Abstract
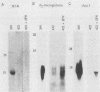
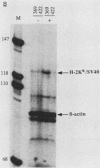
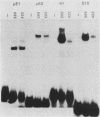
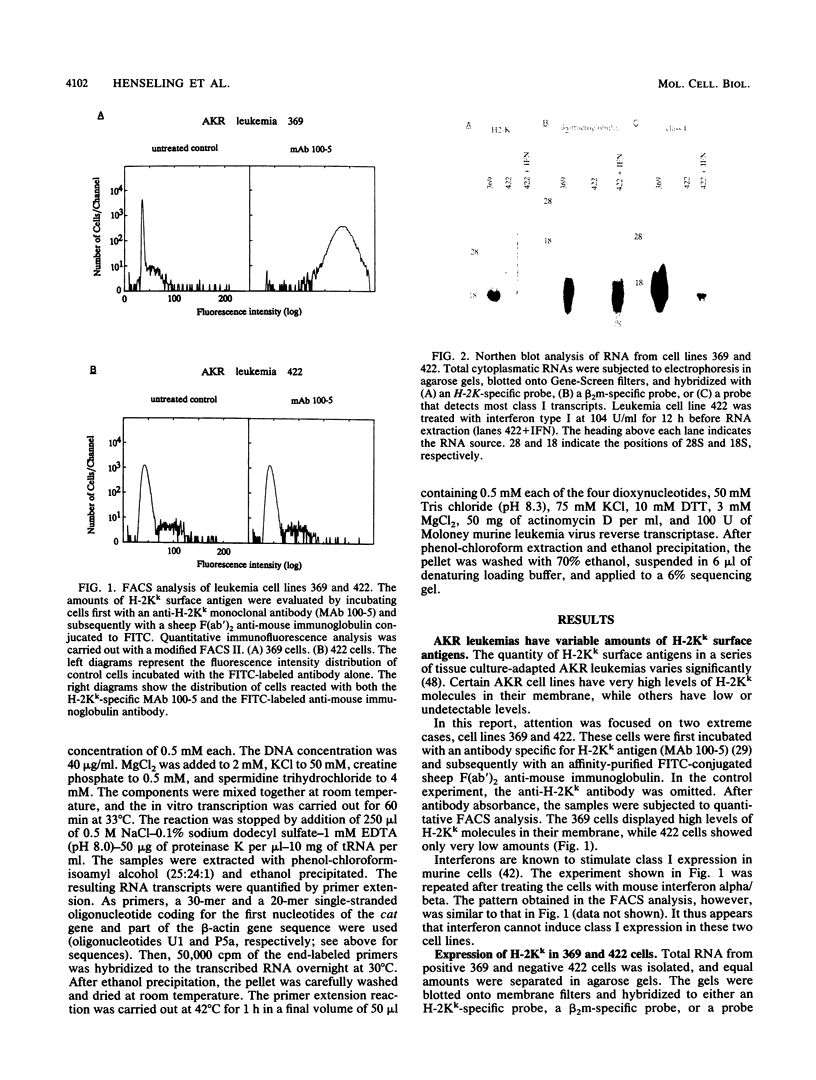
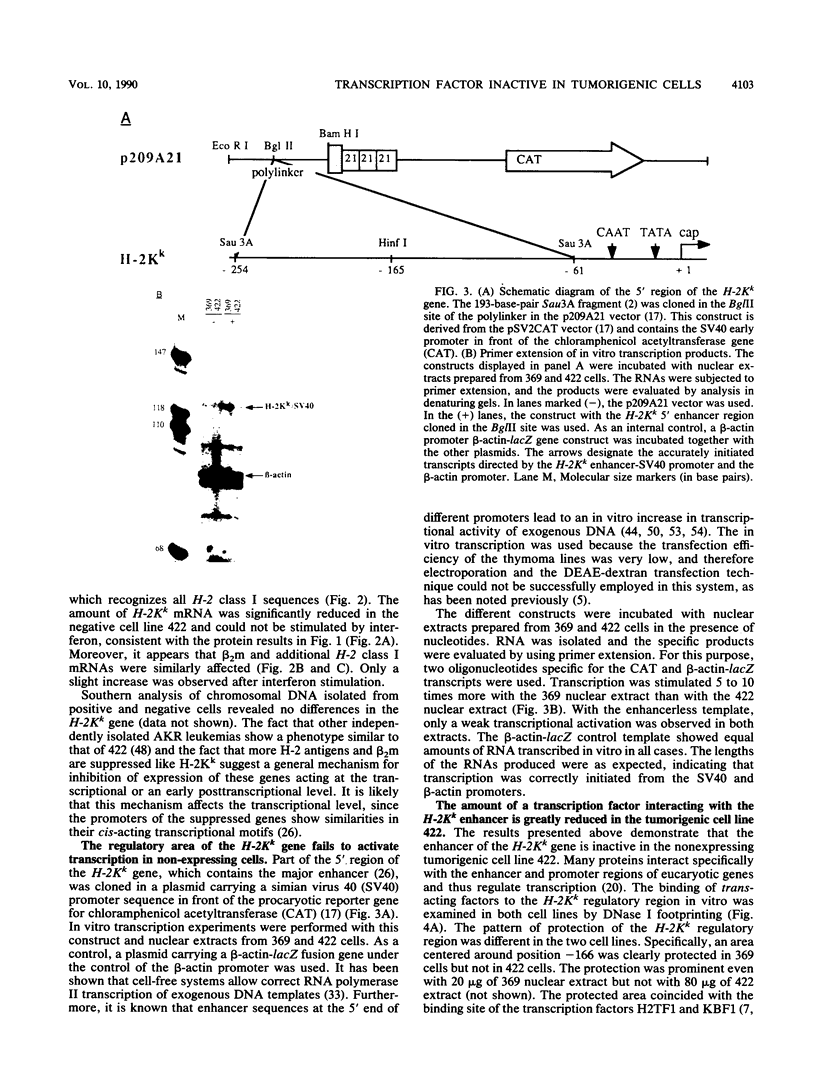
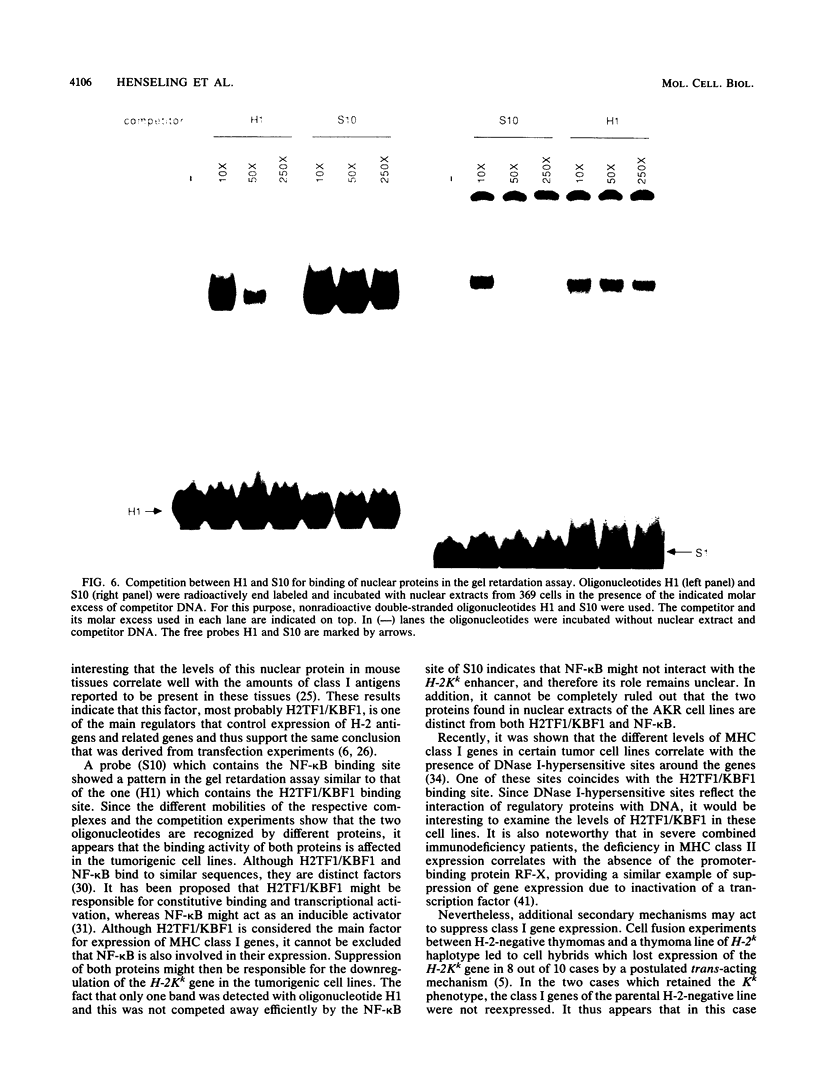
AKR leukemias display different amounts of major histocompatibility complex class I antigens on the cell surface. The absence of H-2Kk molecules correlates with the ability of these cell lines to form tumors in vivo as well as to escape lysis by cytotoxic T lymphocytes in vitro. In this report it is shown that the 5' regulatory area of the H-2Kk gene failed to activate transcription in H-2Kk-negative cells. Examination of the proteins interacting with the H-2Kk enhancer in expressing and nonexpressing cells revealed clear differences. In particular, the level of a nuclear protein interacting at position -166 was greatly reduced in the negative cell lines. A transcription factor, known as H2TF1 or KBF1, has been shown previously to interact with this binding site and to be essential for the expression of certain class I genes as well as the expression of beta 2-microglobulin. These results demonstrate that the molecular mechanism of class I gene suppression in malignant tumor cells is at the level of transcription and is most probably modulated by H2TF1/KBFI. In addition, it is shown that the same transcription factor is only present in mouse tissues expressing class I antigens.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce-Gomez B., Jones E. A., Barnstable C. J., Solomon E., Bodmer W. F. The genetic control of HLA-A and B antigens in somatic cell hybrids: requirement for beta2 microglobulin. Tissue Antigens. 1978 Feb;11(2):96–112. doi: 10.1111/j.1399-0039.1978.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Arnold B., Burgert H. G., Archibald A. L., Kvist S. Complete nucleotide sequence of the murine H-2Kk gene. Comparison of three H-2K locus alleles. Nucleic Acids Res. 1984 Dec 21;12(24):9473–9487. doi: 10.1093/nar/12.24.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augereau P., Chambon P. The mouse immunoglobulin heavy-chain enhancer: effect on transcription in vitro and binding of proteins present in HeLa and lymphoid B cell extracts. EMBO J. 1986 Aug;5(8):1791–1797. doi: 10.1002/j.1460-2075.1986.tb04428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baldacci P., Transy C., Cochet M., Penit C., Israel A., Kourilsky P. A trans-acting mechanism represses the expression of the major transplantation antigens in mouse hybrid thymoma cell lines. J Exp Med. 1986 Sep 1;164(3):677–694. doi: 10.1084/jem.164.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Sharp P. A. Binding of a nuclear factor to a regulatory sequence in the promoter of the mouse H-2Kb class I major histocompatibility gene. Mol Cell Biol. 1987 Jan;7(1):305–313. doi: 10.1128/mcb.7.