Abstract
Objective
To assess anti-psoriatic activity of the methanol extract and the isolated flavonoid quercetin from the rhizome of Smilax china (S. china) Linn.
Methods
Mouse tail test was used for the evaluation of anti-psoriatic activity. Methanol extract (100 and 200 mg/kg b.w.) and isolated flavonoid quercetin (25 and 50 mg/kg b.w.) were tested in Swiss albino mice. Parameters studied in the mouse tail test were changes in epidermal thickness and percentage orthokeratotic values. The anti-inflammatory role of the methanol extract and isolated flavonoid quercetin was evaluated using carrageenan-induced pleurisy in rats. In vitro antiproliferant assay on HaCaT cell lines was also carried out.
Results
The isolated flavonoid quercetin from the rhizome of S. china produced significant orthokeratosis (P<0.01) in the mouse tail test. In epidermal thickness, a significant reduction with respect to control was observed in groups treated with retinoic acid and isolated flavonoid quercetin. The methanol extract (200 mg/kg) and isolated flavonoid quercetin (50 mg/kg) showed anti-inflammatory effect in terms of significant inhibition (P<0.001) in leukocyte migration. Maximum antiproliferant activity was shown by isolated flavonoid quercetin (IC50, 62.42±10.20 µg/mL).
Conclusions
From the above data, the flavonoid quercetin shows significant orthokeratosis, anti-inflammatory and maximum antiproliferant activities. To our knowledge, this is the first report on the anti-psoriatic effect of the flavonoid quercetin which is promising for further investigations to prove its anti-psoriatic activity.
Keywords: Smilax china, Anti-psoriatic, Quercetin, HaCaT cells, Anti-inflammation, Flavonoid, Orthokeratosis, Antiproliferant activity, Rhizome
1. Introduction
Psoriasis is a common genetically determined chronic inflammatory skin disorder characterized by red, scaly and raised patches. It affects mainly knees, elbows and scalp. It is a lifelong disorder with unpredictable remissions and relapses. It affects the patients physically, mentally and socially[1],[2]. In psoriasis, epidermal hyperproliferation, abnormal keratinocyte differentiation, angiogenesis with blood vessel dilatation and excess Th-1 and Th-17 inflammation can be observed[3]. Early and active psoriatic lesions are characterized by intra-epidermal penetration of activated polymorphonuclear leukocytes, which causes uncontrolled production of reactive oxygen species (ROS), leading to peroxidative damage to membranes of the skin and contributing to the exacerbation of lesions. ROS may also activate phospholipase A2 (PLA2) and thus increase the release of mediators of arachidonic acid (AA). Prostaglandin E2 (PGE2) produced by the cyclooxygenase (COX) pathway also contributes to psoriasis by dilating capillaries in the dermis, increasing leukocyte infiltration and stimulating keratinocyte cell growth[4]. However, although genetic, immunological and environmental factors seem to be implicated, the exact cause is not yet known and even today, psoriasis is not well understood[5].
Affordability, availability, and side effects of prolonged use of allopathic drugs still remain a challenge and concern. In this context, flavonoids and polyphenols are proving to be highly effective and are therefore gradually emerging as viable alternatives to conventional drugs for various diseases. Flavonoids are phenolic compounds widely distributed in plants, with more than 7 000 structures and the numbers constantly increasing. A large number of flavonoids have been shown to be potential immunomodulators, acting as anti-inflammatory, antistress, anticancer agents and in various skin diseases[6]. The therapeutic potential of flavonoids and the necessity for scientific validation in popular medicine have prompted increased interest in the field. The rhizome of the plant S. china Linn. is used in various diseases such as rheumatism, gout, epilepsy, skin diseases, chronic nervous diseases, syphilis, flatulence, dyspepsia, colic, neuralgia, constipation, helminthiasis, psoriasis and seminal weakness[7]. In the present study, an attempt was made to evaluate the anti-psoriatic activity of flavonoid-quercetin from the rhizome of Smilax china (S. china) Linn. which is listed in indigenous medicine as having high therapeutic value and is even now being used in various diseases.
2. Materials and methods
2.1. Plant material
The plant specimen for the proposed study was purchased from commercial source in Chennai, Tamil Nadu. It was identified and authenticated by Dr. P Jayaraman, Director, Plant Anatomy Research Center, (PARC) Tambaram, Chennai. A voucher specimen (accession No. 168) was deposited in the Herbarium, Department of Pharmacognosy, School of Pharmaceutical Sciences, Vels University, Tamilnadu, India for future reference.
2.2. Extraction
The rhizome of S. china was shade dried, coarsely powdered and extracted using a Soxhlet apparatus with methanol for 18 h. The extracted solution was filtered and concentrated in a rotary evaporator under reduced pressure (rotary vacuum flash evaporator), freeze-dried and used for the study.
