Abstract
Objective
To investigate the hypoglycemic, hypolipidemic and antioxidant activities of aqueous extract of Terminalia paniculata bark (AETPB) in streptozotocin (STZ)-induced diabetic rats.
Methods
Acute toxicity was studied in rats after the oral administration of AETPB to determine the dose to assess hypoglycemic activity. In rats, diabetes was induced by injection of STZ (60 mg/kg, i.p.) and diabetes was confirmed 72 h after induction, and then allowed for 14 days to stabilize blood glucose level. In diabetic rats, AETPB was orally given for 28 days and its effect on blood glucose and body weight was determined on a weekly basis. At the end of the experimental day, fasting blood sample was collected to estimate the haemoglobin (Hb), glycosylated haemoglobin (HbA1c), serum creatinine, urea, serum glutamate-pyruvate transaminase (SGPT), serum glutamate-oxaloacetate transaminase (SGOT) and insulin levels. The liver and kidney were collected to determine antioxidants levels in diabetic rats.
Results
Oral administration of AETPB did not exhibit toxicity and death at a dose of 2 000 mg/kg. AETPB treated diabetic rats significantly (P<0.001, P<0.01 and P<0.05) reduced elevated blood glucose, HbA1c, creatinine, urea, SGPT and SGOT levels when compared with diabetic control rats. The body weight, Hb, insulin and total protein levels were significantly (P<0.001, P<0.01 and P<0.05) increased in diabetic rats treated with AETPB compared to diabetic control rats. In diabetic rats, AETPB treatment significantly reversed abnormal status of antioxidants and lipid profile levels towards near normal levels compared to diabetic control rats.
Conclusions
Present study results confirm that AETPB possesses significant hypoglycemic, hypolipidemic and antioxidant activities in diabetic condition.
Keywords: Hypoglycemic, Streptozotocin, Terminalia paniculata, Hypolipidemic, Antioxidant, Diabetic, Antidiabetic activity, Blood glucose, SGPT, SGOT, Insulin, Haemoglobin, Antioxidant activity
1. Introduction
Diabetes mellitus is an endocrine metabolic disorder characterized by hyperglycemia, altered lipids, carbohydrates, proteins metabolism and it increases risk of cardiovascular diseases complications[1]. The two forms of diabetes, type 1 and 2, differ in their basic mechanisms of development and in physiologic characteristics such as associations with obesity, age, and insulin. But, both types of the diabetes share the common characteristics of hyperglycemia, microvascular and macrovascular complications. Moreover, the alterations of lipoproteins metabolism are involved to the pathogenesis of the cardiovascular disease in both forms of diabetes in a similar way[2]. Also, diabetes is usually accompanied by increased generation of free radicals or impaired antioxidant defenses. Oxidative stress is also responsible for the development and progression of diabetes and its complications are reported by Maritim et al[3]. Diabetes has a considerable impact on the health, life style, life expectancy of patients and its related complications are major healthcare problems. Currently, diabetes is controlled by handful of available drugs such as oral hypoglycemic agents and insulin, but they have their own limitations. Traditionally, many herbal medicines and medicinal plants have been used for the treatment of diabetes as an alternative medicine[4]. Presence of various phytoconstituents in medicinal plants is thought to act on a different series of targets by multiple modes and mechanisms. Hence, plants have the potential to impart therapeutic effect in complicated disorders like diabetes and its complications[5]. Screening of medicinal plants is one of the alternative and valid approaches in the drug development process because they contain diverse phytoconstituents which may give new drug leads and may be effective and safe in diabetes. In India, traditionally numbers of plants are used to manage the diabetic conditions and their active principles were isolated but few plants have been scientifically studied.
Terminalia paniculata (T. paniculata) Roth (Combretaceae) is a large deciduous tree distributed in western and eastern Ghats, in the semi-evergreen and moist deciduous forests of India. The bark is astringent, bitter, cooling and useful in vitiated conditions of kapha and pitta, cough, bronchitis, strangury, diabetes, skin diseases, leprosy condition[6]. In India, it is traditionally used in the treatment of diabetes and up-to-date literature survey revealed that there is no scientific documentation to claim its efficacy in diabetes. Hence, the present investigation was aimed to study the hypoglycemic, hypolipidemic and free radical scavenging properties of the aqueous extract of T. paniculata bark (AETPB) in streptozotocin (STZ)-induced diabetic rats. The preliminary phytochemical analysis of AETPB showed the presence of carbohydrate, tannins and phenolic compounds[7].
