Abstract
Objective
To investigate the effect of humic acid on nematode infected, resistant and susceptible grapes in relation to lipid peroxidation and antioxidant mechanisms on selected biochemical parameters known as proactive substances.
Methods
The grape rootstocks, superior, superior/freedom and freedom were reacted differently to Meloidogyne incognita and Rotylenchulus reniformis according to rootstock progenitor. Two weeks after inoculation, two commercial products of humic acid were applied at the rate of (2, 4 mL or grams/plant) as soil drench. After 4 months, nematode soil populations were extracted and counted. A subsample of roots from each plant was stained and gall numbers, embedded stages per root were calculated, final population, nematode build up (Pf/Pi), average of eggs/eggmass were estimated. Subsamples of fresh root of each treatment were chemically analyzed.
Results
Freedom reduced significantly the nematode criteria and build up. Humic acid granules appeared to be more suppressive to nematode build up on superior and the higher dose on superior/freedom than liquid treatments. On freedom, all treatments reduced significantly the nematode build up regardless to the material nature. The higher dose was more effective than the lower one. As a result of humic acid applications, the malondialdehyde (MDA) and H2O2 contents were significantly reduced after humic acid treatments while the antioxidant compounds glutathione (GSH), ascorbic acid (ASA) and total phenol contents were significantly increased when compared with check. Antioxidant defense enzymes ascorbate peroxidase (APX), superoxide dismutase (SOD), catalase (CAT) and polyphenol oxidase (PPO)showed significant increase in their specific activities in treated plants compared with nematode treated check.
Conclusions
Humic acid treatments improve the yield of grape by increasing the contents of antioxidant compounds and the specific activities of antioxidant enzymes.
Keywords: Meloidogyne incognita, Rotylenchulus reniformis, Grape, Humic acid, Oxidative stress, Antioxidant enzymes, Lipid peroxidation, Nematode, Grape rootstocks, Antioxidant compounds, Biochemical parameters, Proactive substance, MDA, SOD
1. Introduction
Damage of the root-knot and reniform nematode species to Vitis vinifera (V. vinifera) varieties has been extensively reported[1]–[3].
Plant endoparasitic nematodes, including the potato cyst nematode (Globodera rostochiensis), spend a major part of their life cycles being embedded in the roots of a host plant and are therefore exposed to a variety of host defense responses[4]. These responses may include generation of damaging reactive oxygen species (ROS). ROS such as superoxide anion (O2−), singlet oxygen, hydrogen peroxide (H2O2) and hydroxyl radical (OH−) are produced continuously as byproducts of various metabolic pathways that are localized in different cellular compartments[5]–[8]. However, under stressful conditions, their formation might increase to exceed the antioxidant scavenging capacity, thus creating oxidative stress by reaction and damage to all biomolecules[9]. Compared to animal parasitic nematodes, little is known about the defence proteins employed by plant parasitic nematodes. Superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) activities have been detected in some endoparasitic nematodes but little is known about the roles of these proteins in the host-parasite interaction and none of these proteins has been characterized in detail. There is no information regarding peroxiredoxins in plant parasitic nematodes[10]. The incompatible resistant interactions to nematode infection may include many physiological defense actions, production of H2O2, jasmonic acids, the formation of ROS[11]–[13], different enzymatic and non-enzymatic glutathione and ascorbate[14], increase in peroxidase and polyphenol oxidase level[15], antioxidant properties, and polyphenols[16].
Crops, especially those having Vitis champinii as a progenitor (freedom) have been reported as resistant to different root-knot and reniform nematode species[17],[18]. Growing plants supplemented with fertilizers containing humic acid improved their resistant to nematode infection[19]. Growing resistant grape varieties supplemented with humic acid were supposed to result better nematode management.
The present study aims to investigate the effect of humic acid on nematode infected, resistant and susceptible grapes in relation to lipid peroxidation and oxidant mechanisms on selected biochemical parameters known as proactive substances.
2. Materials and methods
2.1. Nematode species stock cultures
Pure cultures of the root-knot nematode, Meloidogyne incognita (M. incognita) and the reniform nematode, Rotylenchulus reniformis (R. reniformis) were obtained from isolates belonging to the Nematology Research Center, Faculty of Agriculture, Cairo University. Nematode species have been propagated separately, i.e. M. incognita on eggplant cv. Classic and R. reniformis on pigeon pea. The plants were grown in 20 cm diameter clay pots filled with sterilized loamy soil. To avoid contamination, cultures of each species were arranged separately, examined and periodically renewed in order to ensure continuous supplies of inocula for the experimental work.
2.2. Glasshouse experiments
One year old seedlings of grape rootstocks, superior, superior/freedom and freedom with uniform size were obtained from Grape Department, Horticulture Research Institute, Agriculture Research Center and cultivated singly in 20 cm diameter clay pots filled with steam sterilized sandy loam soil (1:1, v/v). One month later, 5 seedlings of each rootstock were inoculated separately with either 5 000 infective stages of M. incognita or R. reniformis by pipetting the nematode water suspension into 4 holes around the root system which was immediately covered with soil. Pots were labeled and arranged randomly on a glasshouse clean bench, receiving similar horticulture treatments. Seedlings were left out after 4 months from inoculation. Soil population was extracted by means and counted[20]. The nematode embedded stages of both species were also counted.