2.3. Phytochemical screening
Qualitative tests for the presence of plant secondary metabolites, such as carbohydrate, alkaloid, tannin, flavonoid, saponin and glycoside, were carried out on the total methanol extract using standard procedure[8].
2.3.1. High performance liquid chromatography (HPLC) analysis
Quantitative analysis of the flavonoid in the sample was performed using HPLC system. Analytical HPLC was performed on (D) a computer-controlled high-pressure-gradient LaChrom-HPLC-system (Merck-Hitachi), containing an Interface L-7000; two pumps L-7100 (one for each eluent); diode array detector L-7450; autosampler L-7200 with 100 µL sample loop; solvent degasser L-7612; high-pressure gradient mixer; Rheodyne injection valve 7725i, 20 µL sample loop; administration of the device, data recording and analysis were performed with the LaChrome Software version 4.0. The column was Thermo ODS Hypersil C18 (250 mm × 4.6 mm, 5 µm) in isocratic mode. The separation was achieved using a mobile phase of acetonitrile: 0.1 M phosphate buffer: glacial acetic acid (15: 85: 1, v/v/v) with pH adjusted to 4.0 using phosphoric acid at a flow-rate of 1.0 mL/min. The effluent was monitored using UV detection at a wavelength of 300 nm. The mobile phase was filtered through 0.45 µm nylon filter prior to use[9]. The percentage content of flavonoids in the rhizome of S. china was calculated.
2.3.2. High performance thin layer chromatography (HPTLC) analysis with known marker quercetin
HPTLC (silica gel G 60F254 TLC plates of E. Merck, layer thickness 0.2 mm) fingerprint profile was established for methanol extract of S. china rhizome. HPTLC was performed on (10 cm × 10 cm) aluminum backed plates coated with silica gel 60F254 (Merck, Mumbai, India). Standard solution of quercetin and sample solution were applied to the plates as bands 8.0 mm wide, 30.0 mm apart, and 10.0 mm from the bottom edge of the same chromatographic plate by use of a Camag (Muttenz, Switzerland) Linomat V sample applicator equipped with a 100 µL Hamilton (USA) syringe. Ascending development to a distance of 80 mm was performed at room temperature (28±2 °C), with toluene: ethyl acetate: formic acid (5: 4: 1) (v/v/v), as mobile phase, in a Camag glass twin-trough chamber previously saturated with mobile phase vapour for 20 min. After development, the plates were dried with a hair dryer and then scanned at 254 nm with a Camag TLC Scanner with WINCAT software, using the deuterium lamp[10]. A stock solution of standard quercetin (100 µg/mL) was prepared in methanol. Different volumes of stock solution 2, 4, 8, 16 and 32 µL, were spotted onto TLC plate to obtain concentrations of 200, 400, 800, 1 600 and 3 200 ng/spot of quercetin. Calibration curve range (200–3 200 ng/spot) for quercetin was found to be linear. The data of peak areas plotted against the corresponding concentrations were collected.
2.3.3. Isolation of quercetin
The rhizome of S. china was separately Soxhlet extracted in 80% methanol (100 mL/g dry weight) on a water bath for 24 h. The extract was concentrated and reconcentrated in petroleum ether (40–60 °C) (fraction-I), ethyl ether (fraction-II) and ethyl acetate (fraction-III) in succession. Each of the steps was repeated three times to ensure complete extraction in each case. Fraction I and II were rejected since they were rich in fatty substances whereas fraction III showed positive result in the test for flavonoids and subjected to TLC.
2.3.4. Thin layer chromatography (TLC)
The ethyl acetate fraction (fraction III) was separately applied 1 cm above the edge of the plates along with standard reference compound (quercetin). These glass plates were developed in an airtight chromatography chamber containing about 200 mL of solvent mixture of n-butanol, acetic acid and water (4: 1: 5, upper layer). Several other solvent mixtures such as ethyl acetate saturated with acetic acid: water (6: 4 v/v) and forestal system (acetic acid, concentrated hydrochloric acid, water, 10: 3: 30 v/v/v) were also tried. The solvent mixture of n-butanol, acetic acid and water (4: 1: 5, upper layer v/v/v) gave the best results in all the samples examined. The developed plates were air dried and visualized under UV light which showed fluorescent spots in the fractions III in all the instances coinciding with those of the standard samples of quercetin (Rf 0.62). The plates were then placed in a chamber saturated with ammonia vapours to observe the colour of spots (quercetin deep yellow). Rf values were calculated for isolated samples and compared with coinciding standard.
