Abstract
Objective
To evaluate the pharmacognostic characters of an important medicinal plant, Holoptelea integrifolia (H. integrifolia) Roxb.
Methods
The pharmacognostic studies were carried out in terms of organoleptic, microscopic, macroscopic and fluorescence analysis.
Results
The characteristic microscopic features of leaves were observed as trichomes, multicellular trichomes, xylem cells, phloem cells, collenchyma, vascular bundles, spongy parenchyma and palisade cells. The characteristic microscopic features of root bark included cork cambium, primary cortex, phloem fibers, medullary rays, endodermis, pericycle and lignified fibers in the transverse section and longitudinal section. The characteristic microscopy of root bark powder showed the presence of cortex cells, sieve tubes, calcium oxalate crystals and lignified fibers. Macroscopic study showed that leaf shape-oval, apex-acute, base-cordate and leaf margin was entire with glabrous surface, bitter taste and characteristic odour. The morphological features of root bark showed deep fissured, rough and firm surface with rhitydome and the periderm parallel to cambium.
Conclusions
Various pharmacognostic characters observed in this study help in the identification and standardization of H. integrifolia.
Keywords: Holoptelea integrifolia, Microscopy, Organoleptic, Fluorescence analysis, Identification, Standardization, Pharmacognostic character
1. Introduction
Over the last decade there has been a growing interest in drugs of plant origin in contrast to the synthetics that are regarded as unsafe to human and environment[1]. Holoptelea integrifolia (H. integrifolia) is a roadside tree of family Ulmaceae and commonly known as Indian elm. It has gray bark covered with blister peeling in corky scales on old trees. In traditional system of medicine, bark and leaves are used as bitter, astringent, acrid, thermogenic, anti-inflammatory, digestive, carminative, laxative, anthelmintic, depurative, repulsive, urinary astringent, and in rheumatism[2],[3]. The bark of H. integrifolia can be easily adulterated with low grade material if the supply of crude drug is inadequate[4]. This adulteration can be prevented by means of various evaluation parameters like microscopic study. Microscopy is an important tool for authentification of crude drugs and study of powdered drugs[5]. It is important to interpret morphological and anatomical descriptions of crude drugs as well as characteristic features of drugs and adulterants of commercial significance[6]. Establishment of the pharmacognostic, morphological and microscopical characters of leaves and bark of the plant will assist in standardization, which can guarantee quality, purity and identification of samples.
2. Materials and methods
2.1. Chemicals
All the chemicals used were of analytical grade and were obtained from E. Merck Limited India and Hi-Media Laboratories, Mumbai, India.
2.2. Procurement of plant materials
Fresh leaves and root bark of H. integrifolia were collected from Ch. Devilal Herbal Nature Park, Chuharpur, Yamunanagar. Identification of the plant was done by Dr. HB Singh, NISCAIR under reference number (NISCAIR/RHMD/Consult/-2010-11/1574/172).
2.3. Organoleptic evaluation
Various sensory parameters of the plant material (such as colour, odour, size, shape, and taste) were studied by organoleptic evaluation.
2.4. Macroscopic evaluation
Various macroscopic characters of fresh leaves of H. integrifolia were recorded such as duration, type of leaf base, presence or absence of petiole and characters of lamina. Lamina consists of characteristic features such as composition, incision, shape, venation, margin, apex, base, surface and texture. The root bark is morphologically studied for its size, shape, surface, fracture and configuration[7].
2.5. Microscopic evaluation
In microscopic evaluation, studies were conducted on both grounds qualitatively and quantitatively. The model of microscope used for study of different characters was SKC-400, Suswox Optik, Sudheer Scientific Works, India.
2.5.1. Qualitative microscopy
In this study, transverse sections of leaf and root bark as well as longitudinal section of root bark were studied under photomicrograph. Staining reagents (such as phloroglucinol-HCl and methyl orange) were used as per standard procedures[8]–[11]. The various identifying characters were studied with or without staining and recorded.
2.5.1.1. Leaf microscopy
In this study, leaf was dipped in chloral hydrate solution for several hours until it lost its colour and pigments. The cube of pith was selected and vertically cut, leaf was inserted and fine sections were obtained. Fine sections mounted on glass slide with help of glycerin without any staining reagent used were placed under microscope. Staining of the fine sections with methyl orange and phloroglucinol was done. Various identifying characters, such as type of trichomes[12] and cell composition[13] were recorded and then photomicrography was done.
2.5.1.2. Root bark microscopy
The root bark was placed in a test tube containing sufficient water and was boiled for few minutes. The softened bark was transversally and longitudinally sliced into fine sections. These fine sections were subjected to staining reagent 0.1% w/v phloroglucinol followed by concentrated hydrochloric acid. The stained and unstained sections were observed under microscope[13],[14]. Different layers of cells and identifying characters were observed and then photomicrography was done.