For testing the effect of humic acid on the root-knot nematode development and reproduction, another 5 seedlings of each rootstock were inoculated with 5 000 J2 of M. incognita/pot. Two weeks after inoculation, two commercial products of humic acid (liquid and granules) were applied at the rate of (2, 4 mL or grams/plant) as soil drench. All treatments were arranged in a fully randomized design on a clean bench in the glasshouse at (32±5) °C receiving similar horticultural treatments. After 4 months, nematode soil populations were extracted and counted using a Hawksley counting slide, under a binocular microscope. A subsample (5 g) of roots from each plant was stained and gall numbers, embedded stages (developmental stages + eggmasses) per root were calculated, final population (embedded stages + nematodes in soil), nematode build up (Pf/Pi), average of eggs/eggmass were estimated.
2.3. Plant chemical analysis
Subsamples of fresh root of each treatment were chemically analyzed as follows.
2.3.1. Preparation of enzyme extracts
Samples of 0.25 g were homogenized in 5 mL of 50 mM phosphate buffer (pH 7.0) containing 1.0 N NaCl, 1% PVP (Sigma) M.W. 40 000, 1 mM ascorbate (Sigma) at 4 °C. After centrifugation at 15 000 × g for 15 min the supernatant was collected.
2.3.2. Assay of protein content
Soluble proteins were measured by the Bio-Rad micro assay according to the method of Bradford with some modifications[21] using crystalline bovine serum albumin as a reference.
2.4. Determination of oxidative burst
2.4.1. Lipid peroxidation
About 0.5 g ground roots was homogenized in 2 mL of 0.1% (w/v) trichloroacetic acid (TCA), followed by centrifugation at 12 000×g for 20 min. The supernatant (1 mL) obtained was mixed with an equal volume of TCA (10%) containing 0.5% (w/v) TBA or no TBA as the blank, and heated at 95 °C for 30 min and then cooled in ice. The reaction product was centrifuged at 12 000×g for 15 min and the supernatant absorbance was measured at 400, 532 and 600 nm. The malondialdehyde (MDA) equivalent was derived from the absorbance[22].
2.4.2. Assay of hydrogen peroxide concentration
Hydrogen peroxide was measured by the method described by Capaldi and Taylor[23], with a slight modification. The ground roots was homogenized in 5% TCA (2.5 mL per 0.5 g powder) with 50 mg active charcoal at 0 °C, and centrifuged for 10 min at 15 000×g. Supernatant was collected, neutralized with 4 N KOH to pH 3.6 and used for H2O2 assay. The reaction mixture contained 200 µL of leaf extract, 100 µL of 3.4 mM 3-methylbenzothiazoline hydrazone (MBTH). The reaction was initiated by adding 500 µL of horseradish peroxidase solution (90 U per 100 mL) in 0.2 M sodium acetate (pH 3.6). 2 min later 1 400 µL of 1 N HCl was added. Absorbance was read at 630 nm after 15 min.
2.4.3. Determination of total glutathione (GSH)
The level of total acid-soluble SH compound (GSH) was determined with Ellman's reagent[24]. The buffer was mixed with 630 µL of 0.5 M K2HPO4 and 25 µL of mM 5, 5′-dithiobis (2-nitrobenzoic acid) (final pH 7). The absorbance at 412 nm was read after 2 min. GSH was used as a standard.
2.4.4. Ascorbic acid (ASA) determination
Levels of ASA followed the procedure as described by Singh et al with few modifications[25]. Briefly, fresh leaf sample of a known weight (1 g) was extracted with 3 mL of 5% (w/v) TCA and centrifuged at 18 000 × g for 15 min. ASA was determined in a reaction mixture consisting of 0.2 mL of supernatant, 0.5 mL of 150 mM phosphate buffer (pH 7.4, containing 5 mM EDTA) and 0.2 mL of deionized water. Colour was developed in reaction mixtures with the addition of 0.4 mL of 10% (w/v) TCA, 0.4 mL of 44% (v/v) phosphoric acid, 0.4 mL of α,α-dipyridyl in 70% (v/v) ethanol and 0.2 mL of 3% (w/v) FeCl3. The reaction mixtures were incubated at 40 °C for 40 min and the absorbance was read at 532 nm.
2.4.5. Assay of phenol
The phenolic assay was conducted following the method of Singleton et al[26]. The samples were homogenized at the rate of 0.1 g per 1 mL of 80% methanol and the methanolic extract was kept in a water bath at 70 °C for 15 min with frequent agitation. One mL of methanolic extract was added to 5 mL of distilled water and 250 mL of Folin-Ciocalteau reagent (1 N) was added and the solution was kept at 25 °C for 30 min. Finally, 1 mL of saturated solution of Na2CO3 and 1 mL of distilled water were added and the reaction mixture was incubated for 1 h at 25 °C. After the blue color development, the absorbance was recorded at 725 nm. The contents of total soluble phenols were calculated according to a standard curve obtained from a Folin-Ciocalteau reaction with a catechol solution. The phenol content was expressed as phenol equivalents in mg/g fresh weight of callus tissues.