Fraction III was hydrolyzed by refluxing with 7% H2SO4 (10 mL/g residue) for 5 h. The mixture was filtered and the filtrate was extracted with ethyl acetate in a separating funnel. The ethyl acetate layer was washed with distilled water till neutrality and dried in vacuo[11]. The residue was then crystallized with chloroform and the sample was subjected to IR, mass and NMR spectral analysis.
2.4. Animals
Healthy male adult albino mice (25–30 g) obtained from the Animal House of Vels University, Tamilnadu, India were used for the study. Animals were housed in polypropylene cages and were left 7 days for acclimatization to animal room maintained under controlled condition [a 12 h light-dark cycle at (22±2 °C)] on standard pellet diet and water ad libitum. All animals were taken care of under ethical consideration as per the guidelines of CPCSEA with due approval from the Institutional Animal Ethics Committee (Registration No. XII/VELS/PCOG/01/2000/CPCSEA/IAEC/22.2.11). Animals were used for acute toxicity study and mouse tail test for psoriasis.
2.5. Acute toxicity
Acute toxicity study-up and down procedure was carried out as per the guidelines by Organization for Economic Co-operation and Development (OECD) 423[12]. Mice (6/group) were divided into five groups. The first 2 groups received oral doses of 1 000, and 2 000 mg/kg of methanol extract and the 3rd and 4th groups received oral doses of 200 and 400 mg/kg of isolated flavonoid quercetin. The fifth group received saline (10 mL/kg) orally. Mortality was assessed 24 h after administration. The animals were also observed for toxic symptoms and mortality was determined 24 h after treatment.
2.6. In vivo anti-psoriatic activity
The mouse-tail model is based on the induction of orthokeratosis in those parts of the adult mouse-tail, which have a normally parakeratotic differentiation.
2.6.1. Perry scientific mouse tail model
This is accepted as a screening method for measuring anti-psoriatic activity of drugs. The basis of this method is that topical treatment of a mouse-tail with anti-psoriatic drugs enhances orthokeratotic cell differentiation in the epidermal scales. This characteristic was utilized for direct measurement of drug efficacy in an animal model. Drugs were applied topically, once daily, 5 times in a week, for 2 weeks. Two hours after the last treatment, the animals were sacrificed; longitudinal sections of the tail skin were made and prepared for histological examination (hematoxylin- eosin staining). As an indicator of orthokeratosis, the number of scale regions with a continuous granular layer was counted and expressed as a percentage of the total number of scale regions per section. Drug activity is defined by the increase in percentage of orthokeratotic regions[13].
2.6.2. Extracts tested
The methanolic extract and isolated flavonoid quercetin from the rhizome of S. china were screened for anti-psoriatic activity. The methanol extract (100 and 200 mg/kg) and isolated flavonoid quercetin (25 and 50 mg/kg) were formulated in the form of a cream, using liquid paraffin (10 mL) and bees wax (3 g) and applied topically. Retino-A 0.05% cream (Tretinoin cream U.S.P.)-Janssen-Cilag Pharmaceuticals (Trademark of Johnson & Johnson, U.S.A.) was used as a standard.
2.6.3. Procedure
Male Wistar rats weighing around 300 g were used. Screening of methanolic extract and isolated flavonoid quercetin (in the form of cream) was carried out with reference to the standard, Retino-A 0.05%. Extract and fraction (in the form of cream) were applied topically, once daily, 5 times a week, for 2 weeks. Drugs were applied topically, once daily, 5 times a week, for 2 weeks. Two hours after the last treatment, animals were sacrificed, longitudinal sections of the tail skin were made and prepared for histological examination (hematoxylin-eosin staining). As an indicator of orthokeratosis, the number of scale regions with a continous granular layer was counted and expressed as a percentage of the total number of scale regions per section. Drug activity is defined by the increase in percentage of orthokeratotic regions. Ten sequential scales were examined for the presence of a granular layer induced in the previously parakeratotic skin areas. The induction of orthokeratosis in those parts of the adult mouse tail, which have a normally parakeratotic differentiation, was quantified measuring the length of the granular layer (A) and the length of the scale (B). The proportion (A/B)×100 represents the % orthokeratosis per scale, and the drug activity (DA) was calculated as follows:
DA = (mean OK of treated group − mean OK of control group × 100) / (100 − mean OK of control group)
Where OK means orthokeratosis.
The measurements were carried out at the border of the scale with a semiautomatic image evaluation unit[14].
2.6.4. Measurement of epidermal thickness
It was obtained by measuring the distance between the dermoepidermal borderline and the beginning of the horny layer. Five measurements per animal were made in every 10 scales and the mean of different animals was calculated. The result of extract and isolated flavonoid quercetin against psoriasis was recoreded.
