Abstract
Objective
To establish the wound healing activity of aqueous and ethanolic extract of roots of Ficus racemosa (F. racemosa).
Methods
Two models were performed to evaluate the wound healing activity i.e. incision and excision models. In incision model the parameter which was carried out was breaking strength of wounded skin. In excision model percentage wound contraction and period of epithelialization were established for both the extracts. Reference standard drug was povidone iodine ointment for comparison with other groups.
Results
From the observation in both two models, aqueous extract of F. racemosa was found to have greater wound healing activity in terms of breaking strength in incision model and percentage wound contraction, period of epithelialization in excision model than that of other groups.
Conclusions
In conclusion, our findings suggest that aqueous extract of F. racemosa possesses better wound healing ability than the ethanolic extract.
Keywords: Ficus racemosa, Incision, Excision, Phytochemical analysis, Povidone iodine
1. Introduction
The wound repair process has three orderly but temporally overlaid stages i.e. inflammation, cell proliferation and tissue regeneration[1]. Wound healing is a process which is fundamentally a connective tissue response. Initial stage of this process involves an acute inflammatory phases followed by synthesis of collagen and other extracellular macromolecules which are later remolded to form scars[2]. Therefore, tissue repair and wound healing are the complex processes that involve a series of biochemical and cellular reactions[3]–[5]. There are several reports stating that the extracts of several plants have wound healing properties[3],[6],[7].
Some plants possessing prohealing activity have been scientifically analyzed. Tridex procumbens, Trigonella foenumgraecum, Leucas lavandulaefolia and Aloe vera have shown promising wound healing activity[8].
Ficus racemosa (F. racemosa) Linn (Moraceae) is an evergreen, moderate to large sized spreading, lactiferous, deciduous tree, without much prominent aerial roots found throughout greater part of India in moist localities and is often cultivated in villages for its edible fruit. The astringent nature of the bark has been employed as a mouth wash in spongy gum and also internally in dysentery, menorrhagia and haemoptysis. All parts of this plant (leaves, fruits, bark, latex, and sap of the root) are medicinally important in the traditional system of medicine in India. The leaves powdered and mixed with honey are given in bilious infections. The bark is antiseptic, antipyretic and vermicidal, and the decoction of bark is used in the treatment of various skin diseases, ulcers and diabetes. It is also used as a poultice in inflammatory swellings/boils and is regarded to be effective in the treatment of piles, dysentry, asthma, gonorrhea, gleets, leucorrhea and urinary diseases[9]. Very little phytochemical work has been carried out on F. recemosa. Many bioactive compounds have been isolated from F. recemosa, e.g., campesterol, hentriacontane, hentriacontanol, kaempferol, stigmasterol, methyl ellagic acid from stems, tetra triterpene, glauanol acetate, racemosic acid from leaves, glauanol, hentriacontane, β-sitosterol, glauanolacetate, glucose, tiglic acid, esters of taraxasterol, lupeolacetate, friedelin, higher hydrocarbons and other phytosterol from fruit, cycloartenol, euphorbol and its hexacosanoate, taraxerone, tinyatoxin from root, euphorbol and its hexacosanate, ingenol and its triacetate, taraxerone from bark[10],[11]. The scientific study of the roots of F. racemosa in wound healing has not been investigated so far. Therefore, the present study was designed to determine the healing activity of roots in the wound model in rats.
2. Materials and methods
2.1. Plant collection
Roots of F. racemosa L. growing in natural habitat in Modasa, Gujarat, India, were collected in October, 2010 and identified by associate professor Dr. MS Jangid, Department of Botany, Modasa, Hemchandra Gujarat University by carrying out macroscopic and microscopic evaluation and were submitted to the institute for future reference purpose.
2.2. Preparation of the root extract
Dried and coarsely (500 g) powdered roots of F. racemosa were extracted with 90% (v/v) ethanol in Soxhlet apparatus for 36 h and aqueous extract was prepared by using maceration technique of extraction. The filtrate was filtered and concentrated on water bath using petridish. The temperature was maintained at 55 °C. The powdered extract was dried and weighed[12].
2.3. Preliminary phytochemical analysis
Preliminary phytochemical studies were performed for testing different chemical groups present in ethanolic and aqueous extracts[13]–[15].