2. Materials and methods
2.1. Plant material and extraction
T. paniculata Roth. (Combretaceae) bark was collected from Annaimalai hills, Coimbatore, Tamil Nadu, India. The specimen was authenticated at Botanical Survey of India (BSI), Coimbatore (BSI/SRC/5/23/09-10/Tech.-813). The separated barks were shade dried, powdered and 100 g of bark powder was soaked in distilled water for 12 h. On the next day, plant material was boiled for 30 min and filtered. This aqueous extract was concentrated, transferred to air-tight glass container and sealed. The container was stored at (2–8 °C) until the completion of pharmacological studies and yield of the extract was 11% (w/w).
2.2. Experimental animals
Female Wistar rats (150–180 g) were used to assess acute toxicity and male Wistar rats (150–200 g) were used to evaluate anti-diabetic activity. All animals were housed in standard laboratory conditions [temperature (22±2 °C) and humidity (45±5)% with 12 h day: 12 h night cycle]. The standard laboratory diet was provided to the animals and they were allowed to drink water ad libitum. Studies were carried out after the approval of Institutional Animal Ethical Committee in accordance with institutional ethical guidelines for the care of laboratory animals of KMCH College of Pharmacy, Coimbatore, India (approval no. KMCRET/Ph.D/5/09).
2.3. Chemicals
The estimation of biochemical parameters was carried out using commercially available kits (Primal Healthcare Limited, Lab Diagnostic Division, Mumbai, India). STZ and other chemicals were procured from Himedia Laboratories, Mumbai, India.
2.4. Acute toxicity study
Acute oral toxicity study was performed as per Organization for Economic Cooperation and Development guidelines 423 (acute toxic classic method)[8]. After the oral administration of AETPB (2 000 mg/kg), animals were observed individually at least once during the first 30 min, periodically during the first 24 h, with special attention given during the first 4 h, and daily thereafter, they were observed for a total of 14 days for toxicity determination.
2.5. Induction of experimental diabetes in rats
STZ was dissolved in freshly prepared 0.1 M cold citrate buffer (pH 4.5) and administered by intraperitoneal route (60 mg/kg) to the overnight fasted rats[9]. After 6 h of STZ injection, rats were received 5% dextrose solution for the next 24 h to prevent STZ induced fatal hypoglycemia as a result of massive pancreatic insulin release after its administration[10]. Diabetes was confirmed 72 h after induction by measurement of tail vein blood glucose levels using glucose meter (Glucocard™ 01-mini, Arkray Factory, Inc., Japan) by glucose oxidase-peroxidase method using strips. Diabetic rats were kept 14 days under standard laboratory condition for the stabilization of blood glucose levels[9]. After 14 days induction of diabetes, blood glucose was again determined and animals with a blood glucose level greater than 250 mg/dL were selected for the study.
2.6. Experimental design for antidiabetic activity
The rats were divided into five groups with six rats in each group. Group 1: normal control rats received propylene glycol (5 mL/kg); group 2: STZ-induced diabetic rats were treated with propylene glycol (5 mL/kg); group 3: STZ-induced diabetic rats were treated with AETPB (100 mg/kg); group 4: STZ-induced diabetic rats were treated with AETPB (200 mg/kg); group 5: STZ-induced diabetic rats were treated with glibenclamide (5 mg/kg)[11]. The vehicle, AETPB and glibenclamide were administered orally to its respective group animals for 28 days. AETPB was dissolved in water and glibenclamide was suspended in propylene glycol just prior to the oral administration of dose throughout the treatment period. The fasting blood glucose level and body weight were estimated every week (0, 7, 14, 21 and 28 day). At the end of the fourth week, vehicle, AETPB and glibenclamide were administered to the overnight fasted animals and after 1 h treatment all animals were anaesthetized with ketamine (100 mg/kg, i.p.). Blood sample was collected through retro-orbital plexus puncture and kept in with or without disodium ethylene diamine tetra acetate for the biochemical parameters estimation.
2.7. Estimation of biochemical parameters
The haemoglobin (Hb) and glycosylated haemoglobin (HbA1c) were estimated using whole blood by commercially available kits. The serum insulin was determined by radioimmunoassay method. The serum lipid profiles such as high density lipoprotein (HDL), total cholesterol (TC) and triglycerides (TG) were estimated using commercially available kits. The serum low density lipoprotein (LDL) and very low density lipoprotein (VLDL) levels were calculated using Friedewald formula: VLDL = TG / 5; LDL = TC − (HDL + VLDL). The serum total protein, creatinine, urea, serum glutamate-pyruvate transaminase (SGPT) and serum glutamate-oxaloacetate transaminase (SGOT) levels were analyzed by commercial kits specific for the test. All above biochemical parameters were estimated using semi-autoanalyzer (Photometer 5010V5+, Germany).