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Sharp P. A. Two transcription factors, NF-kappa B and H2TF1, interact with a single regulatory sequence in the class I major histocompatibility complex promoter. Proc Natl Acad Sci U S A. 1988 Feb;85(3):723–727. doi: 10.1073/pnas.85.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Uehara H., Martinko J., Nathenson S. G. Primary structure of a murine transplantation antigen. Nature. 1981 May 7;291(5810):35–39. doi: 10.1038/291035a0. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984 Sep;38(3):287–292. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- De Baetselier P., Katzav S., Gorelik E., Feldman M., Segal S. Differential expression of H-2 gene products in tumour cells in associated with their metastatogenic properties. Nature. 1980 Nov 13;288(5787):179–181. doi: 10.1038/288179a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Festenstein H., Schmidt W. Variation in MHC antigenic profiles of tumor cells and its biological effects. Immunol Rev. 1981;60:85–127. doi: 10.1111/j.1600-065x.1981.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Stark G. R. alpha-Interferon-induced transcription of HLA and metallothionein genes containing homologous upstream sequences. Nature. 1985 Apr 18;314(6012):637–639. doi: 10.1038/314637a0. [DOI] [PubMed] [Google Scholar]
- Goodenow R. S., Vogel J. M., Linsk R. L. Histocompatibility antigens on murine tumors. Science. 1985 Nov 15;230(4727):777–783. doi: 10.1126/science.2997918. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- Hui K., Grosveld F., Festenstein H. Rejection of transplantable AKR leukaemia cells following MHC DNA-mediated cell transformation. Nature. 1984 Oct 25;311(5988):750–752. doi: 10.1038/311750a0. [DOI] [PubMed] [Google Scholar]
- Hämmerling G. J., Klar D., Pülm W., Momburg F., Moldenhauer G. The influence of major histocompatibility complex class I antigens on tumor growth and metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):245–259. doi: 10.1016/0304-419x(87)90008-4. [DOI] [PubMed] [Google Scholar]
- Israel A., Kimura A., Fournier A., Fellous M., Kourilsky P. Interferon response sequence potentiates activity of an enhancer in the promoter region of a mouse H-2 gene. Nature. 1986 Aug 21;322(6081):743–746. doi: 10.1038/322743a0. [DOI] [PubMed] [Google Scholar]