2.4. High performance thin layer chromatography (HPTLC) profile
Chromatography was performed on (3 cm × 10 cm) HPTLC plates coated with 0.25 mm layer of silica gel 60 F254 (Merck, Germany). Before using, the plates were washed with methanol and activated at 110 °C for 5 min. Samples were applied as 4 mm wide bands and 6 mm apart by using a Camag (Muttenz, switzerland) Linomat IV sample applicator equipped with 100 µL syringer. A constant application rate of 5 µL/sec was used.
2.5. Animals
Wistar albino rats of either sex weighing between (180–200 g) were obtained from Jai Foundation Research, Vapi (Gujarat). The study was approved by the Institutional Ethics Committee for animal experimentation, Vidyabharti Trust College of Pharmacy, Umrakh, Gujarat (VBT/IAEC/11/05/45) and all the procedures on animals were carried out as per CPCSEA guidelines, India. These animals were used for the wound healing activity studies. The animals were stabilized for 1 week. They were maintained in standard conditions at room temperature, (60±5)% relative humidity and 12 h light dark cycle. They were given standard pellet diet and water ad libitum throughout the course of the study. The ethanolic and aqueous extracts of F. recemosa were administered topically to all groups of animals.
2.6. Incision wound model
The rats were anesthetized by administering ketamine (0.5 mL/kg b.w. i.p.). Incision wounds of about 6 cm in length and 2 mm in depth were made with sterile scalpel on the shaved back of the rats 30 min after the administration of ketamine injection. The parted skin was kept together and stitched with black silk at 0.5 cm intervals. Surgical thread (no. 000) and a curved needle (no. 9) were used for stitching. The continuous thread on both wound edges was tightened for good closure of the wounds. The wounds of animals in the different groups were treated with drug topically for a period of 10 days. Rats were divided into four groups with 6 in each group. Rats in group 1 were topically treated with simple ointment and served as control; rats in group 2 were topically treated with povidone lodine ointment; and rats in group 3 and 4 were topically treated with aqueous and ethanolic extract of F. racemosa, respectively. The wounding day was considered as day 0. When wounds were cured thoroughly, the sutures were removed on the 8th post-wounding day and the tensile strength of the skin that is the weight in grams required to break open the wound/skin was measured by tensiometer on the 10th day[16]–[20].
2.7. Excision wound model
A standard wound of uniform 2 cm diameter was formed with the aid of a round seal[21]–[24]. The treatment to the excision wounds of the rats was the same as described above. The percentage wound closure, epithelization time and scar area on complete epithelization were measured.
2.8. Histopathological examinations
Specimen samples of skin tissues from control, standard and treated groups were taken out from the healed wounds of the animals in excision and incision wound models for histopathological examinations. The thin sections were cut and stained with haematoxylin and eosin[25]–[27] and observed under microscope for the histopathological changes such as fibroblast proliferation, collagen formation.
2.9. Statistical analysis
The mean value ± SEM was calculated for each parameter. Results were statistically analyzed by one-way-analysis of-variance (ANOVA) followed by post hoc dunnet's test. P<0.05 was considered as significant.
3. Results
3.1. Phytochemical analysis
Preliminary phytochemical screening of the extract showed that the roots of F. racemosa L. contain saponins, tannins, alkaloids and flavanoids while other constituents like amino acids, carbohydrate are absent. Further the presence of these chemical constituents was confirmed by HPTLC fingerprinting.
