 2009;47:1673–1706. doi: 10.1016/j.freeradbiomed.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butterfield DA, Sultana R. Redox proteomics: understanding oxidative stress in the progression of age related neurodegenerative disorders. Expert Rev Proteomics. 2008;5:157–160. doi: 10.1586/14789450.5.2.157. [DOI] [PubMed] [Google Scholar]
- 5.Butterfield DA, Castenga A, Pocernich CB, Drak J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer's diseases. J Nutr Biochem. 2002;13:444–461. doi: 10.1016/s0955-2863(02)00205-x. [DOI] [PubMed] [Google Scholar]
- 6.Guidi I, Galimberti D, Lonati S, Novembrino C, Bamonti F, Tiriticco M, et al. Oxidative imbalance in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2006;27:262–269. doi: 10.1016/j.neurobiolaging.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 8.Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–790. doi: 10.1053/gast.2003.50087. [DOI] [PubMed] [Google Scholar]
- 9.Ramakrishna BS, Varghese R, Jayakumar S, Mathan M, Balasubramanian KA. Circulating antioxidants in ulcerative colitis and their relationship to disease severity and activity. J Gastroenterol Hepatol. 1997;12:490–494. doi: 10.1111/j.1440-1746.1997.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 10.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Upston JM, Kritharides L, Stocker R. The role of vitamin E in atherosclerosis. Prog Lipid Res. 2003;42:405–422. doi: 10.1016/s0163-7827(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 12.Jainu M, Shyamala Devi CS. In vitro and in vivo evaluation of free radical scavenging potential of Cissus quadrangularis. Afr J Biomed Res. 2005;8:95–99. [Google Scholar]
- 13.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role of inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halliwell B. How to characterize an antioxidant: an update. Biochem Soc Symp. 1995;61:73–101. doi: 10.1042/bss0610073. [DOI] [PubMed] [Google Scholar]
- 15.Buyukokuroglu ME, Gulcin I, Oktay M, Kufrevioglu OI. In vitro antioxidant properties of dantrolene sodium. Pharmacol Res. 2001;44:491–494. doi: 10.1006/phrs.2001.0890. [DOI] [PubMed] [Google Scholar]
- 16.Charmi MT, Lazari D, Karioti A, Skaltsa H, Litina DH, Souleles C. Antioxidant and anti-inflammatory activities of Sideritis perfoliata subsp. perfoliata (Lamiaceae) Phytother Res. 2008;22:450–454. doi: 10.1002/ptr.2333. [DOI] [PubMed] [Google Scholar]
- 17.Unver A, Arslan D, Ozcan MM, Akbulut M. Phenolic content and antioxidant activity of some spices. World Appl Sci J. 2009;6:373–377. [Google Scholar]
- 18.Banerjee D, Chakrabarti S, Hazara AK, Benwerjee S, Ray J, Mukharjee B. Antioxidant activity and total phenolics of some mangroves in sunderbans. Afr J Biotechnol. 2008;7:805–810. [Google Scholar]
- 19.Duthie G, Crozier A. Plant-derived phenolic antioxidants. Curr Opin Clin Nutr Metab Care. 2000;3:447–451. doi: 10.1097/00075197-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Baravalia Y, Kaneria M, Vaghasiya Y, Parekh J, Chanda S. Antioxidant and antibacterial activity of Diospyros ebenum Roxb. leaf extracts. Turk J Biol. 2009;33:159–164. [Google Scholar]
- 21.Kaneria M, Baravalia Y, Vaghasiya Y, Chanda S. Determination of antibacterial and antioxidant potential of some medicinal plants from Saurashtra region, India. Indian J Pharm Sci. 2009;71:406–412. doi: 10.4103/0250-474X.57289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locatelli M, Travaglia F, Coissos JD, Martelli A, Stevigny C, Arlorio M. Total antioxidant activity of hazelnut skin (Nocciola piemonte PGI): impact of different roasting condition. Food Chem. 2010;119:1647–1655. [Google Scholar]
- 23.Rakholiya K, Kaneria M, Chanda S. Vegetable and fruit peels as a novel source of antioxidants. J Med Plant Res. 2011;5:63–71. [Google Scholar]
- 24.Arno MB, Canao A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73:239–244. [Google Scholar]
- 25.Salazar R, Pozos ME, Cordero P, Perez J, Salinas MC, Waksman N. Determination of the antioxidant activity of plants from Northeast Mexico. Pharm Biol. 2008;46:166–170. [Google Scholar]
- 26.Chanda S, Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr J Microbiol Res. 2009;3:981–996. [Google Scholar]
- 27.Anjaria J, Parabia M, Dwivedi S. Ethnovet heritage Indian ethnoveterinary medicines: an overview. 1st ed. Ahmedabad: Pathik Enterprise; 2002. [Google Scholar]
- 28.Parekh J, Chanda S. In vitro antibacterial activity of the crude methanol extract of Woodfordia fructicosa Kurz. flower (Lythraceae) Braz J Microbiol. 2007;38:204–207. [Google Scholar]
- 29.Li HB, Jiang Y, Wong CC, Cheng KW, Chen F. Evaluation of two methods for the extraction of antioxidants from medicinal plants. Anal Bioanal Chem. 2007;388:483–488. doi: 10.1007/s00216-007-1235-x. [DOI] [PubMed] [Google Scholar]
- 30.McDonald S, Prenzler PD, Antolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73–84. [Google Scholar]