- Israël A., Kimura A., Kieran M., Yano O., Kanellopoulos J., Le Bail O., Kourilsky P. A common positive trans-acting factor binds to enhancer sequences in the promoters of mouse H-2 and beta 2-microglobulin genes. Proc Natl Acad Sci U S A. 1987 May;84(9):2653–2657. doi: 10.1073/pnas.84.9.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies W. A., Rüther U., Wagner E. F., Kvist S. Cytolytic T cells recognize a chimeric MHC class I antigen expressed in influenza A infected transgenic mice. EMBO J. 1988 Nov;7(11):3423–3431. doi: 10.1002/j.1460-2075.1988.tb03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Israël A., Le Bail O., Kourilsky P. Detailed analysis of the mouse H-2Kb promoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell. 1986 Jan 31;44(2):261–272. doi: 10.1016/0092-8674(86)90760-9. [DOI] [PubMed] [Google Scholar]
- Korber B., Hood L., Stroynowski I. Regulation of murine class I genes by interferons is controlled by regions located both 5' and 3' to the transcription initiation site. Proc Natl Acad Sci U S A. 1987 May;84(10):3380–3384. doi: 10.1073/pnas.84.10.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet D., Parham P., Strominger J. L. Heavy chain of HLA-A and HLA-B antigens is conformationally labile: a possible role for beta 2-microglobulin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3844–3848. doi: 10.1073/pnas.76.8.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke H., Hammerling G. J., Hohmann C., Rajewsky K. Hybrid cell lines secreting monoclonal antibody specific for major histocompatibility antigens of the mouse. Nature. 1978 Jan 19;271(5642):249–251. doi: 10.1038/271249a0. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Fan C. M., Maniatis T., Baltimore D. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell. 1989 Apr 21;57(2):287–294. doi: 10.1016/0092-8674(89)90966-5. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschek U., Pülm W., Hämmerling G. J. Altered regulation of MHC class I genes in different tumor cell lines is reflected by distinct sets of DNase I hypersensitive sites. EMBO J. 1989 Aug;8(8):2297–2304. doi: 10.1002/j.1460-2075.1989.tb08356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M. S., Weissman I. L. AKR leukemogenesis: identification and biological significance of thymic lymphoma receptors for AKR retroviruses. Cell. 1979 May;17(1):65–75. doi: 10.1016/0092-8674(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Miyazaki J., Appella E., Ozato K. Negative regulation of the major histocompatibility class I gene in undifferentiated embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9537–9541. doi: 10.1073/pnas.83.24.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello D., Daniel F., Baldacci P., Cayre Y., Gachelin G., Kourilsky P. Absence of significant H-2 and beta 2-microglobulin mRNA expression by mouse embryonal carcinoma cells. Nature. 1982 Mar 18;296(5854):260–262. doi: 10.1038/296260a0. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Giacomini P., Bigotti A., Imai K., Nicotra M. R., Ng A. K., Ferrone S. Heterogeneity in the expression of HLA and tumor-associated antigens by surgically removed and cultured breast carcinoma cells. Cancer Res. 1983 Feb;43(2):660–668. [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980 Oct 25;255(20):9678–9684. [PubMed] [Google Scholar]