2.5. Determination of antioxidant defense enzymes specific activities
2.5.1. Assay of SOD specific activity
The specific activity of SOD was assayed by measuring its ability to inhibit the photochemical reduction of NBT[27]. The 3 mL reaction mixture contained 50 mM phosphate buffer pH 7.8, 13 mM methionine, 75 µM NBT, 2 µM riboflavin, 1.0 mM EDTA and 20 µL enzyme extract. Riboflavin was added last and the reaction was initiated by placing the tubes 30 cm below 15 W fluorescent lamps. The reaction was started by switching on the light and was allowed to run for 10 min. Switching off the light stopped the reaction and the tubes were covered with black cloth. Non-illuminated tubes served as control. The absorbance at 560 nm was read. The volume of enzyme extract corresponding to 50% inhibition of the reaction was considered as one enzyme unit.
2.5.2. Assay of APX specific activity
The specific activity of APX was measured by estimating the rate of ascorbate oxidation (extinction coefficient 2.8/mM/cm). The 3 mL reaction mixture contained 50 mM phosphate buffer (pH 7.0), 0.1 mM H2O2, 0.5 mM sodium ascorbate, 0.1 mM EDTA and a suitable aliquot of enzyme extract. The change in absorbance was monitored at 290 nm and enzyme activity was expressed as units min/mg/protein[28].
2.5.3. Assay of CAT specific activity
For measurement of the catalase specific activity the method of Aebi[29] was used. The 3 mL reaction mixture consisted of 50 mM sodium phosphate buffer (pH 7.0), 20 mM H2O2 and a suitable aliquot of enzyme. Decrease in the absorbance was taken at 240 nm (the molar extinction coefficient of H2O2 is 0.04/mM/cm). Enzyme activity was expressed as units min/mg/protein.
2.5.4. Assay of polyphenol oxidase (PPO) specific activity
PPO was assayed by using photochemical method as described by Coseteng et al[30]. The reaction mixture contained 50 mM potassium phosphate buffer (pH 6.2), 250 mM catechol and enzyme extract. The increasing in absorbance at 420 nm was measured. One unit of enzyme activity is defined as the amount of the enzyme that causes an increase of 0.001 absorbance unit per min at 25 °C.
2.6. Statistical analysis
Data were compared by Duncan's multiple range test (DMRT) at the 5% level of probability using MSTAT version 4[31].
3. Results
3.1. M. incognita and R. reniformis reproductivity on grape rootstocks
Data in Table 1 revealed that grapes reacted differently according to the nematode species and rootstock. M. incognita reproduction (as measured by numbers of galls, emdedded stages, build up and eggs/egg mass) was efficient on Vitis vinifera stock (superior) with no significant differences with superior/freedom. Yet, freedom reduced significantly the nematode criteria and build up. The same trend was observed by R. reniformis, however the nematode folded hardly on freedom.
Table 1. Reproductivity of M. incognita and R. reniformis on grape rootstocks.
| Nematode species | Rootstocks | Galls | Embedded stages | Soil population | Final population | Pf/Pi | Eggs/Eggmass | P.R.I. |
| M. incognita | Superior | 756a | 1 004a | 11 560 | 12 564 | 2.51a | 167a | 100 |
| Superior/ Freedom | 517b | 714b | 11 000 | 11 714 | 2.34a | 136b | 93 | |
| Freedom | 382c | 601c | 8 660 | 9 261 | 1.85b | 141b | 74 | |
| R. reniformis | Superior | – | 1 152a | 23 790 | 24 942 | 4.99a | 119a | 100 |
| Superior/ Freedom | – | 702b | 12 800 | 13 502 | 2.70b | 91b | 54 | |
| Freedom | – | 407c | 4 660 | 5 067 | 1.01c | 60c | 20 |
Means followed by the same letter(s) within a column in each block are not significantly different (P≤0.05) according to DMRT.
3.2. Effect of humic acid on M. incognita development and reproduction
Table 2 indicated that most if not all treatments significantly increased M. incognita numbers of galls and embedded stages as compared with the check regardless to humic acid nature, dose or grape rootstock, with the exception superior and superior/freedom treated with 2 and 4 g of humic acid, respectively where the numbers were significantly decreased comparing to other treatments. That reduction was also reflexed significantly on the rate of nematode build up (Pf/Pi) and numbers of eggs/egg mass. It is worthy to notice that freedom achieved the least numbers in such criteria. Humic acid applied at the rate of 2 and 4 g granules appeared to be more suppressive to nematode build up on superior and the higher dose on superior/freedom. Humic acid applied as liquid at 4 mL/plant diminished significantly the nematode build up than 2 mL/plant but not as much as granule application. On freedom, all treatments reduced significantly the nematode build up regardless to the material nature. The higher dose was more effective than the lower one.
