Abstract
Objective
To evaluate the effects of quinine and chloroquine against male mice infected with Plasmodium berghei and their adverse effects on the mice testes.
Methods
In this study, 48 adult male mice, (20–25 g), aged 8 to 12 weeks were divided into four groups. This study was carried out from December 2009 until May 2010 in the School of Public Health, Tehran University of Medical Sciences.
Results
The results showed that 58.33% of mice treated with chloroquine were completely recovered. Parasitemia was 4% on day 8 when compared to that on day 0, whereas it was 9% on day 9. There was no orchitis found in this group. The mortality of mice after exposing to quinine on day 5 was 8.3%, whereas from day 10 to day 14 it was 91.7%. We found 75% orchitis occurred in quinine treated group. There was a significant difference between quinine and chloroquine effects on the parasite and also mice testes (P<0.05).
Conclusions
In this study, It can be concluded that male mice have full resistance to the quinine. Quinine does not only make male mice recover completely, but also cause inflammation on mice testicles tissue.
Keywords: Quinine, Chloroquine, Orchitis, Plasmodium berghei
1. Introduction
Malaria is one of the six major infectious diseases in human communities[1]. Annual mortality was reported to be 1.5 to 2.7 million worldwide and it is one of the four diseases causing deaths of children in Africa[2],[3]. Global strategy on malaria treatment is health management, use of beneficial drugs, vector control and training of health care[4]–[6].
Plasmodium parasites are agents of rodent's malaria[7]. The life cycle is similar to another malaria parasite of mammals[8]. The study on the rodent's malaria can be a good model of human malaria infection. Quinine is an antimalarial drug which is made from Cinchona bark[9]. The mode of action of quinine is similar to chloroquine. For the first time, the effects of quinine on the hexokinase activity of Plasmodium berghei (P. berghei) were reported by Fraser and Kermack in 1957[10].
The first report due to quinine resistance in mice was presented by Thompson et al in 1965[11]. Quinine alone or with other antimalarial medications is used to treat malaria cases especially in pregnant women near delivery[12]. Of course, the presence of Plasmodium falciparum (P. falciparum) has been reported even after treatment with quinine in humans[13]. Although it increases the parasite resistance, it is still regarded as one of the most commonly antimalarial drugs[6],[14]–[17].
There are some reports on the effects of chloroquine on some species of rodents infected by Plasmodium chabaudi, Plasmodium yoelii and P. berghei[18]–[20]. Since chloroquine and quinine are the most common antimalarial drugs, their therapeutic effects and risk of resistance to drugs were studied. In this study, we are aimed to assess the adverse effects of the drugs on testis tissue.
2. Materials and methods
2.1. Drugs
Drugs were prepared by the Diseases Center, Ministry of Health and Medical Education of Iran, and normal saline 0.9% was from Merck Company, Germany.
2.2. Animals
The adult male albino mice, (20–25 g), 8 to 12 weeks old were selected. Animals were housed under 12/12 h light/dark cycle, and were given easy access to food and water. Para aminobenzoic acid was not added to their diets in the animal house.
All rodents were kept based on the recommended maintenance guide and use of animals in institutions, and the Applied Research Ethics National Association (Applied Research Ethics National Association Office of Laboratory Animal Welfare)[21].
2.3. Malaria parasite
P. berghei strain NK65 from the Aberdeen, University of Scotland was prepared with the help of Dr Mohammad-Zadeh from Urmia University of Medical Sciences.
2.4. Experimental design
This study was conducted from December 2009 until May 2010 in the School of Public Health, Tehran University of Medical Sciences. Forty eight adult male mice were divided into four groups with 12 mice in each group. The mice were infected via injection with 0.2 mL suspension of 106 parasitized erythrocytes intraperitoneally. Blood samples were taken from the tail of mice and parasites were determined after the preparation of thin blood smear and staining with the Giemsa. The percentage of contamination was counted using the binocular microscope and a counter. Glass slides were painted with emersion oil and ocular 10×, and 100× fields. The principles of treatment were the proposed protocol of the WHO treatment guidelines and instruments described by Ryley and Petrs in 1970[6],[22]. Chloroquine was administered to the mice in the first group as 20 mg/kg b.w. once a day for four consecutive days. Quinine at a dose of 20 mg/kg b.w. was given to the mice in the second group for the first day and 10 mg/kg b.w. for the second to fourth day. In the third group (positive control), the normal saline 0.9% was used for four consecutive days.
In the fourth group (negative control), the 12 mice selected were not parasitized or given any treatments, in order to evaluate the natural mortality rate of the mice in the animal house. In this study, the mice life span, parasitemia, mortality rate, and adverse effects of the drugs were recorded.