2.8. Determination of antioxidant levels
In all group animals, liver and kidney were collected after the scarification and washed immediately with ice cold saline to remove blood. The antioxidants, superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), reduced glutathione (GSH) and lipid peroxidation by measuring thiobarbituric acid reactive substances (TBARS) were determined in liver and kidney[12].
2.9. Statistical analysis
All the data expressed as mean±SEM were evaluated by one-way analysis of variance (ANOVA), followed by Dunnett's test for multiple comparisons using prism Graphpad version 5.0 and values of P<0.05 were considered as statistically significant.
3. Results
3.1. Acute toxicity study
In rats, oral administration of AETPB at a dose of 2 000 mg/kg did not produce any signs of toxicity and no animals were died up to 14 days. It showed that AETPB was nontoxic in rats up to an oral dose of 2 000 mg/kg bw. Therefore, investigation of hypoglycemic activity was carried out using 100 and 200 mg/kg dose levels.
3.2. Effect of AETPB on blood glucose and body weight
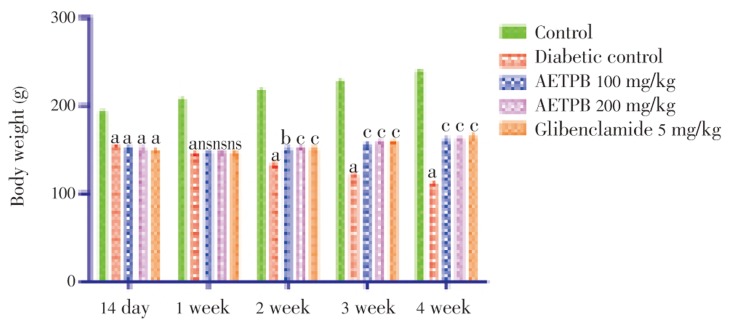
The blood glucose levels were significantly (P<0.001) increased after the administration of STZ compared to the normal control rats. The oral treatment of AETPB 100 and 200 mg/kg and glibenclamide (5 mg/kg) decreased significantly (P<0.001) blood glucose level of the diabetic rats compared to diabetic control rats (Table 1). Administration of STZ significantly (P<0.001) reduced body weight in diabetic rats compared to normal control rats (Figure 1). In diabetic rats, treatment of both doses of AETPB and glibenclamide significantly (P<0.01 and P<0.001) increased body weight compared to diabetic control rats.
Table 1. Hypoglycemic activity of AETPB in STZ-induced diabetic rats (mean±SEM) (n=6).
| Treatments | Dose (mg/kg) | Blood glucose (mg/dL) |
||||
| 14 day of STZ injection | 1 week | 2 week | 3 week | 4 week | ||
| Normal control | CMC (5 mL/kg) | 56.16±2.52 | 65.33±1.78 | 57.00±1.52 | 69.33±2.33 | 66.16±2.70 |
| Diabetic control | CMC (5 mL/kg) | 388.83±6.41a | 369.67±4.72a | 397.83±5.70a | 380.67±5.13a | 395.50±4.76a |
| AETPB | 100 | 379.00±8.71a | 139.50±3.29b | 118.17±3.66b | 106.50±2.71b | 89.33±2.84b |
| AETPB | 200 | 370.83±5.95a | 157.17±3.73b | 129.50±2.37b | 114.67±3.78b | 97.83±1.70b |
| Glibenclamide | 5 | 402.00±6.01a | 272.83±6.22b | 131.67±3.23b | 95.83±2.93b | 81.33±2.67b |
a: P<0.001 diabetic control compared with control group; b: P<0.001 AETPB 100, 200 mg/kg and glibenclamide 5 mg/kg compared with diabetic control group; CMC: carboxymethylcellulose.
Figure 1. Effect of AETPB on body weight in STZ-induced diabetic rats.
All data are expressed in mean±SEM (n=6). a: P<0.001 diabetic control, AETPB 100, 200 mg/kg and glibenclamide 5 mg/kg compared with normal control group; b: P<0.01 AETPB 100 mg/kg compared with diabetic control group; c: P<0.001 AETPB 100, 200 mg/kg and glibenclamide 5 mg/kg compared with diabetic control group; ns: No significance.