Table 2. Reproductivity of M. incognita on grape rootstocks as influenced by addition of humic acid.
| Rootstocks | Treatments | Dose/Plant | Galls | Embedded stages | Soil population | Final population | Pf/Pi | Eggs/Eggmass |
| Superior | Humic acid (liquid) | 2 mL | 826c | 1 099d | 11 400 | 12 499 | 2.50a | 151de |
| 4 mL | 847c | 1 103d | 9 600 | 10 703 | 2.14c | 157cd | ||
| Humic acid (granules) | 2 g | 714d | 895f | 6 920 | 7 815 | 1.56f | 139efg | |
| 4 g | 867c | 1 156cd | 8 440 | 9 596 | 1.92e | 121hi | ||
| Inoculated only | – | 756d | 1 004e | 11 560 | 12 564 | 2.51a | 167c | |
| Superior/ Freedom | Humic acid (liquid) | 2 mL | 923b | 1 230b | 8 620 | 9 850 | 1.97de | 146def |
| 4 mL | 930b | 1 200bc | 7 160 | 8 360 | 1.67f | 149de | ||
| Humic acid (granules) | 2 g | 1 051a | 1 325a | 9 100 | 10 425 | 2.09cd | 226a | |
| 4 g | 734d | 979e | 6 720 | 7 699 | 1.54f | 114ij | ||
| Inoculated only | – | 517ef | 714g | 11 000 | 11 714 | 2.34b | 136fg | |
| Freedom | Humic acid (liquid) | 2 mL | 469fg | 730g | 7 580 | 8 310 | 1.66f | 124hi |
| 4 mL | 416gh | 572h | 6 480 | 7 052 | 1.41g | 130gf | ||
| Humic acid (granules) | 2 g | 559e | 672g | 7 420 | 8 092 | 1.62f | 179b | |
| 4 g | 348i | 554h | 6 380 | 6 934 | 1.39g | 104j | ||
| Inoculated only | – | 382hi | 601h | 8 660 | 9 261 | 1.85e | 141efg |
Means followed by the same letter(s) within a column in each block are not significantly different (P≤0.05) according to DMRT.
3.3. Effect of nematode infection on grape root contents of lipid peroxidation (MDA) and H2O2 content
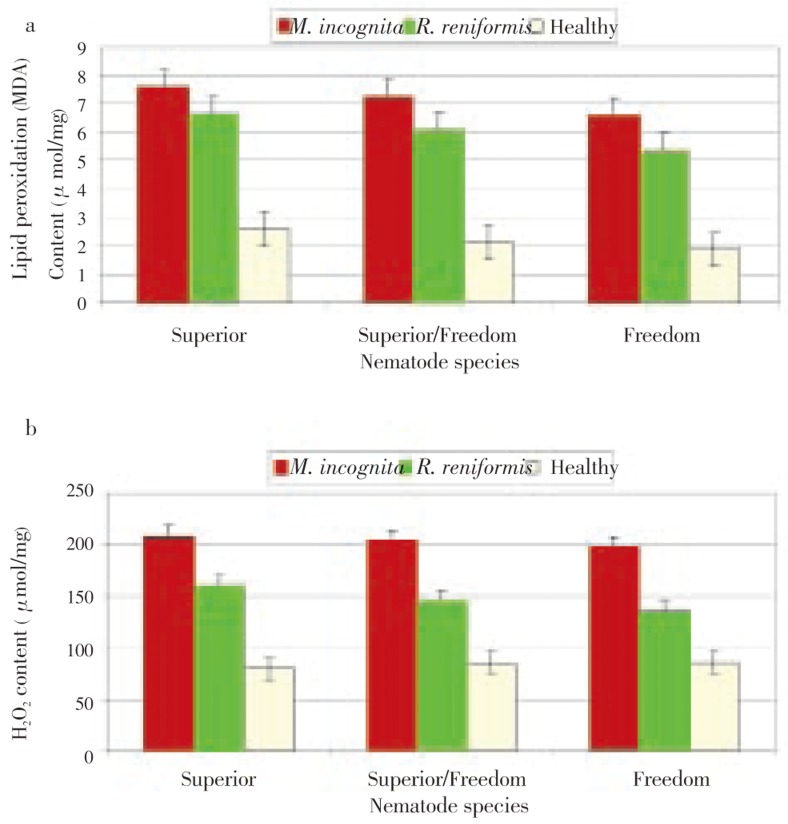
Results showed that nematode infection (M. incognita or R. reniforms) significantly increased root contents of both lipid peroxidation (MDA) and H2O2 in roots of all rootstocks (superior, superior/freedom and freedom) in comparison with the healthy plants (Figure 1). M. incognita infection clearly reduced the content of MDA and H2O2 than did R. reniformis especially in superior treatments.
Figure 1. Effect of M. incognita or R. reniformis on grape root contents of lipid peroxidation (MDA) (a) and hydrogen peroxide (H2O2) (b).
Each value is expressed as mean±standard deviation (n=3).
3.4. Effect of nematode infection on grape root contents of non-enzymatic and specific activity of enzymatic antioxidant defense system
Data in Table 3 indicated that, the nematode infection significantly enhanced the contents of both non-enzymatic antioxidant total GSH, TAA and TPH and enzymatic antioxidant specific activity APX, SOD, CAT and PPO in all rootstocks treatments compared with healthy plants.