3.2. Incision wound model
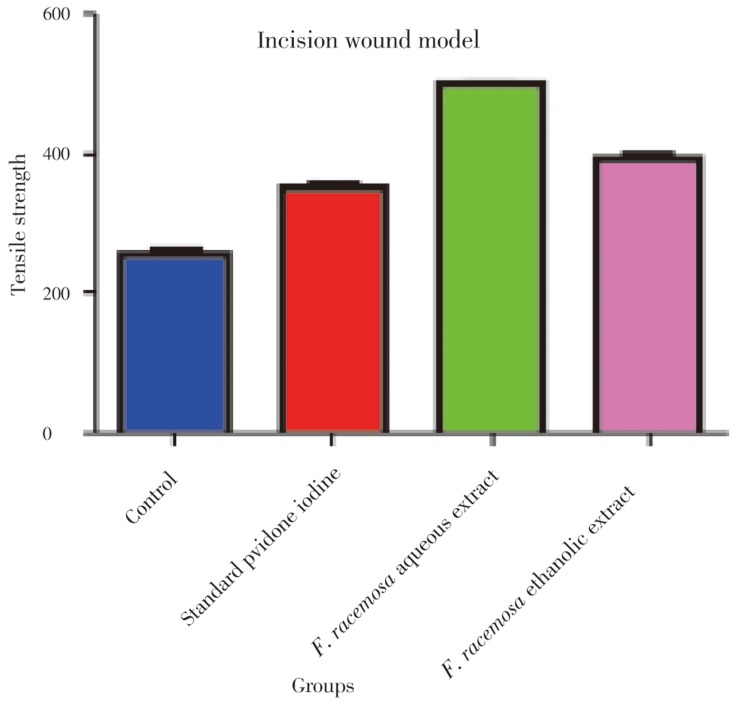
The breaking strength of the incision wounds was increased in drug treated groups to significant extent, i.e. (257.50±2.81) in control was increased up to (497.20±3.03) with F. racemosa aquous extract, (394.70±6.61) with F. racemosa ethanolic extract. The results were also comparable to standard drug i.e. povidone iodine, with breaking strength of (352.00±2.83). The difference between rats treated with drugs and control was statistically significant (P<0.05). Measurement of breaking strength in incision model was shown in Figure 1.
Figure 1. Measurement of breaking strength in incision model.
3.3. Excision wound model
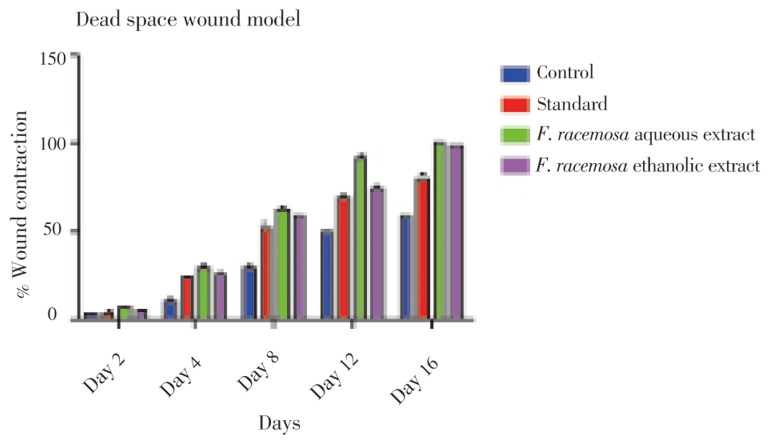
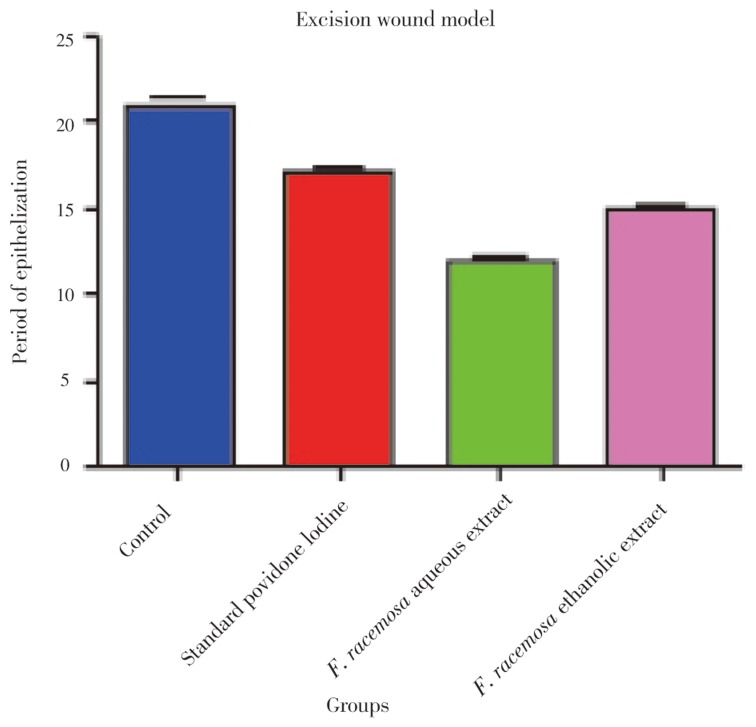
The results of the progress of the wound healing induced by F. racemosa extract ointments (aqueous and ethanolic), simple ointment and povidone iodine (standard drug) were shown in Table 1, Figure 2 and 3. It was observed that the wound contracting ability of both extracts was significantly greater than that of the control (i.e. simple ointment treated group). The aqueous extract ointment treated groups showed significant wound healing from the fourth day onwards, which was comparable to that of the standard drug, i.e. povidone iodine ointment. The wound closure time was lesser, as well as the percentage of wound contraction was much more with the aqueous extract ointment treated group [12 days for 100% contraction which was almost better than that of the povidone iodine (17 days) treated group]. Ethanolic extract ointment treated animals showed significant wound contraction from the fourth day onwards and achieved 100% contraction with the wound closure time of 14.17 days.