- 31.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 32.McCune LM, Johns T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J Ethnopharmacol. 2002;82:197–205. doi: 10.1016/s0378-8741(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 33.Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988;37:837–841. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- 34.Shimada K, Fulikawa K, Yahara K, Nakamura T. Antioxidative properties of Xanthem autoxidation of soyabean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. [Google Scholar]
- 35.De Boer HJ, Kool A, broberg A, Mziray WR, Hedberg J, Levenfors JJ. Antifungal and antibacterial activity of some herbal remedies from Tanzania. J Ethnopharmacol. 2005;96:461–469. doi: 10.1016/j.jep.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 36.Parekh J, Chanda S. In vitro antimicrobial activities of extracts of Launaea procumbens Roxb. (Labiateae), Vitis vinifera L. (Vitaceae) and Cyperus rotundus L. (Cyperaceae) Afr J Biomed Res. 2006;9:89–93. [Google Scholar]
- 37.Chandarana H, Baluja S, Chanda S. Comparison of antibacterial activities of selected species of Zingiberaceae family and some synthetic compounds. Turk J Biol. 2005;29:83–97. [Google Scholar]
- 38.Razali N, Razab R, Junit SM, Aziz AA. Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidantle) Food Chem. 2008;111:38–44. [Google Scholar]
- 39.Rivero RM, Ruiz JM, Gria PC, Lopez-Lefevr LR, Sanchez E, Romero L. Resistance to cold and heat stress: accumulatoin of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001;160:315–321. doi: 10.1016/s0168-9452(00)00395-2. [DOI] [PubMed] [Google Scholar]
- 40.Chandini SK, Ganesan P, Bhaskar N. In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem. 2008;107:707–713. [Google Scholar]
- 41.Rice-Evans C, Miller N, Paganga G. Structure-antioxidant activity relationships of flavanoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 42.Kumar RA, Rajkumar V, Guha G, Mathew L. Therapeutic potentials of Oroxylum indicum bark extracts. Chin J Nat Med. 2010;8:121–126. [Google Scholar]
- 43.Rajkumar V, Guha G, Kumar RA. Antioxidant and anti-neoplastic activities of Picrorhiza kurroa extracts. Food Chem Toxicol. 2011;49:363–369. doi: 10.1016/j.fct.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Zhu KX, Lian CX, Guo XN, Peng W, Zhou HM. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 2011;126:1122–1126. [Google Scholar]
- 45.Yu L, Haley S, Perret J, Harris M, Wilson J, Qian M. Free radical scavenging properties of wheat extracts. J Agric Food Chem. 2002;50:1619–1624. doi: 10.1021/jf010964p. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Wang M, Rosen RE, Ho CT. DPPH radical scavenging active components from Polygonum multiflorum Thumb. J Agric Food Chem. 1999;47:2226–2228. doi: 10.1021/jf990092f. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Moreno C, Larrauri JA, Saura-Calixto F. Free radical scavenging capacity of selected red rose and white wines. J Sci Food Agric. 1999;79:1301–1304. doi: 10.1021/jf980607n. [DOI] [PubMed] [Google Scholar]
- 48.Duan XJ, Zhang WW, Li XM, Wang BG. Evolution of antioxidant property of extract and fractions obtained from a red alga Polysiphonia urceolata. Food Chem. 2006;95:37–43. [Google Scholar]
- 49.Argolo ACC, Sant'Ana AEG, Pletsch M, Coelho LCB. Antioxidant activity of leaf extracts from Bauhinia monandra. Bioresour Technol. 2004;95:229–233. doi: 10.1016/j.biortech.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Roginsky V, Lissi EA. Review of methods to determine chain breaking antioxidant activity in food. Food Chem. 2005;92:235–254. [Google Scholar]
- 51.Park PJ, Shidi F, Yoen YJ. Antioxidantactivities of enzymatic extraccs from and edible seaweed Sarassum horneri using ESR spectroscopy. J Food Lipids. 2004;11:15–27. [Google Scholar]
- 52.Pan Y, Wang K, Huang S, Wang H, Mu X, He C, et al. Antioxidant activity of microwave assisted extract of longan (Dimocarpus longan Lour.) peel. Food Chem. 2008;106:1264–1270. [Google Scholar]
- 53.Fu M, Mao L. In vitro antioxidant activities of five cultivars of daylily flowers from China. Nat Prod Res. 2008;22:584–591. doi: 10.1080/14786410701592828. [DOI] [PubMed] [Google Scholar]
- 54.Lee JC, Kim HR, Kim J, Jang YS. Antioxidant property of ethanol extract of the stem of Opuntia ficus-indica var. saboten. J Agric Food Chem. 2002;50:6490–6496. doi: 10.1021/jf020388c. [DOI] [PubMed] [Google Scholar]
- 55.Saxena S, Hajare SN, More V, Kumar S, Wadhawan S, Mishra BB, et al. Antioxidant and radioprotective properties of commercially grown litchi (Litchi chinensis) from India. Food Chem. 2011;126:39–45. [Google Scholar]
- 56.Wu CR, Lin WH, Hseu YC, Lien JC, Lin YT, Kuo TP, et al. Evaluation of the antioxidant activity of five endemic Ligustrum species leaves from Taiwan flora in vitro. Food Chem. 2011;127:564–571. doi: 10.1016/j.foodchem.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 57.Gulcin I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006;78:803–811. doi: 10.1016/j.lfs.2005.05.103. [DOI] [PubMed] [Google Scholar]