Table 3. Effect of M. incognita and R. reniformis on reduced GSH, total ascorbic acid (TAA) and total phenols (TPH), and on the activities of APX, SOD, CAT and PPO in grape roots.
| Rootstocks | Nematode species | GSH (A) | TAA (B) | TPH (B) | APX (C) | SOD (C) | CAT (D) | PPO (C) |
| Superior | M. incognita | 5.11f | 8.85f | 4.36f | 13.41d | 192.91e | 43.03c | 11.59d |
| R. reniformis | 9.31c | 14.30a | 8.11b | 29.10b | 245.20b | 68.96a | 35.05b | |
| Healthy | 2.42h | 3.23h | 1.06h | 8.03e | 112.80i | 20.21g | 2.53e | |
| Superior/Freedom | M. incognita | 6.16d | 10.45d | 4.95d | 12.87d | 176.83f | 34.67e | 10.66d |
| R. reniformis | 10.39a | 12.78c | 7.29c | 26.75c | 227.11c | 65.50b | 32.40c | |
| Healthy | 2.31h | 3.55g | 1.37g | 7.85e | 127.84h | 20.10g | 3.05e | |
| Freedom | M. incognita | 5.96e | 9.95e | 4.85e | 14.26d | 199.90d | 38.16d | 13.19d |
| R. reniformis | 9.98b | 13.94b | 8.95a | 35.42a | 297.50a | 69.29a | 38.03a | |
| Healthy | 2.67g | 3.06h | 1.31g | 7.25e | 136.42g | 24.91f | 2.28e |
A: µmol/g FW; B: mg/g FW; C: unit/mg protein; D: µmol/mg protein/min. Means followed by the same letter(s) within a column in each block are not significantly different (P≤0.05) according to DMRT.
3.5. Effect of humic acid on infected grape root contents of lipid peroxidation (MDA) and H2O2 content
All infected grape rootstocks and treated with humic acid had lower contents of both lipid peroxidation (MDA) and H2O2 (Table 4). The higher concentrations of humic acid (4 mL and 4 g) were the best treatments for decreasing the MDA and H2O2 contents. It decreased the content of MDA and H2O2 by (59%, 58% and 54%, 52%), respectively in superior rootstock, (69%, 69% and 53%, 53%), respectively in superior/freedom rootstock and (65%, 62% and 45.5%, 47%), respectively in freedom rootstock.
Table 4. Effect of humic acid on MDA, H2O2, reduced GSH, TAA and TPH, and on the specific activities of APX, SOD, CAT and PPO in infected grape roots.
| Rootstocks | Treatments | Dose/Plant | MDA (A) | H2O2 (A) | GSH (A) | TAA (B) | TPH (B) | APX (C) | SOD (C) | CAT (C) | PPO (C) |
| Superior | Humic acid (liquid) | 2 mL | 3.36e | 134.28e | 10.24g | 15.90c | 9.04h | 14.83efg | 212.50j | 38.43m | 14.43j |
| 4 mL | 2.14k | 122.42f | 15.71b | 19.70b | 11.84o | 30.84d | 282.85e | 78.19c | 41.95d | ||
| Humic acid (granule) | 2 g | 3.99d | 139.81d | 10.26g | 15.57cd | 8.93i | 14.43efg | 209.47k | 45.23i | 11.56l | |
| 4 g | 3.23f | 116.33g | 15.63b | 19.89b | 11.53e | 32.03d | 275.47f | 77.21d | 34.20f | ||
| Inoculated only | – | 7.62a | 288.07a | 5.11l | 8.85i | 4.36n | 13.41fgh | 192.91m | 43.03k | 11.59l | |
| Healthy | – | 2.61i | 80.05o | 2.42n | 3.23jk | 1.06p | 8.03i | 112.80q | 20.21q | 2.53o | |
| Superior/Freedom | Humic acid (liquid) | 2 mL | 3.03g | 109.58i | 11.50c | 14.14e | 8.07j | 15.05efg | 224.74g | 49.94g | 16.24h |
| 4 mL | 2.27j | 95.63m | 16.84a | 21.54a | 14.41a | 41.93a | 349.91a | 89.34a | 44.49b | ||
| Humic acid (granules) | 2 g | 3.07g | 106.93k | 11.34d | 13.93e | 7.83k | 16.17e | 217.26i | 46.50h | 16.92g | |
| 4 g | 2.33j | 96.14m | 16.73a | 21.61a | 14.35a | 37.52c | 344.89b | 84.93b | 46.42a | ||
| Inoculated only | – | 7.25b | 203.09b | 6.16j | 10.45g | 4.95l | 12.87gh | 176.83n | 34.67o | 10.66m | |
| Healthy | – | 2.12k | 85.17n | 2.31n | 3.55j | 1.37o | 7.85i | 127.84p | 20.10q | 3.05n | |
| Freedom | Humic acid (liquid) | 2 mL | 3.04g | 115.33h | 10.88f | 11.13f | 9.71g | 15.60ef | 220.17h | 44.01j | 15.44i |
| 4 mL | 2.33j | 107.59j | 6.48i | 15.30d | 12.23c | 40.83ab | 330.02c | 73.30f | 39.73e | ||
| Humic acid (granules) | 2 g | 2.74h | 109.18i | 11.08e | 10.79fg | 9.89f | 15.72ef | 216.84i | 39.94l | 14.45j | |
| 4 g | 2.51i | 104.73l | 6.68h | 15.27d | 12.48b | 38.63bc | 329.05d | 76.09e | 43.09c | ||
| Inoculated only | – | 6.60c | 197.40c | 5.96k | 9.95h | 4.85m | 14.26efg | 199.90l | 38.16n | 13.19k | |
| Healthy | – | 1.89l | 85.60n | 2.67m | 3.06k | 1.31o | 7.25i | 136.42o | 24.91p | 2.28p |
A: µmol/g FW; B: mg/g FW; C: unit/mg protein. Means followed by the same letter(s) within a column in each block are not significantly different (P≤0.05) according to DMRT.