2.7. Anti-inflammatory activity
The 7 groups of rats were pre-treated with methanol extract (100 and 200 mg/kg), isolated flavonoid quercetin (25 and 50 mg/kg), indomethacin (5 mg/kg, p.o.) and celecoxib (10 mg/kg, p.o.) as the standard drugs and water (0.1 mL, p.o.) as the control. Thirty minutes later, all animals received an intrapleural injection of carrageenan (200 µg/animal). Four hours later the animals were anesthetized with ethyl ether. The pleural exudate was collected, transferred to a 15 mL conical centrifuge tube and the total volume was determined. The pleural cavity was washed with 1.0 mL saline containing heparin (10 IU/mL). The number of migrating leukocytes in the exudate was determined with a Neubauer chamber[15]. Results were expressed as mean ± SEM of exudate pleural volume, and of counts of total leukocytes.
2.8. In vitro anti-psoriatic activity
In vitro anti-psoriatic activity was carried out in HaCaT human keratinocyte cell line[16]. Human HaCaT keratinocytes were obtained from NCCS, Pune, India. The cells were seeded at a concentration of 1.0×105 cells/mL in a 96 well microtitre plate and grown in Dulbecco's modified Eagle's medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (BioWest). After 24 h, the supernatant was decanted and the monolayer was washed once. Then 100 µL of test substance in various concentrations was added to the cells in microtitre plates. Test compounds were prepared in dimethyl sulphoxide (DMSO) and then diluted with DMEM; the final concentration of DMSO was 0.2% in the culture medium. Each sample concentration was tested in triplicates. Controls were performed with DMSO or medium alone. Asiaticoside (Sigma) was used as positive control. The plates were then incubated at 37 °C for 3 days in 5% CO2 atmosphere. Antiproliferant activiy was assessed by performing the Sulphorhodamine B (SRB) assay. Cells were fixed by adding 25 µL of ice-cold 50% trichloroacetic acid on top of the growth medium and the plates were incubated at 4 °C for 1 h, after which plates were washed to remove traces of medium, drug and serum. SRB stain (50 µL; 0.4% in 1% acetic acid) (Sigma) was added to each well and left in contact with the cells for 30 min after which they were washed with 1% acetic acid, rinsing 4 times until only dye adhering to the cells was left. The plates were then dried and 100 µL of 10 mM Tris buffer (Sigma) added to each well to solubilise the dye. The plates were shaken gently for 5 min and absorbance was read at 550 nm using a micro plate reader (Biorad, USA).
2.9. Statistical analysis
Level of significance of all the parameters was expressed as the arithmetic mean ± SE and was analyzed by one-way analysis of variance (ANOVA), followed by Dunnett's “t” test. P value less than 0.05 (P<0.05) was the critical criterion for statistical significance.
3. Results
3.1. Phytochemical screening
Preliminary phytochemical screening of methanol extract showed the presence of flavonoids, saponins, sterols, tannins and carbohydrates.
3.2. HPLC analysis
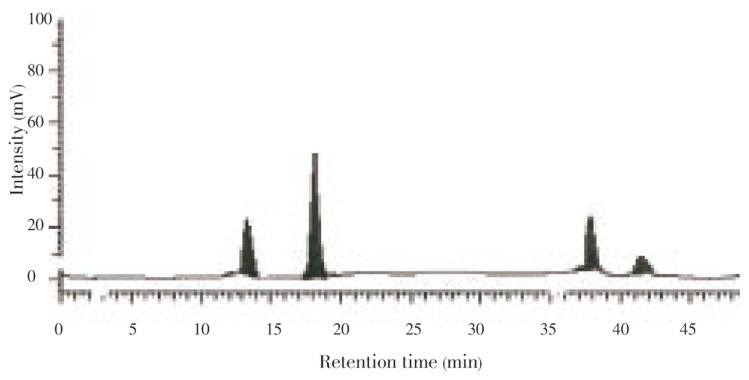
HPLC chromatograms from the rhizome of S. china Linn showed 17 components. The main difference was in peak eluted at 13.45, 41.44, 11.67, and 39.34 min, respectively. In the present investigation, 4 flavonoids were quantified at 254 nm using peak area by comparison to a calibration curve derived from the standard quercetin (0.968% w/w), kaempferol (0.082% w/w), myricetin (0.468% w/w), and formononetin (0.268% w/w) (Figure 1).
Figure 1. HPLC chromatograms of flavonoids.