2.5. Statistical analysis
The data were analyzed by one way ANOVA test and SPSS software. P value less than 0.05 was considered as a significant value.
3. Results
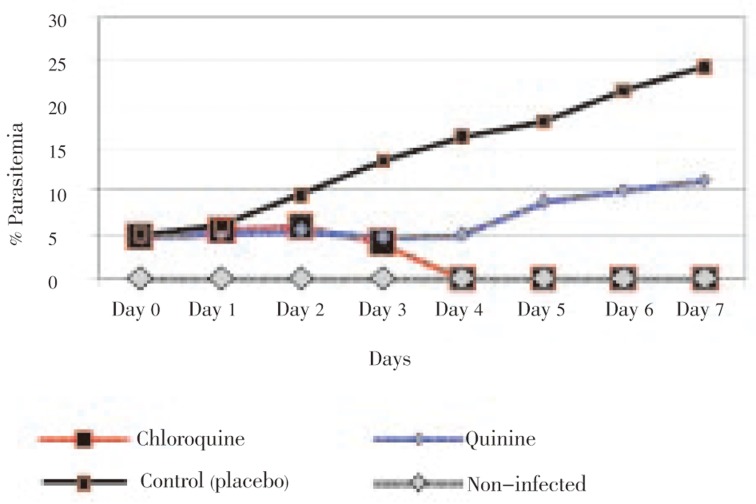
In chloroquine treated group, after injection, parasitemia was reduced gradually. On day 4 (24 h after the last treatment dose), it reached zero and still remained zero until day 7 (three days after treatment). About, 58.33% mice were fully recovered and were still alive until 28 days, whereas the rests were dead after 26 until 28 days (Figure 1). In this group, parasitemia was 4% on day 8 when comparing to that before treatment. The orchitis and urinary tract discharge were not found among the treated mice. In addition, the signs of necrosis and inflammation were not observed at the point of the needle injection site over the past few days after the last injection.
Figure 1. Rate of parasitemia decline with chloroquine and quinine drugs comparing with control and non-infected groups.
Parasitemia was not significantly reduced after the injection of quinine and it was increased four-day post treatments (Figure 1). The increase of parasitemia was found to be 5%, and 11.2% four, and seven-day post treatments, respectively. The mortality of the mice was found to be 8.3% and 91.7% five and ten to fourteen-day post treatments, respectively. Orchitis and discharge of uro-genitally tract infections were observed in 75% of the samples treated.
The percentage of parasitemia increased in the third group (receiving placebo). This index was 16.4%, and 17.9% on day 4 and 5, respectively, whereas it was found to be 26.4% eight-day post treatments. There was a significant difference between the first and third group (P<0.05). There were no secretions from infected orchitis or uro-genital discharge among the mice in the third group when compared to the second group.
The average of parasitemia percentage was 2.5% among the mice on day 4 of the first and second group, whereas it was found to be 16.4% in the third group (P<0.05). The average of parasitemia percentage was 9.2% among the samples treated by chloroquine and quinine eight-day post treatments, whereas it was calculated as 26.4% in the third group (P<0.05). Among the drugs used in rodent's malaria treatment, chloroquine was the most effective on P. berghei.
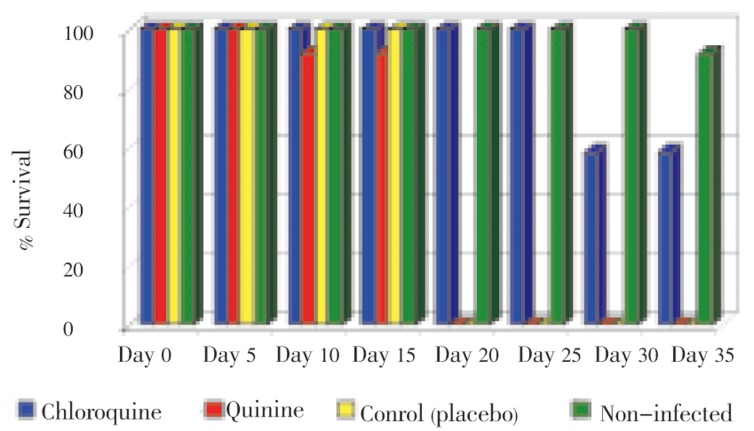
No adverse effect of chloroquine was observed on the mice testes. Survival rate of mice was shown in Figure 2. Average life span was 15 days in the third group. Among the treated groups, the average of life span in the first group was longer than that of the other groups.
Figure 2. Comparison of survival rate of mice in chloroquine, quinine, placebo control and non-infected groups.