2.5.1.3. Powder microscopy
The dried root bark was powdered and studied under microscope. Different staining reagents (such as iodine for detection of starch grains and phloroglucinol for detection of lignified components) were used. A little quantity of root bark powder was taken onto a microscopic slide, 1–2 drops of 0.1% w/v phloroglucinol solution and a drop of concentrated hydrochloric acid were added and covered with a cover slip. The slide preparation was mounted in glycerol and examined under microscope. The presence of starch grain and calcium oxalate crystal was detected by the formation of blue colour on addition of 2–3 drops of 0.01 M iodine solution[15]. The characteristic structures and cell components were observed and their photographs were taken using photomicrography.
2.5.2. Quantitative microscopy
2.5.2.1. Determination of stomatal number and stomatal index
Stomatal number is the average number of stomata per square millimeter of epidermis. The percentage proportion of the ultimate divisions of the epidermis of a leaf which can be converted into stomata is termed as stomatal index. Stomatal index can be calculated by using following equation:
I = S / E + S×100, where, I = stomatal index, S = number of stomata per mm2 and E = number of ordinary epidermal cells per mm2. A piece of leaf was cleaned and the upper and lower epidermis was peeled out separately by means of forceps. It was kept on slide and mounted in glycerin water. Camera lucida was attached and drawing board was placed for drawing the cells. A square of 1 mm by means of stage micrometer was drawn on it. The slide with cleared leaf was placed on the stage and the epidermal cells and stomata were traced. The number of stomata and the number of epidermal cells in each field were counted. The numbers of stomata were counted as stomatal number and the stomatal index using the above formula was calculated separately for upper and lower surface.
2.5.2.2. Determination of vein-islet and vein termination number
Vein islet is the minute area of photosynthetic tissue encircled by the ultimate division of the conducting strands. Vein termination number is the number of veinlet terminations per mm of leaf surface. A piece of the leaf was cleared by boiling in chloral hydrate solution and camera lucida and drawings board were arranged and 1 mm line was drawn with help of stage mm. A square was constructed on this line in the centre of the field. The slide was placed on the stage. The veins included within the square were traced off, completing the outline of those islets which overlap two adjustment side of the square. The average number of vein islet from the four adjoining square, to get the value for one square mm was calculated[16]. The number of veinlet termination present within the square was counted and the average number of veinlet termination number from the four adjoining square to get the value for 1 square mm was found known as vein termination number.
2.5.2.3. Determination of palisade ratio
A piece of the leaf was boiled in chloral hydrate and was placed under microscope. Camera lucida and drawing board were arranged and the outline of four cells of the epidermis was traced using 4 mm objective. Then, palisade layer was focused down and sufficient cells for covering the tracing of the epidermal cells were traced off. The outline of those palisade cells which were intersected by the epidermal walls was completed. The palisade cells under the four epidermal cells (including cells which are more than half and excluding cells which are less than half within the area of epidermal cells) were counted. The determination for five groups of four epidermal cells from different part of the leaf was repeated. The average number of cells beneath epidermal cells was calculated known as palisade ratio.
2.6. Fluorescence analysis
Fluorescence analysis of powder of root bark was done by standard procedure[17]. In this analysis the root bark and its powder were treated with various acidic and basic solvents and were then observed in UV/ visible chamber under visible, short wave and long wave regions simultaneously[18],[19]. Fluorescence is an important phenomenon exhibited by various chemical constituents show fluorescence in the visible range in day light. The UV light produces fluorescence in many natural products (e.g. alkaloids like berberine) which do not visibly fluorescence in day light. If the substances themselves are not fluorescent, they may often be converted into fluorescent derivatives by applying different reagents hence some crude drugs are often assessed qualitatively in this way and it is an important parameter of pharmacognostical evaluation[20]. The changes in appearance and colour were observed and recorded.
3. Results
3.1. Organoleptic evaluation
The organoleptic features of the leaves indicated dark green colour on upper surface and light green colour on lower surface. The powder of leaf appeared green in colour, coarse in texture, slightly aromatic and unpleasant in odour with tingling and burning taste. The stem was brown in colour, with bitter taste and smooth texture. The root bark and its powder were whitish grey in colour, astringent taste and disagreeable odour.
3.2. Macroscopic evaluation
The macroscopic evaluation involved study of morphological characters and taxonomy. The morphological characters of leaves were observed as deciduous, leaf base rounded to (sub) cordate and symmetric petiole. The composition of lamina was found to be simple, pinnate reticulate venation, entire margin, acuminate apex, sub-ribs alternate, surface glabrous and texture subcoriaceous. The size of leaves varied from 1.5 to 8.5 cm in length and 2.5 cm. The morphological features of root bark were observed as silver grey deep fissured, rough and firm externally. The inner surface of bark showed white colour when fresh and dark brown when dried. The configuration of surface showed some rectangular rhitydomes (old dead phloem cells) and periderm parallel to cambium but to a short distance where they deviated from obliquely to normally. The circumference of bark was about 84 cm, average thickness of fresh bark (3 to 19 mm) and dried bark was 1.5 to 15 mm.