3.6. Effect of humic acid on infected grape root contents of non-enzymatic and activity of enzymatic antioxidant defense system
Humic acid treatments improved the levels of non-enzymatic and enzymatic antioxidant molecules in all infected grape rootstocks, especially the higher concentrations of humic acid (4 mL and 4 g) (Table 4). Significant increment was observed in non-enzymatic antioxidant contents (GSH, TAA and TPH) of all rootstocks. In addition, the antioxidant enzyme activity (APX, SOD and CAT) was enhanced markedly with humic concentrations and showed its maximum at the higher concentrations of humic (4 mL and 4 g). While the PPO activity showed 2–3 times increment at humic concentrations (4 mL and 4 g) in comparison to the low concentrations of humic (2 mL and 2 g).
4. Discussion
Compared to the susceptible V. vinifera stock (superior), the nematodes development and reproduction were variable according to nematode species and rootstock progenitor[18].
Generally, humic acid treatments either liquid or granules increased significantly the galls and embedded stages numbers[19]. That increase was governed by the grape rootstock progenitor. But that was not the case with the nematode soil population, build up and eggs/eggmass. These criteria were significantly decreased in most treatments. It seems that some humic acid affects the nematode fertility and consequently its fecundity. The reduction in rates of such nematode criteria was also influenced with rootstock resistance. The lowest rates were achieved by freedom at the higher doses of humic acid.
The increase of proteins and fatty acids in root tissues as a result of treating plants with organic acids[18],[19] may enhance some biochemical compounds able to retard nematode reproduction.
Incompatible resistant interactions between plant and pathogen are often determined by the formation of ROS by the pathogen[12]. ROS, such as H2O2, is some of the most damaging stressors in plants. Thus, H2O2 from the oxidative stress plays a key role in the orchestration of a localized hypersensitive response during the expression of plant disease resistance[32]. ROS induced lipid peroxidation may be one of the mechanisms accounting for cell death[33]. Our results agreed with those facts and showed that, in comparison with healthy plants, nematode infections by M. incognita or R. reniforms on grape root treatments (superior, superior/freedom and freedom) enhanced the content of MDA and H2O2. Our results also indicated that plants possess both enzymatic and non-enzymatic antioxidant defense systems to counteract ROS under nematode infections. The significant increase of non-enzymatic antioxidant such as GSH, TAA and TPH contents may be driven by enhanced of MDA and H2O2 formation in the nematode infections. However, GSH may play a protective role in scavenging of singlet oxygen, peroxides and hydroxyl radicals and is involved in recycling reduced of ASA in the ascorbate-glutathione cycle[34]. On the other hand, the significant increase in the TPH contents could be affected as strong antioxidant natural products induced under oxidative stress condition controlling the oxidative damage. These data were in accordance with Goodman et al[35], who found that, multifold increase of phenols after challenging with elicitation may be due to the excess production of H2O2 in elicited plant cells through increased respiration[36] or due to the activation of hexose-monophosphate pathway, acetate pathway and release of bound phenols by hydrolytic enzymes. In addition, the nematode infection caused marked increase in the specific activity of antioxidant enzymes (APX, SOD, and CAT) which are involved in scavenging excess ROS in plant cells[14]. CAT and APX play an essential role in scavenging from the H2O2 toxicity. The combined action of CAT and SOD converts the toxic O2− and H2O2 to water and molecular oxygen (O2), thus averting the cellular damage under unfavorable conditions like infection by nematodes, salt stress, Fe deficiency, cadmium stress, lead toxicity and ionizing radiation[37]–[42]. While the increase in PPO specific activity after nematodes infection may be due to autooxidation of the total phenol substrate which interact with H2O2 and may prevent nematode spread to healthy tissue[43].
Humic acid treatments on grape roots infected by M. incogenta reduced the contents of lipid peroxidation and H2O2 in all rootstocks by improving the contents of non-enzymatic antioxidants (GSH, TAA and TPH) and increase the specific activities of PPO and antioxidants enzymes (APX, SOD and CAT) and reached its maximum induction at the higher treatments (4 mL and 4 g). Similar results were reported in response to salt and drought stress[37],[44]. In addition, our results indicated that the humic treatments (4 mL and 4 g) at superior/freedom rootstock produce the most suitable plant treatments to reduce the ROS, lipid peroxidation formation and improve the induction of antioxidant defense system. Similar results were reported in response to sodium nitroprusside (SNP; nitric oxide donor) treatment against drought stress[44].
It is commonly accepted now that humic acid treatments may play an important role in antioxidant defense system of plants, it is supposed that low concentration of humic acid might be a signal to induce the expression of many antioxidative molecules and enzymes and reduce the ROS in plant cells[45].
Reduction of ROS such as H2O2 and lipid peroxidation levels by induction the levels of antioxidant non-enzymatic such as (GSH, TAA and TPH) and enzymes molecules, such as APX which scavenges potentially harmful H2O2 from plant cells by utilizing ascorbate as a very important reducing substrate for H2O2 detoxification in the photosynthetic organism, SOD, which catalyses the dismutation of O2− into oxygen and hydrogen peroxide and CAT, which converts H2O2 to water and oxygen, may also be integral to the development of anti-nematode defense[11]. Our results were in agreement with Sun et al[46], who found that humic acid treatments improved the yield of grape by increasing the activity of antioxidant enzymes.