3.3. Effect of AETPB on Hb, HbA1c and serum insulin levels
Induction of diabetes significantly (P<0.001) increased the level of HbA1c, but decreased the level of Hb and serum insulin compared to normal control rats. In diabetic rats, treatment of AETPB 100 and 200 mg/kg dose and glibenclamide significantly (P<0.001 and P<0.01) reduced elevated HbA1c levels and increased Hb and serum insulin levels towards normal levels compared to diabetic control rats (Table 2). Although, in diabetic rats, administration of AETPB 200 mg/kg showed some higher efficacy than AETPB 100 mg/kg but failed to show statistical significance.
Table 2. Effect of AETPB on Hb, HbA1c, serum insulin in STZ-induced diabetic rats (mean±SEM) (n=6).
| Treatments | Dose (mg/kg) | Hb (g/dL) | HbA1c (%) | Serum insulin (µIU/mL) |
| Normal control | CMC (5 mL/kg) | 14.05±0.20 | 6.32±0.06 | 2.07±0.04 |
| Diabetic control | CMC (5 mL/kg) | 7.85±0.22a | 11.16±0.31a | 0.56±0.01a |
| AETPB | 100 | 12.87±0.12b | 9.73±0.19b | 0.82±0.02b |
| AETPB | 200 | 13.02±0.10b | 9.88±0.22c | 1.48±0.04b |
| Glibenclamide | 5 | 13.60±0.16b | 8.23±0.25b | 1.91±0.05b |
a: P<0.001 diabetic control compared with control group; b: P<0.001 AETPB 100, 200 mg/kg and glibenclamide 5 mg/kg compared with diabetic control group; c: P< 0.01 AETPB 200 mg/kg compared with diabetic control group; CMC: carboxymethylcellulose.
3.4. Effect of AETPB on creatinine, total protein, SGOT, SGPT and urea levels
Table 3 represented the efficacy of AETPB on serum SGOT, SGPT, total protein, creatinine and urea in diabetic rats. The above biochemical parameters were significantly (P<0.001) altered in STZ-induced diabetic rats compared to normal control rats. In diabetic rats, administration of both doses of AETPB and glibenclamide significantly (P<0.001, P<0.01 and P<0.05) reduced SGOT, SGPT, creatinine and urea levels compared to diabetic control rats. The AETPB 200 mg/kg treatment showed significantly (P<0.05 and P<0.001) higher reduction of SGOT, SGPT levels compared to AETPB 100 mg/kg dose. Administration of AETPB and glibenclamide showed significant (P<0.001, P<0.01 and P<0.05) increases in total protein levels compared to diabetic control rats.
Table 3. Effect of AETPB on serum biochemical parameters in STZ-induced diabetic rats (mean±SEM) (n=6).
| Treatments | Dose (mg/kg) | SGOT (U/L) | SGPT (U/L) | Total protein (g/dL) | Creatinine (mg/dL) | Urea (g/dL) |
| Normal control | CMC (5 mL/kg) | 150.48±2.01 | 60.00±1.52 | 7.95±0.13 | 0.53±0.02 | 38.36±1.11 |
| Diabetic control | CMC (5 mL/kg) | 256.83±4.38a | 164.67±3.86a | 6.38±0.12a | 0.78±0.03a | 130.23±2.73a |
| AETPB | 100 | 175.17±2.91b | 127.33±2.07b | 7.12±0.20e | 0.55±0.02b | 61.60±1.39b |
| AETPB | 200 | 163.33±2.36b,c | 105.00±4.12b,d | 7.01±0.14f | 0.60±0.02b | 68.07±2.01b |
| Glibenclamide | 5 | 156.50±3.23b | 90.50±2.10b,d | 7.73±0.15b | 0.51±0.01b | 56.72±1.72b |
a: P<0.001 diabetic control compared with control group; b: P<0.001 AETPB 100, 200 mg/kg and glibenclamide 5 mg/kg compared with diabetic control group; c: P<0.05 AETPB 200 mg/kg compared with AETPB 100 mg/kg group; d: P<0.001 AETPB 200 mg/kg and glibenclamide 5 mg/kg compared with AETPB 100 mg/kg group; e: P<0.01 AETPB 100 mg/kg compared with diabetic control group; f: P<0.05 AETPB 200 mg/kg compared with diabetic control group; CMC: carboxymethylcellulose.