Table 1. Percentage wound contraction and period of epithelialization in excision wound model (mean±SE) (n=6).
| Groups | % Wound contraction in days |
Period of epithelialization (days) | ||||
| Day 2 | Day 4 | Day 8 | Day 12 | Day 16 | ||
| Control (simple ointment) (topically) | 4.16±3.87 | 11.83±2.24 | 30.00±1.23 | 50.00±2.54 | 60.00±1.32 | 21.00±0.37 |
| Povidone iodine (topically) | 5.08±1.23 | 24.16±1.54* | 53.33±3.67* | 69.16±3.30* | 80.83±1.23* | 17.00±0.37* |
| F. racemosa aqueous extract (topically) | 7.50±0.14 | 30.83±0.23* | 63.33±0.64* | 92.50±0.12* | 100.00±0.00* | 12.00±0.26* |
| F. racemosa ethanolic extract (topically) | 5.90±0.13 | 27.00±2.31* | 59.00±1.23* | 75.00±0.32* | 98.33±1.32* | 15.00±0.26* |
*: P<0.05 when compared with control group.
Figure 2. Measurement of percentage wound contraction in excision model.
Figure 3. Measurement of period of epithelialization in excision model.
3.4. Histopathological examinations
In standard drug and F. racemosa extracts treated albino rats, excision and incision type of wounds showed significant healing as in fibroblasts cells, collagen fibres and new blood vesicles in Figure 4 and 5 while in control rats wounds showed incomplete healing in Figure 6. Control group showed the slightest wound healing ability when compared to extracts and reference ointment treated groups. Fibroblast cells, collagen fibres and blood vessels are prominently present in standard and extracts treated group as compared to control.
Figure 4. Histopathological characteristics of healed tissue on the 10th day by treatment with standard ointment.
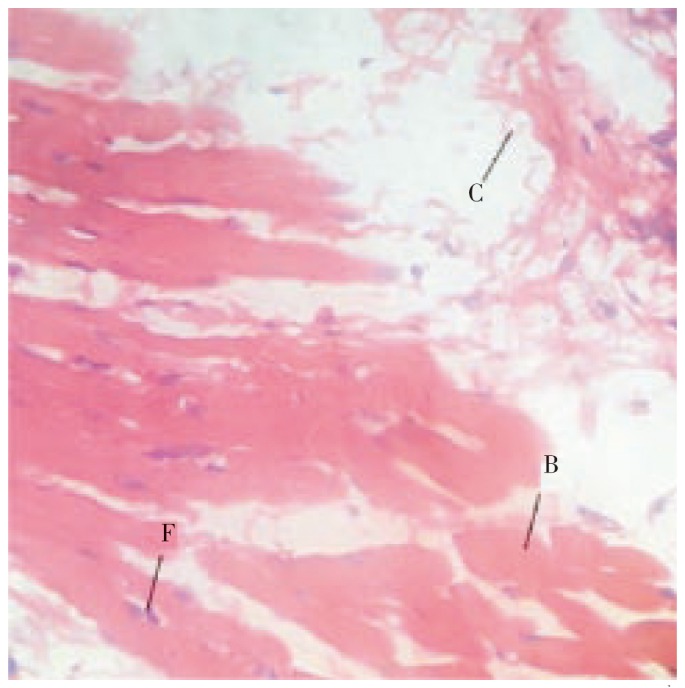
Figure shows increased fibroblasts cells (F), collagen fibres (C) and blood vesicles (B) in incision wound.