3.3. HPTLC analysis
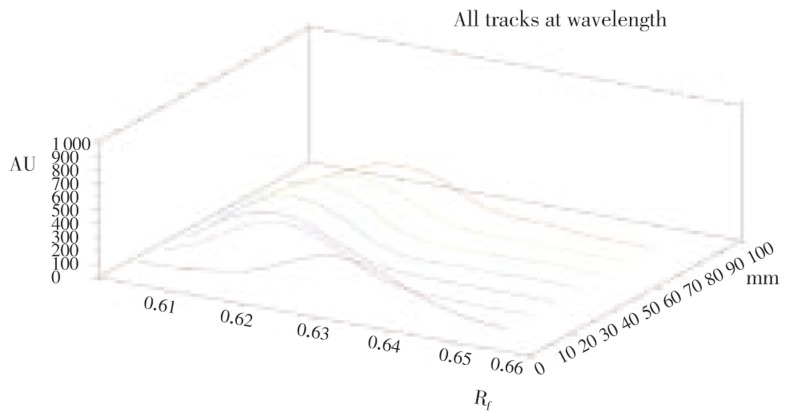
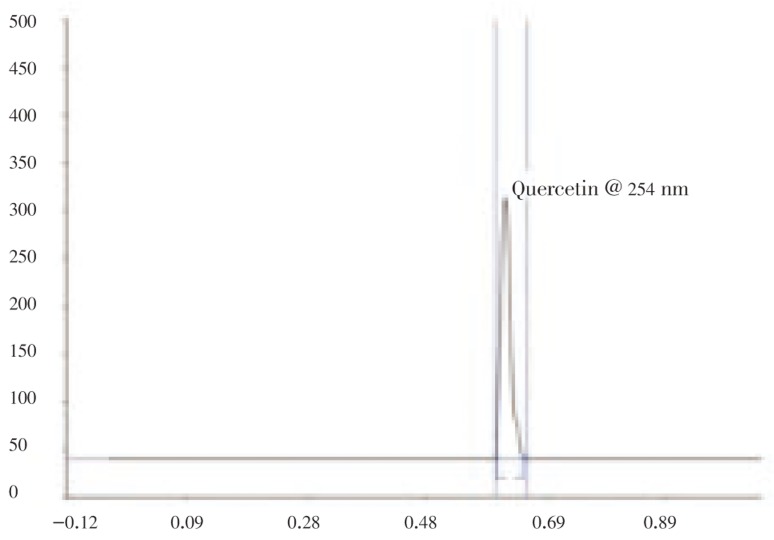
In HPTLC chromatogram, all tracks at wave length were shown in Figure 2. The Rf value of standard quercetin was found to be 0.61 and peak area was 3 226.1 (Figure 3). The methanol extract of S. china Linn showed nine peaks, the fifth peak Rf value (0.61) was coinciding with standard Rf value and its peak area was 3 127.7 (Figure 4). The Rf value of standard quercetin was found to be 0.62 and peak area was 4 443.8. The concentration of quercetin was found to be 0.298 mg/g.
Figure 2. HPTLC chromatogram of all tracks.
Figure 3. Chromatogram of methanol extract.
Figure 4. Chromatogram of standard quercetin.
3.4. Isolation of quercetin
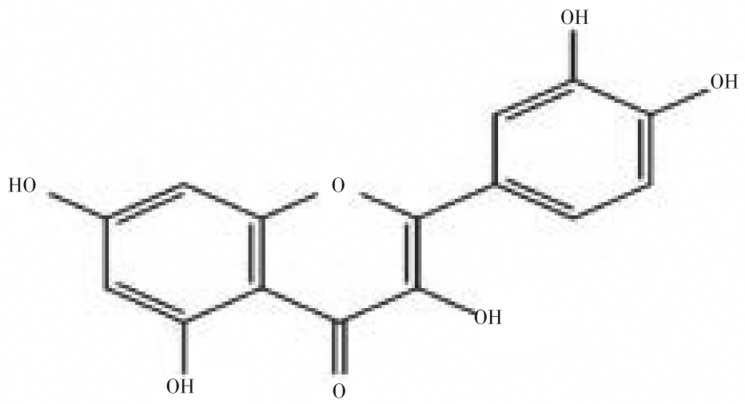
Compound was obtained as a pale yellow powder of melting point 300 °C (decomposed) and EI-MS m/z: 302 [M]+. The Rf value (0.82) of quercetin isolated from the samples coincided with the Rf value of standard quercetin. IR absorption bands at 3 293.82, 1 616.06, 1 511.92 and 1 166.72 were consistent with the presence of hydroxyl, carbonyl, aromatic ring and ether groups, respectively. The 1H-NMR (400MHz, CD3OD): δ 4.53 (1H, s, H-6), 4.90 (1H, s, H-8), 5.90 (1H, d, J=8.4, H-5′), 6.79 (1H, d, J=8.6, H-6′), 7.02 (1H, s, H-2′). The 13C-NMR (100MHz, CD3OD): δ 71.26 (C-8), 72.26 (C-6), 81.51 (C-10), 83.55 (C-2′), 83.70 (C-5′), 94.90 (C-6′), 95.78 (C-1′), 95.92 (C-3), 100.44 (C-3′), 148.19 (C-4′), 148.93 (C-2), 158.41 (C-9), 162.67 (C-5), 165.72 (C-7), 177.50 (C-4). From these results, the compound was considered as quercetin and its chemical structure was shown in Figure 5.