3.3. Microscopic evaluation
3.3.1. Leaf microscopy
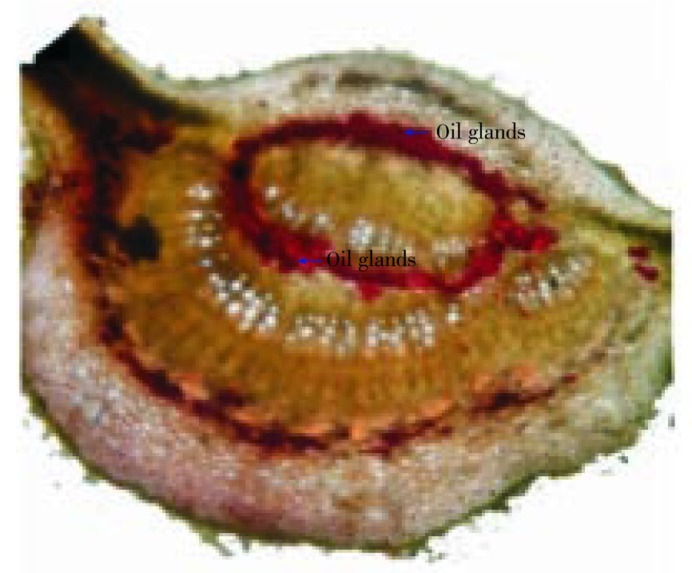
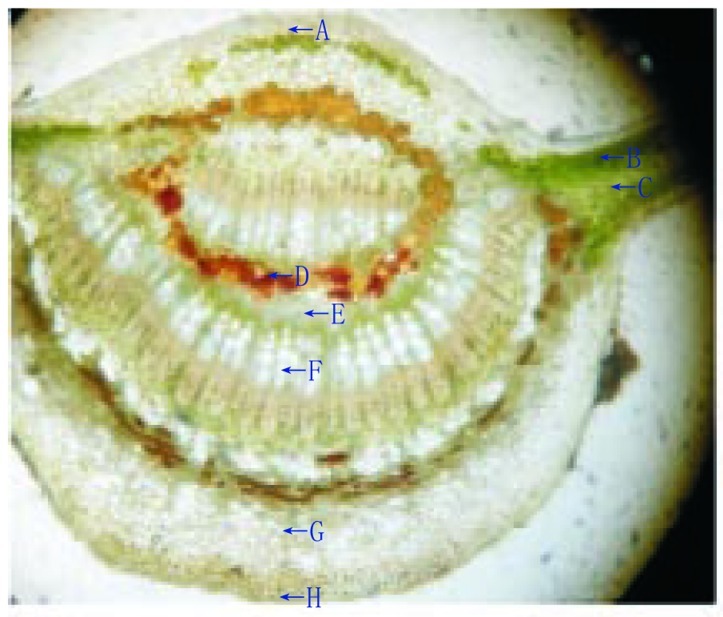
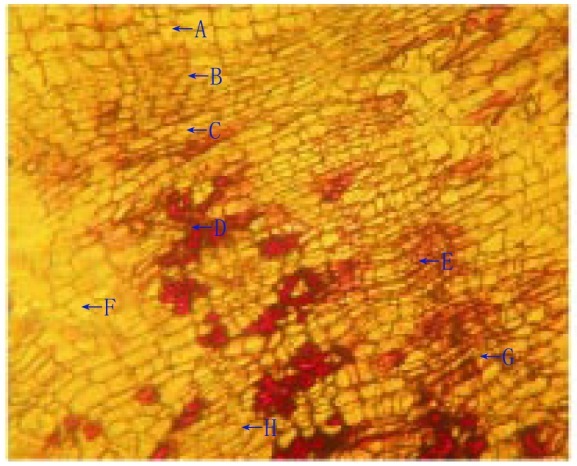
Lamina of the leaf was dorsiventral type. The upper epidermis consisted of small polygonal paranchymatous cells which appeared wavy in outline and after epidermis, chlolenchyma cells were present. The chlolenchyma cells were further seen as upper palisade and lower spongy tissue. The palisade with a single layer of regular, long, columnar cells, beneath which, 3 to 4 layered mass ofclosely packed cells filled with chloroplast was present. The cortex consisted of 4 to 5 layers of paranchymatous cells in the midrib region. The vascular bundle was ovoid in shape, collateral and below them a zone of sclerenchymatous tissues was present. In between the upper epidermis and the vascular bundle, 5 to 6 layers of irregular shaped collenchyma cells were present. Oil glands were also present between them which were detected by red colour imparted by them when subjected to methyl orange (Figure 1). Xylem cells (lignified) were seen on the upper side whereas phloem was seen towards the lower side of the epidermis. There were 2 to 3 layers of cambium in between the xylem and phloem. The phloem consisted of sieve tubes, companioncells and phloem parenchyma whereas xylem consisted of scalariform xylem vessels and parenchyma. Transverse section of leaf was presented in Figure 2. The uniseriate covering and multicellular trichomes were present on both surfaces of leaf, more along the midrib region and less along the lamina. The covering trichomes were simple, uniseriate, unbranched and unicellular with blunt apex and smooth walls (Figure 3).