Figure 5. Histopathological characteristics of rat skin on the 10th day by treatment with F. racemosa extracts.
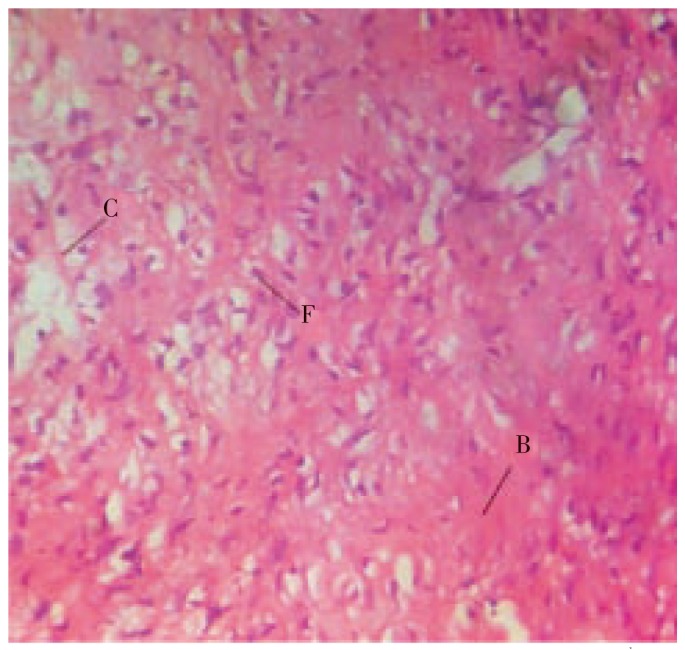
Figure shows increased fibroblasts cells (F), collagen fibres (C) and blood vesicles (B) in incision wound.
Figure 6. Histopathological characteristics of rat skin on the 10th day by treatment with ointment base.
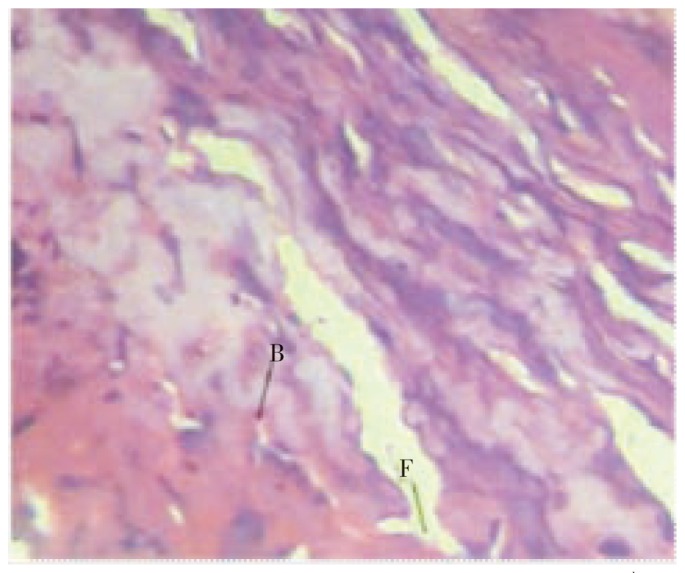
Figure shows poor fibroblast cells (F) and blood vessels (B) in incision wound.
4. Discussion
Wound healing is stepwise process, which consists of different phases such as hemostasis, inflammation, proliferative and remodeling or maturation. The genetic response regulating the body's own cellular resistance mechanisms contributes to the wound and its repair[28]. Therefore, in this study two different models were used to establish the healing potentials of aqueous and ethanolic extracts of F. racemosa on various phases.
In incision wound, the increase in tensile strength of treated wounds may be due to the increase in collagen concentration and stabilization of the fibres. A healing tissue synthesizes collagen, which is a constituent of growing cell[29]. Increase in blood vessels and role of antioxidants were experimentally proved[30].