Figure 5. Structure of quercetin.
3.5. TLC analysis
TLC of total methanolic extract, ethyl acetate fraction and standard (quercetin) was shown in Figure 6.
Figure 6. TLC of total methanolic extract, ethyl acetate fraction and standard (quercetin).

3.6. Acute toxicity
The methanolic extract and isolated flavonoid quercetin from the rhizome of S. china were found to be safe in the doses used. No abnormality in the gross behavioral studies and no mortality were noted in all the tested doses.
3.7. Evaluation for anti-psoriatic activity
The methanolic extract and isolated flavonoid quercetin from the rhizome of S. china were screened for their possible anti-psoriatic activity using Perry's scientific mouse tail model. Extract and isolated flavonoid quercetin were applied topically in the form of a cream. Drug activity is defined by the increase in percentage of orthokeratotic regions. These are the regions in a cell having no nucleus and involved in protection from invaders like micro-organisms, UV rays, weak acids and bases. Methanolic extract (100 and 200 mg/kg) increased the orthokeratotic regions by 19.06% and 26.86%, respectively, whereas 32.18% and 39.80% by isolated flavonoid quercetin (25 and 50 mg/kg) in comparison to normal. The standard drug Retin-A showed the increase by 65.06% (Table 1 and Figure 7).
Table 1. Effect of methanol extract and isolated flavonoid quercetin on the degree of orthokeratosis, epidermal thickness and the ‘drug activity’ in the mouse tail test (mean±SEM) (n=6).
| Treatments | Orthokeratosis (%) | Activity (%) | Change in epidermal thickness (%) |
| Saline | 17.07±3.20 | – | – |
| Retinoic acid (0.1 mg/kg) | 65.06±1.09** | 57** | 5 |
| Methanol extract (100 mg/kg) | 19.06±2.90 | 2 | 0 |
| Methanol extract (200 mg/kg) | 26.86±3.10 | 11* | 2 |
| Isolated flavonoid quercetin (25 mg/kg) | 32.18±3.10** | 18** | 2 |
| Isolated flavonoid quercetin (50 mg/kg) | 39.80±1.60** | 27** | 3 |
Statistical significant test for comparison was done by ANOVA, followed by Dunnet's ‘t’ test (n=6). **: P<0.01, *: P<0.05 when compared with control.
Figure 7. Longitudinal histological sections through the skin of mouse tails treated topically for 2 weeks, HE staining (original magnification 40×).
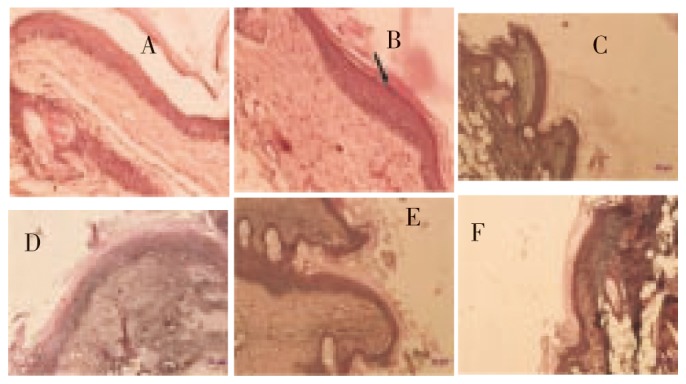
A: Vehicle control; B: Retinoic acid 0.5 mg/kg; C: Methanol extract 100 mg/kg; D: Methanol extract 200 mg/kg; E: Isolated flavonoid quercetin 25 mg/kg; F: Isolated flavonoid quercetin 50 mg/kg. Note that the retinoic acid induced orthogranulosis (indicated by an arrow) is clearly seen over the whole horizontal length of the scale, whereas a granular layer is missing in most parts of the control specimen.
3.8. Carrageenan induced pleurisy in rats
Effect of methanol extract and isolated flavonoid quercetin on leukocyte migration by the carageenan induced pleurisy in mice was shown in Table 2. Among the tested drugs, isolated flavonoid quercetin (25 and 50 mg/kg) showed significant (P<0.001) inhibition of leukocyte migration and significant (P<0.05) inhibition of the formation of pleural exudates.