Figure 1. Red colour imparted by oil glands when subjected to methyl orange.
Figure 2. Transverse section of H. integrifolia leaf.
A: Upper epidermis; B: Spongy parenchyma; C: Palisade cells; D: Oil glands; E: Phloem; F: Xylem; G: Collenchyma; H: Lower epidermis.
Figure 3. Multi-cellular and uniseriate covering trichomes.

3.3.2. Root bark microscopy
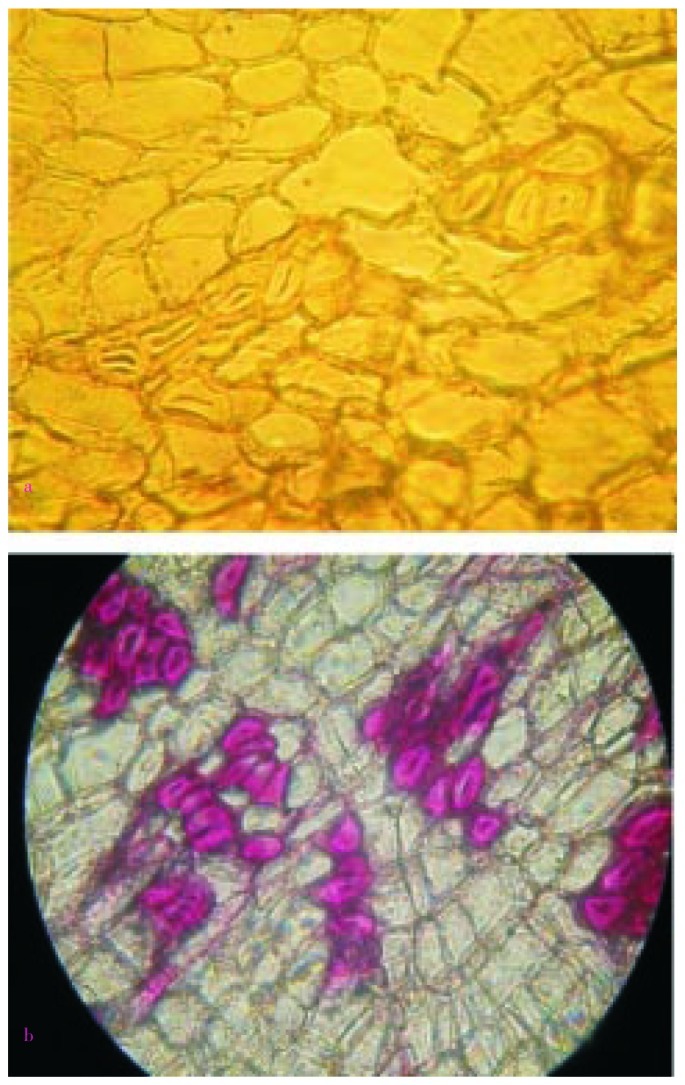
The transverse section of root bark of H. integrifolia showed the arrangement of different types of cells in the periderm. The section stained with phloroglucinol was presented in Figure 4. The cork cells in outermost layers were tangentially elongated and arranged in regular radial rows. The cortex was composed of rectangular cells tangentially elongated, thin walled and compactly arranged. Cortex cells and cork cells under better magnification (400×) were presented in Figure 5a and 5b, respectively. The cells present in cork cambium were arranged in 2–3 layers and resembled with outer cork. Secondary phloem had patches of lignified fibers (10–30 fibers form a patch). Phloem fibers unstained and stained with phloroglucinol were presented in Figure 6a and 6b, respectively. The fibers were polygonal, thick walled with small lumen and patches of fibers were altered with other phloem elements like phloem parenchyma, sieve elements and companion cell. Groups of cortical sclerenchyma were also present commonly in inner phloem than outer one. Cells of phloem consisted prismatic crystals throughout the phloem zone, phloem rays and starch grains. Medullary rays, the principal components of phloem were present as well. The rays are presented in Figure 7a and 7b under magnification 100× and 400×, respectively. Longitudinal section showed the presence of cork cells arranged in uppermost 5–6 layers, cortex cells in 4–5 layers beneath them.