From the observations, it was evident that F. racemosa possesses a definite potential healing action. The breaking strength of the incision wounds was increased in aqueous and ethanolic extract treated groups.
In excision wound healing model the aqueous and ethanolic extract of the root of the plant F. racemosa showed significant increase in percentage closure by enhanced epithelialization. This enhanced epithelialization may be due to the effect of F. racemosa extracts on enhanced collagen synthesis. The higher breaking strength indicates better healing of wounds. Thus it supports the wound healing activity of F. racemosa.
Recent studies with other plant extracts have shown that phytochemical constituents like flavanoids, alkaloids, saponins and tannins[31] are known to promote the wound-healing process. The study reveals that both aqueous and ethanolic extracts treated groups possess good wound healing properties which may be attributed to the individual or combined action of phytoconstituents like, flavanoids, alkaloids, saponins and tannins present in it.
Acknowledgments
The authors are thankful to the Managing Trustee and Vidyabharti Trust College of Pharmacy, Surat, India for providing financial support and facility to carry out this research.
Footnotes
Foundation Project: This work was financially supported by Vidyabharti Trust College of Pharmacy, Surat, India (grant No. VBT/IAEC/11/05/45).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Murti K, Lambole V, Panchal M, Shah M. Healing promoting potentials of roots of Scoparia dulcis in albino rats. Pharmacologia. 2011;2(12):369–373. [Google Scholar]
- 2.Charde RM, Dhongade HJ, Charde MS, Kasture AV. Evaluation of antioxidant, wound healing and anti-inflammatory activity of ethanolic extract of leaves of Ficus religiosa. Int J Pharm Sci Res. 2010;19(5):73–82. [Google Scholar]
- 3.Sadaf F, Saleem R, Ahmed M. Healing potential of cream containing extract of Sphaeranthus indicus on dermal wounds in Guinea pigs. J Ethnopharmacol. 2006;107:161–163. doi: 10.1016/j.jep.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Ayyanar M, Ignacimuthu S. Herbal medicines for wound healing among tribal people in Southern India: ethnobotanical and scientific evidences. Int J Appl Res Nat Prod. 2009;2(3):29–42. [Google Scholar]
- 5.Wild T, Rahbarnia A, Kellner M, Sobotka L, Eberlein T. Basics in nutrition and wound healing. Nutrition. 2010;26:862–866. doi: 10.1016/j.nut.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Süntar IP, Akkol EK, Yalçin FN, Koca U, Keleş H, Yesilada E. Wound healing potential of Sambucus ebulus L. leaves and isolation of an active component, quercetin 3-O-glucoside. J Ethnopharmacol. 2010;129:106–114. doi: 10.1016/j.jep.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 7.Zhai Z, Haney DM, Wu L, Solco AK, Murphy PA, Wurtele ES, et al. Alcohol extract of Echinacea pallida reverses stress-delayed wound healing in mice. Phytomedicine. 2009;16:669–678. doi: 10.1016/j.phymed.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murti K, Lambole V, Gajera V, Panchal M. Exploration of healing promoting potentials of roots of Ficus religiosa. Pharmacologia. 2011;2(12):374–378. [Google Scholar]
- 9.Murti K, Kumar U, Lambole V. Pharmacological properties of Ficus racemosa-a review. Pharmacologyonline. 2010;2:802–807. [Google Scholar]
- 10.Joseph B, Raj SJ. Phytopharmacological properties of Ficus racemosa Linn-an overview. Int J Pharm Sci Rev Res. 2010;3(2):134–138. [Google Scholar]
- 11.Padmaa M. Phytochemicals in Ficus recemosa. Nat Prod Radiance. 2009;8(1):84–90. [Google Scholar]
- 12.Murti K, Kumar U. Reversal of dexamethasone depressant action in wound healing by Ficus benghalensis L. roots. Am J Pharmacol Toxicol. 2011;6(2):68–75. [Google Scholar]