In the fourth group 99% of the mice died after 35 days, whereas, all the mice died after 14 days in the third group. The life span of mice was found to be 26 days approximately in the first group, while samples in the second group survived until the end of the fourth day.
4. Discussion
The purpose of this study was the effect of quinine and chloroquine drugs on the malaria and their adverse effects on the mice which were infected with P. berghei. Generally, the symptoms of malaria in human include headache, muscle pain, stress, nausea, vomiting, fatigue, weakness and in extreme cases of cerebral malaria[23]. There are many reports due to effects of some chemical and natural compound with different doses against P. berghei[24],[25].
The subcutaneous dose of 2 mg/kg of quinine had no therapeutic effect while the dose of 850–950 mg/kg reported the lethal dose[26],[27]. In our study, a dose of 50 mg/kg of quinine had no therapeutic effect that expresses resistance of the drug on P. berghei. There are some reports due to adverse side effects of quinine that can cause hypoglycaemia, agranulocytosis, bleeding disorders, cardiovascular disease, blindness and death[28],[29].
The adverse effects of quinine on rodents have been investigated in many studies[9],[30]–[36].
In this study for the first time, the adverse effects of chloroquine and quinine as orchitis and uro-genitaly discharge in male mice were investigated. Signs and symptoms such as inflammation, swelling, redness and discharge of the testis were found among the 75% of the samples treated with quinine. These factors with resistance of P. berghei to quinine have been considered the main causes of mice death in this investigation. By now, there are many reports due to chloroquine treatment with different doses against P. berghei[37]–[39].
Despite the increasing reports due to resistance of parasite to chloroquine in some parts of the world[6],[16], this drug remains one of the most common to malaria treatment[14],[15].
In our study, although the relative resistance of P. berghei to chloroquine was observed but there are no adverse effects found among the samples. In our study, 58.33% of the cases were fully recovered and the rest died due to increasing the parasitemia and no adverse effects of the drug on the mice testes were observed.
In conclusion, although, quinine associated with chloroquine has been administrated to breakdown of drug resistance[6],[40]–[42], but this investigation indicated that P. berghei was quite resistant to quinine but tolerant to chloroquine. With regard to the significance of malaria in the world and treatment of high-risk age group, the use of alternative drugs is suggested.
Acknowledgments
The authors express thanks to all the professors and colleagues who helped us in this investigation. Sincerely thanks also extend to Dr. Mohammad-zadeh, Urmia University of Medical Sciences. To prepare the parasit, Dr. Azari-Hamidian, Dr. Omrani, Dr. Akhavan, Ms. Nikpour, Mr. Skandari, Ms. Erffani, Mr. Issazadeh for cooporation during the study. This study was financially supported by Tehran University of Medical Sciences with grant No. 240.1932.
Footnotes
Foundation Project: This work was financially supported by Tehran University of Medical Sciences (grant No. 240.1932).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.WHO . Global malaria control and elimination: report of a technical review. Geneva: WHO; 2008. [Online] Available from: http://www.who.int/malaria/docs/elimination/MalariaControlEliminationMeeting.pdf [Accessed on 10 Aug, 2011] [Google Scholar]
- 2.WHO . Malaria elimination, a field manual for low and moderate endemic countries. Geneva: WHO; 2007. [Online] Available from: http://www.who.int/malaria/docs/elimination/MalariaElimination_BD.pdf [Accessed on 12 Aug, 2011] [Google Scholar]
- 3.WHO . Global malaria control and elimination: report of a meeting on containment of artemisinin tolerance. Geneva: WHO; 2008. [Online] Available from: http://www.who.int/malaria/docs/drugresistance/Malaria_Artemisinin.pdf [Accessed on 15 Aug, 2011] [Google Scholar]
- 4.Basco LK. Field application of in vitro assays for the sensitivity of human malaria parasites to antimalarial drugs. Geneva: WHO; 2007. [Online] Available from: http://www.who.int/malaria/docs/drugresistance/OMS_FieldApplicationInVitroAssays.pdf. [Accessed on 20 July, 2011] [Google Scholar]