Figure 4. Transverse section of root bark of H. integrifolia.
A: Cork; B: Cortex cells; C: Cork cambium or phellogen; D: Groups of cortical sclerenchyma; E: Inner part of primary cortex; F: Endodermis; G: Primary phloem; H: Pericycle; I: Phelloderm or secondary cortex; J: Periderm.
Figure 5. Microscopic observation of cortex and cork cells.
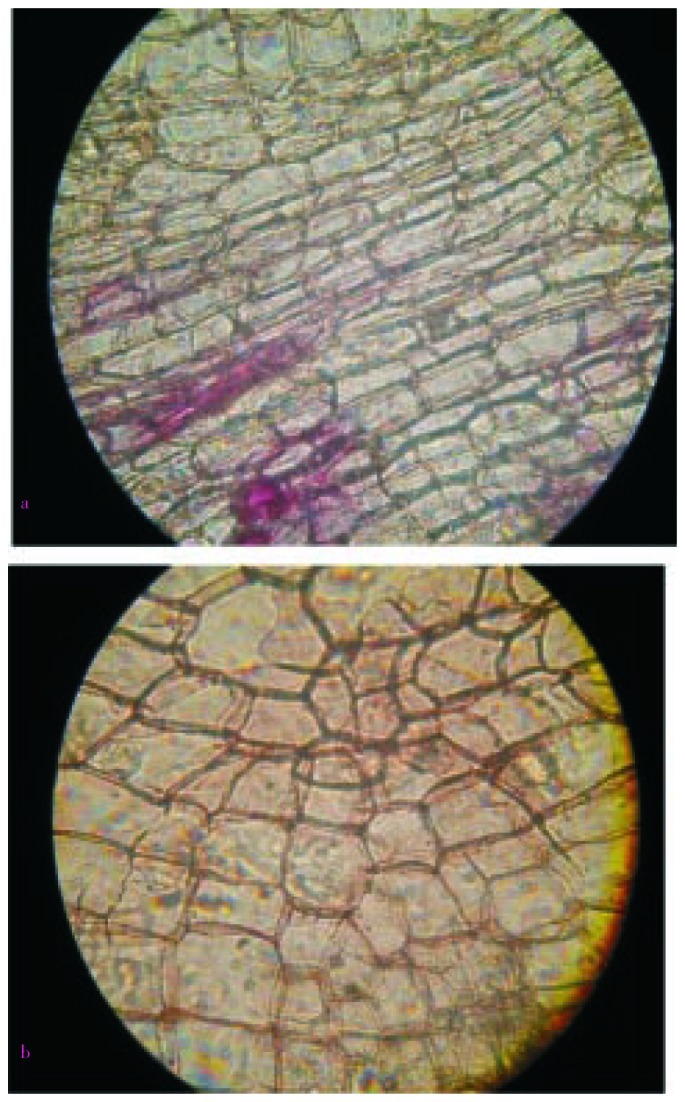
a: Cortex cells (magnification 400×); b: Cork cells (magnification 400×).
Figure 6. Microscopic observation of phloem fibers.
a: Phloem fibers without stain; b: Lignified phloem fibers.
Figure 7. Microscopic observation of medullary rays.
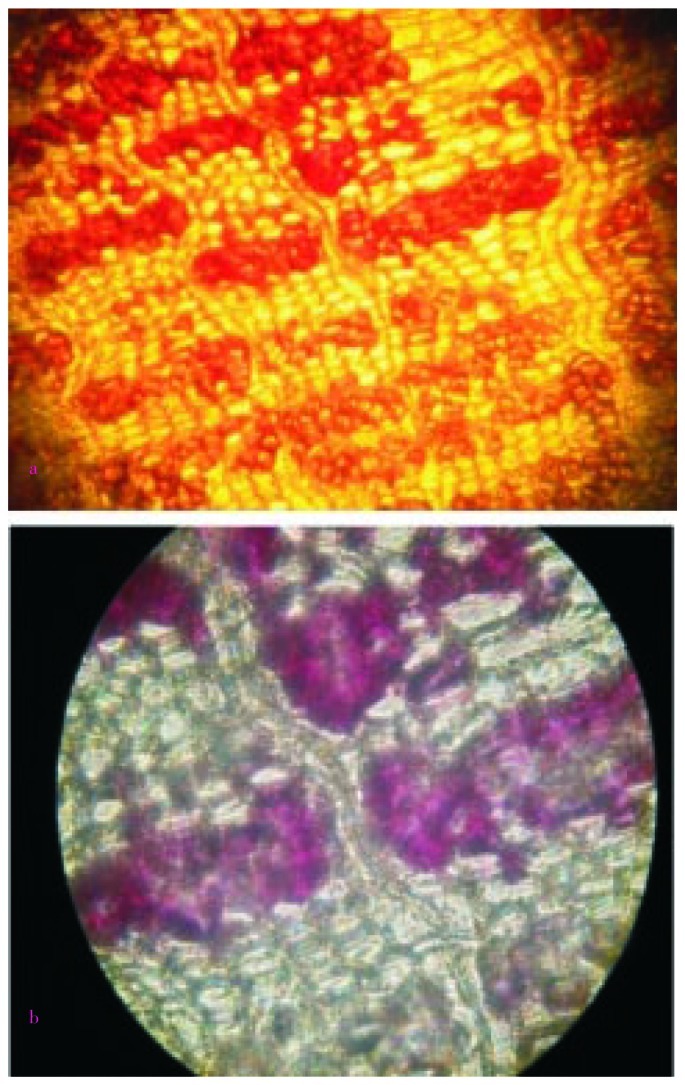
a: Medullary rays under resolution 100×; b: Medullary rays under resolution 400×.