Acknowledgments
Authors would like to thank the Management of the Faculty of Agriculture and, Cairo University for ongoing cooperation to support research and provide funds and facilities necessary to achieve the desired goals of research.
Footnotes
Foundation Project: This work was financially supported by Management of the Faculty of Agriculture and Cario University.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Eastwell KC, Sholberg PL, Sayler RJ. Characterizing potential bacterial agents for suppression of Rhizobium vitis, causal agent of crown gall disease in grapevines. Crop Prot. 2006;25:1191–1200. [Google Scholar]
- 2.Ahmad F, Siddiqui MD. Management of the root-knot nematode Meloidogyne incognita in tomato. Pak J Nematol. 2009;27(2):369–373. [Google Scholar]
- 3.Zhu KJ, Wang B, Fang WZ, Iuo DM. Suppressive capacity of compost extracts and compost tea against the root-knot nematode Meloidogyne javanica on tomato in potting tests. J Yangtze Univ. 2006;1:116–118. [Google Scholar]
- 4.Miyake C, Shinzaki Y, Nishioka M, Horiguchi S, Tomizawa K. Photoinactivation of ascorbate peroxidase in isolated tobacco chloroplasts: Galdieria partita APX maintains the electron flux through thewater-water cycle in transplantomic plants. Plant Cell Physiol. 2006;47:200–210. doi: 10.1093/pcp/pci235. [DOI] [PubMed] [Google Scholar]
- 5.Reddy AM, Kumar SG, Jyothsnakumari G, Thimmanaik S, Sudhakar C. Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.) Chemosphere. 2005;60:97–104. doi: 10.1016/j.chemosphere.2004.11.092. [DOI] [PubMed] [Google Scholar]
- 6.Afify AMR, El-Beltagi HS, Fayed SA, Shalaby EA. Acaricidal activity of successive extracts from Syzygium cumini L. Skeels (Pomposia) against Tetranychus urticae Koch. Asian Pac J Trop Biomed. 2011;1(5):359–364. doi: 10.1016/S2221-1691(11)60080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Beltagi HS, Ahmed OK, El-Desouky W. Effect of low doses γ-irradiation on oxidative stress and secondary metabolites production of rosemary (Rosmarinus officinalis L.) callus culture. Radiat Phys Chem. 2011;80(9):965–973. [Google Scholar]
- 8.Afify AMR, El-Beltagi HS. Effect of the insecticide cyanophos on liver function in adult male rats. Fresenius Environ Bull. 2011;20(4a):1084–1088. [Google Scholar]
- 9.Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, et al. Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol. 2006;291:R491–R511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 10.Dubreuil G, Magliano M, Deleury E, Abad P, Rosso MN. Transcriptome analysis of root-knot nematode functions induced in the early stages of parasitism. New Phytol. 2007;176:426–436. doi: 10.1111/j.1469-8137.2007.02181.x. [DOI] [PubMed] [Google Scholar]
- 11.Michalak A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud. 2006;15(4):523–530. [Google Scholar]
- 12.Delibes A, López-Braña I, Moreno-Vázquez SJ, Martín-Sánchez A. Characterization and selection of hexaploid wheats containing resistance to Heterodera avenae or Mayetiola destructor introgressed from Aegilops. Span J Agric Res. 2008;6:81–87. [Google Scholar]
- 13.Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura E, Kato Y, Sakamoto W. Comparative transcriptome analysis of green/white variegated sectors in Arabidopsis yellow variegated2: responses to oxidative and other stresses in white sectors. J Exp Bot. 2010;61(9):2433–2445. doi: 10.1093/jxb/erq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CC, Slesak I, Jorda L, Sotnikov A, Melzer M, Miszalski Z, et al. Arabidopsis chloroplastic glutathione peroxidases play a role in cross talk between photo-oxidative stress and immune responses. Plant Physiol. 2009;150:670–683. doi: 10.1104/pp.109.135566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wuyts N, Lognay G, Swennen R, De Waele D. Nematode infection and reproduction in transgenic and mutant Arabidopsis and tobacco with an altered phenylpropanoid metabolism. J Exp Bot. 2006;57:2825–2835. doi: 10.1093/jxb/erl044. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Zhang X. Effects of two composted plant pesticide residues incorporated with Trichoderma viride on root-knot nematode in ballon flower. Agric Sci China. 2009;8(4):447–454. [Google Scholar]
- 18.Al-Sayed AA, Kheir AM, El-Naggar HI, Kesba HH. Could other Vitis species be helpful in nematode management in Egypt's sand soil viticultures? Bull Fac Agric Cairo Univ. 2005;56:393–406. [Google Scholar]
- 19.Kesba HH, Al-Shalaby Mona EM. Survival and reproduction of Meloidogyne incognita on tomato as affected by humic acid. Nematology. 2008;10:243–249. [Google Scholar]