- 5.WHO . Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. Geneva: WHO; 2008. [Online] Available from: http://www.who.int/malaria/docs/drugresistance/MalariaGenotyping.pdf [Accessed on 18 Sep, 2011] [Google Scholar]
- 6.WHO . Guidelines for the treatment of malaria. 2nd ed. Geneva, Switzerland: WHO; 2010. [Online] Available from: http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html. [Accessed on 13 Sep, 2011] [Google Scholar]
- 7.Laroque A, Min-Oo G, Tam M, Radovanovic I, Stevenson MM, Gros P. Genetic control of susceptibility to infection with Plasmodium chabaudi AS in inbred mouse strains. Genes Immun. 2011 doi: 10.1038/gene.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langhorne J, Buffet P, Galinski M, Good M, Harty J, Leroy D, et al. The relevance of non-human rimate and rodent malaria models for humans. Malar J. 2011;10:23. doi: 10.1186/1475-2875-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ajibade AJ, Adenowo TK, Akintunde OW, Fakunle PB, Oyewo OO, Ashamu EA, et al. Suppression of exploration and locomotion in adult Wistar rats following quinine administration. J Neurosci Behav Health. 2011;3(3):32–37. [Google Scholar]
- 10.Fraser DM, Kermack WO. The inhibitory action of some antimalarial drugs and related compounds on the hexokinase of yeast and of Plasmodium berghei. Br J Pharmacol Chemother. 1957;12(1):16–23. doi: 10.1111/j.1476-5381.1957.tb01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson PE, Bayles A, Olszewski B, Waitz JA. Quinine-resistant Plasmodium berghei in mice. Science. 1965;148:1240–1241. doi: 10.1126/science.148.3674.1240. [DOI] [PubMed] [Google Scholar]
- 12.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7(2):93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 13.Menendez C, Fleming AF, Alonso PL. Malaria-related anaemia. Parasitol Today. 2000;16(11):469–476. doi: 10.1016/s0169-4758(00)01774-9. [DOI] [PubMed] [Google Scholar]
- 14.Iyawe HOT, Onigbinde AO. Impact of Plasmodium berghei and chloroquine on haematological and antioxidants indices in mice. Asian J Biochem. 2009;4(1):30–35. [Google Scholar]
- 15.Lalloo DG, Shingadia D, Pasvol G, Chiodini PL, Whitty CJ, Beeching NJ, et al. UK malaria treatment guidelines. J Infect. 2007;54(2):111–121. doi: 10.1016/j.jinf.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Liñares GE, Rodriguez JB. Current status and progresses made in malaria chemotherapy. Curr Med Chem. 2007;14:289–314. doi: 10.2174/092986707779941096. [DOI] [PubMed] [Google Scholar]
- 17.Sweetman SC. Martindale: the complete drug reference. 36th ed. London: Pharmaceutical Press; 2008. pp. 594–615. [Google Scholar]
- 18.He Z, Qin L, Chen L, Peng N, You J, Chen X. Synergy of human immunodeficiency virus protease inhibitors with chloroquine against Plasmodium falciparum in vitro and Plasmodium chabaudi in vivo. Antimicrob Agents Chemother. 2008;52:2653–2656. doi: 10.1128/AAC.01329-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira MR, Henrich PP, Sidhu AB, Johnson D, Hardink J, Van Deusen J, et al. In vivo and in vitro antimalarial properties of azithromycin-chloroquine combinations that include the resistance reversal agent amlodipine. Antimicrob Agents Chemother. 2011;55(7):3115–3124. doi: 10.1128/AAC.01566-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghobakclou N, Nateghpour M, Rezael S, Hajaran H, Mohebali M, Abedkhojasteh H. Variation of the chloroquine resistance transporter (CRT) gene in chloroquine-resistant and chloroquine-sensitive Plasmodium berghei. Iran J Parasitol. 2008;3(4):39–44. [Google Scholar]
- 21.Office of Laboratory Animal Welfare of National Institutes of Health Institutional animal care and use committee guidebook. 2nd ed. 2002. [Online] Available from: http://ftp.grants.nih.gov/IACUC/GuideBook.pdf [Accessed on 19 Apr, 2011]
- 22.Ryley JF, Peters W. The antimalarail activity of some quinolon esteras. Ann J Trop Med Parasit. 1970;84(2):209. doi: 10.1080/00034983.1970.11686683. [DOI] [PubMed] [Google Scholar]
- 23.Ibezim EC, Odo U. Current trends in malaria chemotherapy. Afr J Biotechnol. 2008;7:349–356. [Google Scholar]