3.3.3. Powder microscopy
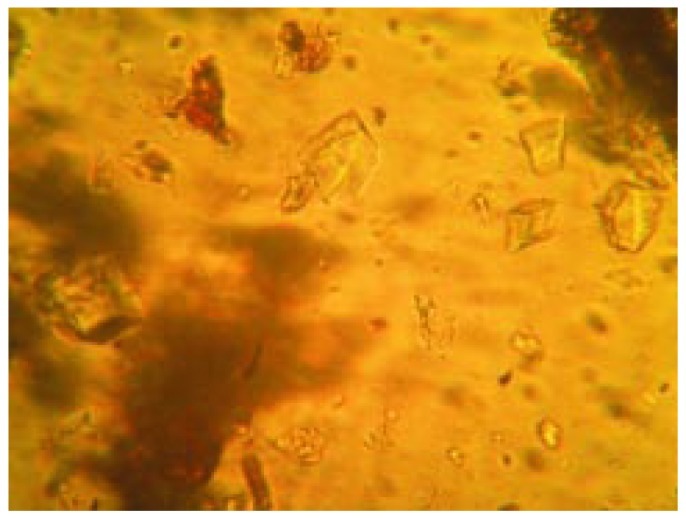
Powdered root bark of H. integrifolia under microscopic investigation showed sieve tubes, lignified fibers and cortex cells (Figure 8, 9 and 10). Calcium oxalate crystals are the calcium salts of oxalic acid produced during biosynthesis of primary and secondary metabolites. The crystals of tetragonal type were observed which have been presented in Figure 11.
Figure 8. Microscopic observation of sieve tubes.
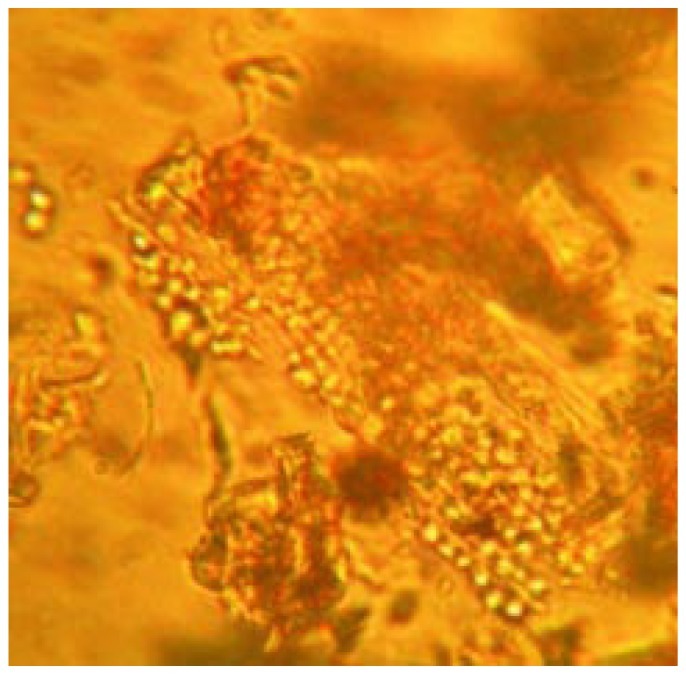
Figure 9. Microscopic observation of lignified fibers.