- Ozato K., Wan Y. J., Orrison B. M. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2427–2431. doi: 10.1073/pnas.82.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W., Satola S., Sanchez C. H., Amaldi I., Lisowska-Grospierre B., Griscelli C., Hadam M. R., Mach B. Congenital immunodeficiency with a regulatory defect in MHC class II gene expression lacks a specific HLA-DR promoter binding protein, RF-X. Cell. 1988 Jun 17;53(6):897–906. doi: 10.1016/s0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Rosa F., Le Bouteiller P. P., Abadie A., Mishal Z., Lemonnier F. A., Bourrel D., Lamotte M., Kalil J., Jordan B., Fellous M. HLA class I genes integrated into murine cells are inducible by interferon. Eur J Immunol. 1983 Jun;13(6):495–499. doi: 10.1002/eji.1830130612. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Wright S., Cedar H., Flavell R., Grosveld F. Regulated expression of an introduced MHC H-2K bm1 gene in murine embryonal carcinoma cells. Nature. 1984 Aug 2;310(5976):415–418. doi: 10.1038/310415a0. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Wildeman A., Chambon P. A trans-acting factor is responsible for the simian virus 40 enhancer activity in vitro. Nature. 1985 Feb 7;313(6002):458–463. doi: 10.1038/313458a0. [DOI] [PubMed] [Google Scholar]
- Schlokat U., Bohmann D., Schöler H., Gruss P. Nuclear factors binding specific sequences within the immunoglobulin enhancer interact differentially with other enhancer elements. EMBO J. 1986 Dec 1;5(12):3251–3258. doi: 10.1002/j.1460-2075.1986.tb04636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W., Festenstein H. Resistance to cell-mediated cytotoxicity is correlated with reduction of H-2K gene products in AKR leukemia. Immunogenetics. 1982;16(3):257–264. doi: 10.1007/BF00343314. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Henseling U., Bevec D., Alonzo A. D., Festenstein H. Control of synthesis and expression of H-2 heavy chain and beta-2 microglobulin in AKR leukemias. Immunogenetics. 1985;22(5):483–494. doi: 10.1007/BF00418093. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Leben L., Atfield G., Festenstein H. Variation of expression of histocompatibility antigens on tumor cells: absence of H-2Kk-gene products from a gross-virus-induced leukemia in BALB.K. Immunogenetics. 