- 24.Zakaria NA, Embi N, Sidek HM. Suppression of Plasmodium berghei parasitemia by LiCl in an animal infection model. Trop Biomed. 2010;27(3):624–631. [PubMed] [Google Scholar]
- 25.Balogun EA, Adebayo JO, Zailani AH, Kolawole OM, Ademowo OG. Activity of ethanolic extract of Clerodendrum violaceum leaves against Plasmodium berghei in mice. Agric Biol J North Am. 2009;3:307–312. [Google Scholar]
- 26.Aviado DM, Rosen R, Dacanay H, Plotkin SH. Antimalarial and antiarrhythmic activity of plant extracts. I. Cinchona and quinine in Plasmodium berghei in immature rats. Med Exp Int J Exp Med. 1969;19(2):79–94. doi: 10.1159/000137183. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs RL. Selection of strains of Plasmodium berghei resistant to quinine, chloroquine, and pyrimethamine. J Parasitol. 1965;51(3):481–482. [PubMed] [Google Scholar]
- 28.Boland ME, Roper SM, Henry JA. Complications of quinine poisoning. Lancet. 1985;1(8425):384–385. doi: 10.1016/s0140-6736(85)91398-4. [DOI] [PubMed] [Google Scholar]
- 29.Cramer JP, López-Vélez R, Burchard GD, Grobusch MP, de Vries PJ. Treatment of imported severe malaria with artesunate instead of quinine-more evidence needed? Malar J. 2011;10:256. doi: 10.1186/1475-2875-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borovskaya TG, Gol'dberg ED, Abramova EV, Fomina TI, Tkachenko SB. Effect of quinine on the morphology of mouse testes. Bull Exp Biol Med. 2000;130(10):994–996. [PubMed] [Google Scholar]
- 31.Osinubi AA, Ajala MO, Noronha CC, Okanlawon AO. Quinine lowers serum and testicular testosterone in adult Sprague-Dawley rats. Afr J Med Med Sci. 2006;35(4):425–430. [PubMed] [Google Scholar]
- 32.Osinubi AA, Daramola AO, Noronha CC, Okanlawon AO, Ashiru OA. The effect of quinine and ascorbic acid on rat testes. West Afr J Med. 2007;26(3):217–221. doi: 10.4314/wajm.v26i3.28313. [DOI] [PubMed] [Google Scholar]
- 33.Crum NF, Gable P. Quinine-induced hemolytic-uremic syndrome. South Med J. 2000;93:726–728. [PubMed] [Google Scholar]
- 34.Yeung CH, Sonnenberg-Riethmacher E, Cooper TG. Infertile spermatozoa of c-ros tyrosine kinase receptor knockout mice show flagellar angulation and maturational defects in cell volume regulatory mechanisms. Biol Reprod. 1999;61:1062–1069. doi: 10.1095/biolreprod61.4.1062. [DOI] [PubMed] [Google Scholar]
- 35.Yeung CH, Wagenfeld A, Nieschlag E, Cooper TG. The cause of infertility of male c-ros tyrosine kinase receptor knockout mice. Biol Reprod. 2000;63:612–618. doi: 10.1095/biolreprod63.2.612. [DOI] [PubMed] [Google Scholar]
- 36.Yeung CH, Anapolski M, Sipilä P, Wagenfeld A, Poutanen M, Huhtaniemi I, et al. Sperm volume regulation: maturational changes in fertile and infertile transgenic mice and association with kinematics and tail angulation. Biol Reprod. 2002;67:269–275. doi: 10.1095/biolreprod67.1.269. [DOI] [PubMed] [Google Scholar]
- 37.Friesen J, Matuschewski K. Comparative efficacy of pre-erythrocytic whole organism vaccine strategies against the malaria parasite. Vaccine. 2011;29(40):7002–7008. doi: 10.1016/j.vaccine.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 38.Goodwin LG. Response of Plasmodium berghei to antimalarial drugs. Nature. 1949;164(4183):1133. doi: 10.1038/1641133a0. [DOI] [PubMed] [Google Scholar]
- 39.Yamada Y, Hidefumi K, Shion H, Oshikata M, Haramaki Y. Distribution of chloroquine in ocular tissue of pigmented rat using matrix-assisted laser desorption/ionization imaging quadrupole time-of-flight tandem mass spectrometry. Rapid Commun Mass Spectrom. 2011;25(11):1600–1608. doi: 10.1002/rcm.5021. [DOI] [PubMed] [Google Scholar]
- 40.WHO . Guidelines for the treatment of malaria. 2nd ed. Geneva: WHO; 2010. pp. 19–21. [Online] Available from: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf [Accessed on 25 Oct, 2011] [Google Scholar]
- 41.CDC Malaria Hotline Guidelines for treatment of malaria in the United States. [Online] Available from: http://www.cdc.gov/malaria/pdf/treatmenttable.pdf [Accessed on 1 June, 2009]
- 42.Directorate General of Health Services . National drug policy on malaria. Directorate of national vector borne disease control programe. New Delhi: Ministry of Health and Family Welfare; 2010. [Online] Available from: http://nvbdcp.gov.in/Doc/drug-policy-2010.pdf [Accessed on 10 Mar, 2010] [Google Scholar]