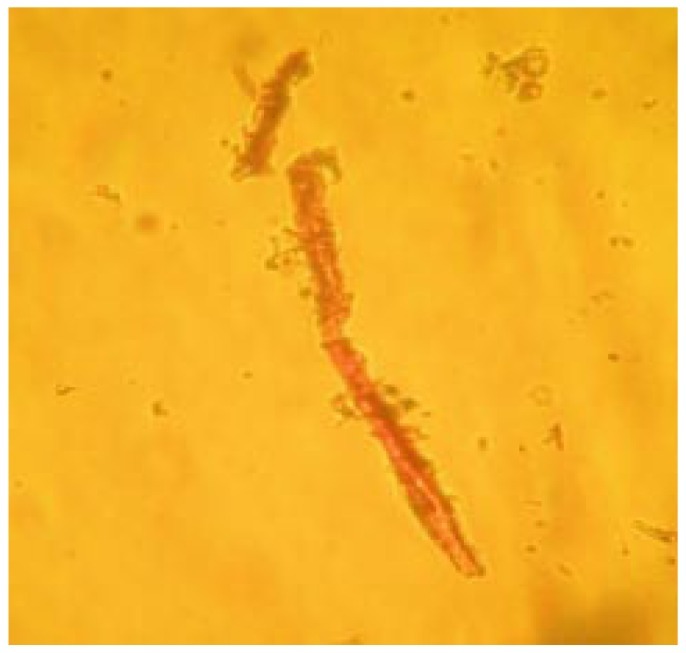
Figure 10. Microscopic observation of cortex cells.
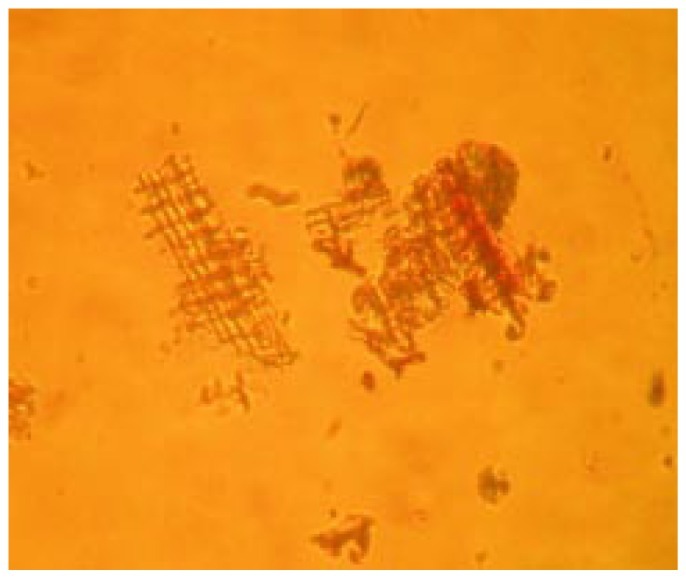
Figure 11. Microscopic observation of calcium oxalate crystals.
3.3.4. Quantitative microscopy
Anomocytic types of stomata were present on the lower surface of leaves. The stomatal number of upper surface and lower surface was found as 3 and 6, respectively. The stomatal indexes of upper surface and lower surface were found 12.3 and 16.5, respectively. The vein islet and vein termination were calculated as 12 and 14. The palisade ratio was found to be 5.2.
3.4. Fluorescence analysis
The results of fluorescence analysis were presented in Table 1.
Table 1. Fluorescence analysis of H. integrifolia root bark powder.
| Treatment | Visible light | Under UV light |
|
| Short wavelength (254 nm) | Long wavelength (366 nm) | ||
| Powder | Cream brown | Cane color | Off white |
| Powder + methanol | Yellowish brown | Greenish black | Ceramic yellow |
| Powder + picric acid | Yellowish brown | Yellowish green | Brownish black |
| Powder + ammonia | Dark brown | Mehndi green | Ceramic brown |
| Powder + nitric acid | Reddish brown | Blackish green | Blackish brown |
| Powder + petroleum ether | Light brown | Blackish green | Ceramic brown |
| Powder + 50% HCl | Mud brown | Greenish black | Coffee brown |
| Powder + 50% H2SO4 | Brown | Ceramic green | Coffee brown |
| Powder + potassium hydroxide | Dark brown | Greenish black | Off white |
4. Discussion
Standardization is an important tool for herbal drugs in order to establish their identity, purity, safety and quality. In order to standardize a drug, various macroscopic, microscopic, fluorescence analysis are done. Microscopic method is one of the cheapest and simplest methods to start with establishing the correct identification of the source material[21]. Morphological and microscopical studies of the leaf will enable to identify the crude drug. The quantitative determination of some pharmacognostical parameters is useful for setting standards for crude drugs. Stomatal number, stomatal index value and palisade ratio, vein islet and vein termination value determination are equally important in the evaluation of crude drugs. These values help in the evaluation of purity of drugs[22]. The information obtained from preliminary phytochemical screening will be useful in finding out the quality of the drug. Morphological and microscopic studies of root bark act as a reliable aid for detecting adulteration. These simple but reliable standards will be useful to a lay person in using the drug as a home remedy. These studies can also help the manufacturers for identification and selection of the raw material for drug production.