- 13.Mohammed A. Text book of pharmacognosy. New Delhi: CBS Publishers and Distributors; 2006. [Google Scholar]
- 14.Kokane DD, More RY, Kale MB, Nehete MN, Mehendale PC, Gadgoli CH. Evaluation of wound healing activity of root of Mimosa pudica. J Ethnopharmacol. 2009;124:311–315. doi: 10.1016/j.jep.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 15.Sachin J, Neetesh J, Tiwari A, Balekar N, Jain DK. Simple evaluation of wound healing activity of polyherbal formulation of roots of Ageratum conyzoides Linn. Asian J Res Chem. 2009;2(2):135–138. [Google Scholar]
- 16.Kiran K, Asad M. Wound healing activity of Sesamum indicum L seed and oil in rats. Indian J Exp Biol. 2008;46(11):777–782. [PubMed] [Google Scholar]
- 17.Zhaia Z, Haney DM. Alcohol extract of Echinacea pallida reverses stress-delayed wound healing in mice. Phytomedicine. 2009;16:669–678. doi: 10.1016/j.phymed.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattanayaka SP, Sunita P. Wound healing, anti-microbial and antioxidant potential of Dendrophthoe falcata (L.f) Ettingsh. J Ethnopharmacol. 2008;120:241–247. doi: 10.1016/j.jep.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Nayak BS, Vinutha B, Geetha B, Sudha B. Experimental evaluation of Pentas lanceolata flowers for wound healing activity in rats. Fitoterapia. 2005;76:671–675. doi: 10.1016/j.fitote.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Jagtap NS, Khadabadi SS, Farooqui IA, Nalamwar VP, Sawarkar HA. Development and evaluation of herbal wound healing formulations. Int J Pharmtech Res. 2009;1(4):1104–1108. [Google Scholar]
- 21.Deshmukha PT, Fernandesb J, Atul A, Toppo E. Wound healing activity of Calotropis gigantea root bark in rats. J Ethnopharmacol. 2009;125:178–181. doi: 10.1016/j.jep.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Vinothapooshan G, Sundar K. Wound healing effect of various extracts of Mimosa pudica. Pharmacologyonline. 2010;1:307–315. [Google Scholar]
- 23.Adiga S, Tomar P, Rajput RR. Effect of Punica granatum peel aqueous extract on normal and dexamethasone suppressed wound healing in Wistar rats. Int J Pharm Sci Rev Res. 2010;5(2):34–37. [Google Scholar]
- 24.Barua CC, Telukdar A, Begum SA, Sarma DK, Pathak DC, Barua AG, et al. Wound healing activity of methanolic extract of leaves of Alternanthera brasiliana Kuntz using in vivo and in vitro model. Indian J Exp Biol. 2009;47:1001–1005. [PubMed] [Google Scholar]
- 25.Shivhare Y, Singour PK, Patil UK, Pawar RS. Wound healing potential of methanolic extract of Trichosanthes dioica Roxb (fruits) in rats. J Ethnopharmacol. 2010;127:614–619. doi: 10.1016/j.jep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Bhat RS, Shankrappa J, Shivakumar HG. Formulation and evaluation of polyherbal wound treatments. Asian J Pharm Sci. 2007;2(1):11–17. [Google Scholar]
- 27.Lodhi S, Singhai AK. Preliminary pharmacological evaluation of Martynia annua Linn leaves for wound healing. Asian Pac J Trop Biomed. 2011;1:421–427. doi: 10.1016/S2221-1691(11)60093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charles VM, Rusell RCG, Williams NS. Short practice of surgery. 22nd ed. London: Champan and Hall; 2006. pp. 9–11. [Google Scholar]
- 29.Pattanayak SP, Sunita P. Wound healing, anti-microbial and antioxidant potential of Dendrophthoe falcata (L.f) Ettingsh. J Ethnopharmacol. 2008;120:241–247. doi: 10.1016/j.jep.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Allen LV, Popovich NG, Ansel HC. Ansel's pharmaceutical dosage form and drug delivery system. Baltimore: Lippincort Williams & Wilkins; 2005. pp. 278–281. [Google Scholar]
- 31.Murti K, Lambole V, Panchal M, Shah M, Gajera V. Evaluation of wound healing activity of polyherbal formulation in rats. Res J Pharmacogn Phytochem. 2011;3(3):112–115. [Google Scholar]