- 20.Hooper DJ, Hallmann J, Subbotin SA. Methods for extraction, processing and detection of plant and soil nematodes. In: Luc M, Sikora RA, Bridge J, editors. Plant parasitic nematodes in subtropical and tropical agriculture. Wallingford: CABI Publishing; 2005. pp. 53–86. [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid per-oxidation in plant tissues containing anthocyanin and other interfering com-pounds. Planta. 1999;207:604–611. doi: 10.1007/s00425-017-2699-3. [DOI] [PubMed] [Google Scholar]
- 23.Capaldi DJ, Taylor KE. A new peroxidase color reaction: oxidative coupling of 3-methyl-2-benzothiazolinone hydrazone (MBTH) with its formaldehyde azine application to glucose and choline oxidases. Anal Biochem. 1983;129:329–336. doi: 10.1016/0003-2697(83)90558-4. [DOI] [PubMed] [Google Scholar]
- 24.De Vos CH, Vonk MJ, Vooijs R, Henk S. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucbalus. Plant Physiol. 1992;98:859–858. doi: 10.1104/pp.98.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh N, Ma LQ, Srivastava M, Rathinasabapathi B. Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci. 2006;170(2):274–282. [Google Scholar]
- 26.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 27.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 28.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:860–867. [Google Scholar]
- 29.Aebi H. Catalase. In: Bergmeyer H, editor. Methods of enzymatic analysis. Weinheim, Adamse: Verlag Chemie; 1983. pp. 273–277. [Google Scholar]
- 30.Coseteng MY, Lee CY. Change in apple polyphenol oxidase and polyphenol concentrations in relation to degree of browning. J Food Sci. 1987;52:985–989. [Google Scholar]
- 31.Software program for the design and analysis of agronomic research experiments. Michigan: Michigan State University; 1987. MSTAT Version 4. [Google Scholar]
- 32.Horvathova J, Suhaj M, Polovka M, Brezov V, Simko P. The influence of gamma-irradiation on the formation of free radicals and antioxidant status of oregano (Origanum vulgare L.) Czech J Food Sci. 2007;25:131–143. [Google Scholar]
- 33.Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol. 1999;57:231–245. doi: 10.1016/s0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa T, Shigeoka S. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci Biotechnol Biochem. 2008;72:1143–1154. doi: 10.1271/bbb.80062. [DOI] [PubMed] [Google Scholar]
- 35.Goodman RN, Kiraly E, Zaitlin M. The biochemistry and physiology of infections in plant diseases. Princeton: Van Nostrand; 1967. p. 534. [Google Scholar]
- 36.Lee JW, Kim JK, Srinivasan P, Choi J, Kim JH, Han SB, et al. Effect of gamma irradiation on microbial analysis, antioxidant activity, sugar content and color of ready-to-use tamarind juice during storage. LWT-Food Sci Technol. 2009;42:101–105. [Google Scholar]
- 37.El-Beltagi HS, Salama ZA, El-Hariri DM. Some biochemical markers for evaluation of flax cultivars under salt stress conditions. J Nat Fiber. 2008;5(4):316–330. [Google Scholar]
- 38.Mohamed AA, El-Beltagi HS, Rashed MM. Cadmium stress induced change in some hydrolytic enzymes, free radical formation and ultrastructural disorders in radish plant. Electron J Environ Agric Food Chem. 2009;8(10):969–983. [Google Scholar]
- 39.Salama ZA, El-Beltagi HS, El-Hariri DM. Effect of Fe deficiency on antioxidant system in leaves of three flax cultivars. Not Bot Hort Agrobot Cluj. 2009;37(1):122–128. [Google Scholar]
- 40.El-Beltagi HS, Mohamed AA, Rashed MM. Response of antioxidative enzymes to cadmium stress in leaves and roots of radish. Not Sci Biol. 2010;2(4):76–82. [Google Scholar]
- 41.El-Beltagi HS, Mohamed AA. Changes in non protein thiols, some antioxidant enzymes activities and ultrastructural alterations in radish plants (Raphanus sativus L.) grown under lead toxicity. Not Bot Hort Agrobot Cluj. 2010;38(3):76–85. [Google Scholar]
- 42.Aly AA, El-Beltagi HS. Influence of ionizing irradiation on the antioxidant enzymes of Vicia faba L. Grasas Y Aceites. 2010;61(3):288–294. [Google Scholar]
- 43.Wuyts N, Dirk DW, Rony S. Activity of phenylalanine ammonia-lyase, peroxidase and polyphenol oxidase in roots of banana (Musa acuminata AAA, cvs Grande Naine and Yangambi km5) before and after infection with Radopholus similis. Nematology. 2006;8:201–209. [Google Scholar]
- 44.Shehab GG, Kansowa OA, El-Beltagi HS. Effects of various chemical agents for alleviation of drought stress in rice plants (Oryza sativa L.) Not Bot Hort Agrobot Cluj. 2010;38(1):139–148. [Google Scholar]
- 45.Karasyova TA, Klose EO, Menzel R, Steinberg CE. Natural organic matter differently modulates growth of two closely related coccal green algal species. Environ Sci Pollut Res Int. 2007;14:88–93. doi: 10.1065/espr2006.06.317. [DOI] [PubMed] [Google Scholar]
- 46.Sun Z, Xue S, Liang W, Liu Y. Effects of different application rates of humic acid compound fertilizer on pepper and its mechanism of anti-senility and incremental yield. Ying Yong Sheng Tai Xue Bao. 2004;15:81–84. [PubMed] [Google Scholar]