In conclusion, the parameters which are reported here can be considered as distinctive enough to identify and decide the authenticity of this drug in herbal industry/trade and this can be included as microscopic standards in Indian Herbal Pharmacopeia.
Acknowledgments
The study was financially supported by University Grants Committee (UGC), New Delhi (grant No. 39-955/2010 SR).
Footnotes
Foundation Project: This work was financially supported by University Grants Committee, New Delhi (grant No. 39-955/2010SR).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Joy PP, Thomas J, Mathew S, Skaria BP. Medicinal plants. Calcutta: Naya Prokash; 2001. pp. 449–632. [Google Scholar]
- 2.Warrier PK, Nambir VPK, Ramankutty C. Indian medicinal plants-a compendium of 500 species. Chennai: Orient Longman Pvt. Ltd; 2004. [Google Scholar]
- 3.Pullaiah T. Encylopaedia of world medicinal plants. New Delhi: Regency Publications; 2006. pp. 1095–1097. [Google Scholar]
- 4.Kare MA, Dhabe AS, Bhutkar AS. Studies on crude drug bark-Holoptelea integrifolia (Roxb.) Planch. J Exp Sci. 2011;2(3):9–11. [Google Scholar]
- 5.Rangari VD. Pharmacognosy and phytochemistry. 1st ed. New Delhi: Career Publications; 2006. pp. 103–115. [Google Scholar]
- 6.Evans WC. Trease and evans pharmacognosy. 16th ed. Edingburgh: Saunders Elsevier; 2009. pp. 541–570. [Google Scholar]
- 7.Yunus MFLS, Yunus D. Systematic bark morphology of some tropical trees. Bot J Linn Soc. 1990;103:367–377. [Google Scholar]
- 8.Pandya DJ, Desai TR, Nadpara NP, Mehta HA, Modi AM. Pharmacognostic study and establishment of quality parameters of leaves of Bombax insigne Linn. Int J Pharmacogn Phytochem Res. 2010;2(3):1–5. [Google Scholar]
- 9.Kokate CK. Practical pharmacognosy. New Delhi: Vallabh Prakashan; 2005. p. p. 107, 108, 115–120, 122–123. [Google Scholar]
- 10.Wallis TE. Text book of pharmacognosy. 5th ed. New Delhi: CBS Publishers & Distributers; 1985. pp. 68–101. [Google Scholar]
- 11.Ali M. Text book of pharmacognosy. New Delhi: CBS Publishers & Distributers; 2008. pp. 181–211. [Google Scholar]
- 12.Gokhale SB, Kokate CK. Practical pharmacognosy. 12th ed. Pune: Nirali Prakashan; 2008. p. 24. [Google Scholar]
- 13.Khandelwal KR. Practical pharmacognosy. 18th ed. Pune: Nirali publication; 2007. pp. 10–14. [Google Scholar]
- 14.Gupta AK, Tandon N, Sharma M. Quality standards of Indian medicinal plants. New Delhi: Indian Council of Medical Research; 2008. pp. 246–255. [Google Scholar]
- 15.Thitikornpong W, Phadungcharoen T, Sukrong S. Pharmacognostic evaluations of Lagerstroemia speciosa leaves. J Med Plant Res. 2011;5(8):1330–1337. [Google Scholar]
- 16.Srinivasa B, Kumar A, Prabhakarn V, Lakshman K, Nandeesh R, Subramanyam P, et al. Pharmacognostical studies of Portulaca oleracea Linn. Braz J Pharmacogn. 2008;18(4):527–531. [Google Scholar]
- 17.Edwin S, Joshi SB, Jain DC. Comparative pharmacognostic studies on root powder of Plumbago zeylanica and Plumbago rosea. Indian J Nat Prod. 2008;24(2):27–29. [Google Scholar]
- 18.Najafi S, Deokule SS. Pharmacognostic study of Tylophora dalzellii Hook.f. J Med Plant Res. 2010;4(5):403–406. [Google Scholar]
- 19.Chase CR, Pratt RJ. Flourescence of powdered vegetables drugs. Indian J Exp Biol. 1949;33(6):428–432. [Google Scholar]
- 20.Ansari SH. Essential of pharmacognosy. 1st ed. New Delhi: Birla publications Pvt. Ltd; 2006. [Google Scholar]
- 21.Singh S, Manchawal L, Chauhan MG. Pharmacognostic study of male leaves of Trichosanthes dioica Roxb. with special emphasis on microscopic technique. J Pharmacogn Phytother. 2010;2(5):71–75. [Google Scholar]
- 22.Kumar S, Kumar V, Prakash O. Microscopic evaluation and physiochemical analysis of Dillenia indica leaf. Asian Pac J Trop Biomed. 2011;1:337–340. doi: 10.1016/S2221-1691(11)60076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]