1981;14(3-4):323–339. doi: 10.1007/BF00342201. [DOI] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Cell type-specific transcriptional enhancement in vitro requires the presence of trans-acting factors. EMBO J. 1985 Nov;4(11):3005–3013. doi: 10.1002/j.1460-2075.1985.tb04036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Hatzopoulos A. K., Balling R., Suzuki N., Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989 Sep;8(9):2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. In vitro transcription of immunoglobulin genes in a B-cell extract: effects of enhancer and promoter sequences. Mol Cell Biol. 1987 May;7(5):1989–1994. doi: 10.1128/mcb.7.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant A., Bohmann D., Zentgraf H., Weiher H., Keller W. A transcription enhancer acts in vitro over distances of hundreds of base-pairs on both circular and linear templates but not on chromatin-reconstituted DNA. J Mol Biol. 1984 Dec 15;180(3):577–600. doi: 10.1016/0022-2836(84)90028-7. [DOI] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Frelinger J. G., Fisher D., Hunkapiller T., Pereira D., Weissman S. M., Uehara H., Nathenson S., Hood L. Three cDNA clones encoding mouse transplantation antigens: homology to immunoglobulin genes. Cell. 1981 Apr;24(1):125–134. doi: 10.1016/0092-8674(81)90508-0. [DOI] [PubMed] [Google Scholar]
- Travers P. J., Arklie J. L., Trowsdale J., Patillo R. A., Bodmer W. F. Lack of expression of HLA-ABC antigens in choriocarcinoma and other human tumor cell lines. Natl Cancer Inst Monogr. 1982;60:175–180. [PubMed] [Google Scholar]
- Wallich R., Bulbuc N., Hämmerling G. J., Katzav S., Segal S., Feldman M. Abrogation of metastatic properties of tumour cells by de novo expression of H-2K antigens following H-2 gene transfection. Nature. 1985 May 23;315(6017):301–305. doi: 10.1038/315301a0. [DOI] [PubMed] [Google Scholar]
- Weinberger J., Baltimore D., Sharp P. A. Distinct factors bind to apparently homologous sequences in the immunoglobulin heavy-chain enhancer. 1986 Aug 28-Sep 3Nature. 322(6082):846–848. doi: 10.1038/322846a0. [DOI] [PubMed] [Google Scholar]
- Wildeman A. G., Sassone-Corsi P., Grundström T., Zenke M., Chambon P. Stimulation of in vitro transcription from the SV40 early promoter by the enhancer involves a specific trans-acting factor. EMBO J. 1984 Dec 20;3(13):3129–3133. doi: 10.1002/j.1460-2075.1984.tb02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J. H., Kvist S., Dobberstein B. Identification of an H-2Kd gene using a specific cDNA probe. EMBO J. 1982;1(4):467–471. doi: 10.1002/j.1460-2075.1982.tb01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano O., Kanellopoulos J., Kieran M., Le Bail O., Israël A., Kourilsky P. Purification of KBF1, a common factor binding to both H-2 and beta 2-microglobulin enhancers. EMBO J. 1987 Nov;6(11):3317–3324. doi: 10.1002/j.1460-2075.1987.tb02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]