Table 2. Effect of methanol extract and isolated flavonoid quercetin on pleurisy induced by intrapleural injection of carrageenan in rats (mean±SEM) (n=6).
| Groups | Exudate volume (mL) | % Inhibition of edema | Leukocyte count (cells/mm3)× 103 |
| Control | 0.90±0.11 | – | 16.50±0.50 |
| Indomethacin (5 mg/kg) | 0.50±0.03* | 44.5 | 10.10±0.35*** |
| Celecoxib (10 mg/kg) | 0.49±0.03** | 45.6 | 9.20±0.40*** |
| Methanol extract (100 mg/kg) | 0.68±1.40 | 34.2 | 14.70±0.70 |
| Methanol extract (200 mg/kg) | 0.58±2.08 | 40.2 | 13.00±0.21*** |
| Isolated flavonoid quercetin (25 mg/kg) | 0.59±0.20 | 38.4 | 14.90±2.38 |
| Isolated flavonoid quercetin (50 mg/kg) | 0.53±1.02* | 50.0 | 13.00±1.30*** |
Statistical significant test for comparison was done by ANOVA, followed by Dunnet's ‘t’ test (n=6). *: P<0.05, **: P<0.01, ***: P<0.001 when compared with control.
3.9. In vitro anti-psoriatic activity
The cytotoxic effect of methanol extract and isolated flavonoid quercetin was evaluated using HaCaT cells, a rapidly multiplying human keratinocyte cell line, as a model of epidermal hyperproliferation in psoriasis. The methanol extract and isolated flavonoid quercetin showed appreciable antiproliferant activity in HaCaT cell line. The isolated flavonoid quercetin (62.42±10.20 µg/mL) was found to have more potent antiproliferant activity than methanol extract (102.50±10.00 µg/mL). The results were validated using asiaticoside as positive control. Asiaticoside showed a potent activity with IC50 value of 31.40 µg/mL.
4. Discussion
The therapeutic potential of flavonoids and the necessity for scientific validation in popular medicine have prompted increased interest in the field. Quercetin, a flavonol occurring in fruit and vegetables is a food component with proven beneficial impact on health[17]. Quercetin has also been demonstrated to display the antiviral, antibacterial, anticarcinogenic and anti-inflammatory effects[18]. Quercetin was isolated from the rhizome of S. china and evaluated for antipsoriatic efficacy using mouse tail test. In the mouse tail test, isolated flavonoid quercetin (25 and 50 mg/kg) produced significant orthokeratosis when compared to control. The isolated flavonoid quercetin also showed significant change in epidermal thickness compared to control while methanolic extract did not produce any significant change in epidermal thickness. Granular layer of the epidermis is greatly reduced or absent in psoriatic lesions[19]. Parakeratotic condition is seen in the adult mouse tail which is one of the hallmarks of psoriasis[20]. Induction of orthokeratosis in the adult mouse tail is the basis behind the mouse tail test. Many drugs presently used in the treatment of psoriasis have been evaluated by the mouse tail test and were found to have shown good efficacies[21].
The formation of exudate in the pleural cavity and leukocyte migration were produced by intrapleural injection of carrageenan and this method was a preclinical model of airway inflammation where type 1 proinflammatory cytokines such as interleukin (IL)-1 and TNF-α play a key pathogenic role[22],[23]. Non-steroidal anti-inflammatory drugs, such as indomethacin, inhibit the accumulation of exudates and mobilization of leukocytes between 3 and 6 h after application of carrageenan[24]. Isolated flavonoid quercetin showed similar anti-inflammatory effects to COX antagonist (indomethacin) and COX-2 selective antagonist (celecoxib) suggesting that it may represent a novel strategy for the modulation of inflammatory response in psoriasis.
Drugs that act by multiple mechanisms in combating psoriasis are more important than drugs acting by any single mechanism. This is because psoriasis is a recurrent chronic inflammatory skin disorder with multiple etiologies. From the above data, the flavonoid quercetin showed significant orthokeratosis, anti-inflammatory and maximum antiproliferant activity. To our knowledge, this is the first report on the anti-psoriatic effect of the flavonoid quercetin which is promising for further investigations to prove its anti-psoriatic activity.
Acknowledgments
This study was supported by the grants from Vels University, Tamilnadu, India. Authors are extremely grateful to our Chancellor Dr. Ishari K Ganesh, Vels University, for the facilities provided to complete this work successfully.
Footnotes
Foundation Project: This work was financially supported by Vels University, Tamilnadu, India.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest. 2004;113(12):1664–1675. doi: 10.1172/JCI22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 3.Azfar RS, Gelfand JM. Psoriasis and metabolic disease: epidemiology and pathophysiology. Curr Opin Rheumatol. 2008;20(4):416–422. doi: 10.1097/BOR.0b013e3283031c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amigo M, Paya M, De Rosa S, Terencio MC. Antipsoriatic effects of avarol-3′-thiosalicylate are mediated by inhibition of TNF-α generation and NF-κB activation in mouse skin. Br J Pharmacol. 2007;152(3):353–365. doi: 10.1038/sj.bjp.0707394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowcock A, Krueger J. Getting under the skin: the immunopathogenesis of psoriasis. Nat Rev Immunol. 2005;5:699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- 6.Crozier A, Jaganath IB, Clifford MN. Clifford dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 7.Variers VPS. Indian medicinal plants: a compendium of 500 species. Madras: Orient Longman Limited; 1996. p. 143. [Google Scholar]
- 8.Khandelwal KR. Practical pharmacognosy techniques and experiments. Pune: Nirali Prakashan; 2004. pp. 149–153. [Google Scholar]
- 9.Rajalakshmi PV, Senthil KK. Direct HPLC analysis of quercetin in exudates of Abutilon indicum (Linn). Malvaceae. J Pharm Sci Technol. 2009;1(2):80–83. [Google Scholar]
- 10.Rich E, Schibli A. HPTLC for the analysis of medicinal plants. New York: Thieme Medical Publisher, Inc; 2008. [Google Scholar]
- 11.Meena MC, Patni V. Isolation and identification of flavonoid “quercetin” from Citrullus colocynthis (Linn.) Schrad. Asian J Exp Sci. 2008;22(1):137–142. [Google Scholar]
- 12.Lipnic RL, Cotruvo JA, Hill RN, Bruce RD, Stitzel KA, Waker AP, et al. Comparison of the up and down conventional LD50 and fixed dose acute toxicity procedure. Fundam Chem Toxicol. 1995;3:223–231. doi: 10.1016/0278-6915(94)00136-c. [DOI] [PubMed] [Google Scholar]
- 13.Vogel GH, Vogel WH. Drug discovery and evaluation: pharmacological assays. 2nd ed. Germany: Springer-Berlag; 2002. p. 1325. [Google Scholar]
- 14.Bosman B, Matthiesen T, Hess V, Friderichs E. A quantitative method for measuring antipsoriatic activity of drugs by the mouse tail test. Skin Pharmacol. 1992;5:41–48. doi: 10.1159/000211016. [DOI] [PubMed] [Google Scholar]
- 15.Balasubramanian A, Ramalingam K, Krishnan S, Christina AJM. Anti-inflammatory activity of Morus indica Linn. Iran J Pharmacol Ther. 2005;4(1):13–15. [Google Scholar]
- 16.Saelee C, Thongrakard V, Tencomnao T. Effects of Thai medicinal herb extracts with anti-psoriatic activity on the expression on NF-κB signaling biomarkers in HaCaT keratinocytes. Molecules. 2011;16:3908–3932. doi: 10.3390/molecules16053908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaur C, Kapoor HC. Antioxidants in fruits and vegetables-the millennium's health. Int J Food Sci Technol. 2001;36:703–725. [Google Scholar]
- 18.Materska M. Quercetin and its derivatives: chemical structure and bioactivity-a review. Pol J Food Nutr Sci. 2008;58(4):407–413. [Google Scholar]
- 19.Vogel GH. Drug discovery and evaluation: pharmacological assays. 3rd ed. New York: Springer; 2008. [Google Scholar]
- 20.Danilenko DM. Preclinical models of psoriasis. Vet Pathol. 2008;45:563–575. doi: 10.1354/vp.45-4-563. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida H, Ishikawa T, Hosoai H, Suzukawa M, Ayaori M, Hisada T, et al. Inhibitory effect of tea flavonoids on the ability of cells to oxidize low density lipoprotein. Biochem Pharmacol. 1999;58:1695–1703. doi: 10.1016/s0006-2952(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 22.Arul B, Kothai R, Sureshkumar K, Christina AJM. Anti-inflammatory activity of Coldeian procumbens Linn. Pak J Pharm Sci. 2005;18(3):17–20. [PubMed] [Google Scholar]
- 23.Ciomar A, Amado B, Cuman RKN. Anti-inflammatory and antinociceptive activities of eugenol essential oil in experimental animal models. Braz J Pharmacogn. 2009;19(1B):212–217. [Google Scholar]
- 24.Almeida AP, Bayer BM, Horakova Z, Beaven MA. Influence of indomethacin and other anti-inflammatory drugs on mobilization and production of neutrophils: studies with carrageenan induced inflammation in rats. J Pharmacol Exp Ther. 1980;214:74–79. [PubMed] [Google Scholar]