3.5. Effect of AETPB on lipid profiles
In STZ-induced diabetic rats, TC, TG, LDL, and VLDL levels were increased and HDL level was decreased significantly (P<0.001) compared to normal control rats. In diabetic rats, administration of AETPB 100 and 200 mg/kg dose showed significant (P<0.001) reduction in elevated TC, TG, LDL and VLDL levels compared to diabetic control rats. Also, a significantly (P<0.05) increased level of HDL was observed in diabetic rats treated with both doses of AETPB and glibenclamide compared to diabetic control rats (Table 4).
Table 4. Hypolipidemic activity of AETPB in STZ-induced diabetic rats (mean±SEM) (n=6).
| Treatments | Dose (mg/kg) | Serum lipid profile (mg/dL) |
||||
| TC | TG | HDL | LDL | VLDL | ||
| Normal control | CMC (5 mL/kg) | 103.13±2.39 | 112.23±2.17 | 49.60±1.86 | 31.02±0.81 | 22.45±0.43 |
| Diabetic control | CMC (5 mL/kg) | 154.50±3.82a | 160.40±4.13a | 36.13±1.02a | 87.72±2.25a | 30.82±0.64a |
| AETPB | 100 | 114.77±3.05b | 129.73±2.71b | 41.48±0.92c | 47.37±1.95b | 25.94±0.54b |
| AETPB | 200 | 120.57±2.81b | 133.40±3.41b | 42.85±1.61c | 53.03±2.14b | 26.68±0.40b |
| Glibenclamide | 5 | 108.10±3.62b | 122.63±2.88b | 46.88±1.50b | 36.69±1.41b | 24.52±0.58b |
a: P<0.001 diabetic control compared with control group; b: P<0.001 AETPB 100 and 200 mg/kg, glibenclamide 5 mg/kg compared with diabetic control group; c: P<0.05 AETPB 100 and 200 mg/kg compared with diabetic control group; CMC: carboxymethylcellulose.
3.6. Antioxidant activity of AETPB
The antioxidant activity of AETPB in liver and kidney was studied in diabetic rats and the data were given in Table 5. After the induction of diabetes by STZ, significantly (P<0.001) decreased levels of SOD, CAT, GPx, reduced GSH and increased level of TBARS in liver and kidney were observed compared to normal control rats. These altered above antioxidant levels were reversed significantly (P<0.001, P<0.01 and P<0.05) to near normal levels after the administration of AETPB 100 and 200 mg/kg dose and glibenclamide 5 mg/kg dose compared to diabetic control rats.
Table 5. Antioxidant activity of AETPB on liver and kidney in STZ-induced diabetic rats (mean±SEM) (n=6).
| Organs | Treatments | Dose (mg/kg) | SOD (U/mg protein) | GPx (µg of GSH utilized/min/mg protein) | CAT (µM of H2O2/min/mg protein) | GSH (µg of GSH/mg protein) | TBARS (µM of MDA /min/mg protein) |
| Liver | Normal control | CMC (5 mL/kg) | 114.94±2.39 | 33.42±0.95 | 31.47±0.84 | 242.30±3.51 | 3.97±0.09 |
| Diabetic control | CMC (5 mL/kg) | 82.55±1.74a | 25.13±0.42a | 23.83±0.33a | 212.47±1.37a | 7.40±0.15a | |
| AETPB | 100 | 99.43±2.23b | 30.48±0.93b | 28.21±0.56b | 239.70±3.11b | 4.29±0.13b | |
| AETPB | 200 | 104.51±3.18b | 28.44±0.45d | 26.81±0.50c | 237.43±3.08b | 4.53±0.14b | |
| Glibenclamide | 5 | 108.67±3.66b | 31.02±0.66b | 30.38±0.66b | 245.86±2.34b | 4.24±0.10b | |
| Kidney | Normal control | CMC (5 mL/kg) | 149.79±2.08 | 32.64±0.98 | 29.90±0.66 | 333.96±5.62 | 5.08±0.08 |
| Diabetic control | CMC (5 mL/kg) | 118.62±1.35a | 25.89±0.71a | 21.34±0.85a | 302.19±3.15a | 7.28±0.13a | |
| AETPB | 100 | 134.00±3.46c | 29.04±0.68ns | 27.78±0.46b | 325.73±5.01c | 5.95±0.23b | |
| AETPB | 200 | 130.09±3.95d | 30.02±1.01d | 28.62±0.69b | 327.38±6.38c | 5.44±0.11b | |
| Glibenclamide | 5 | 145.91±2.94b | 31.53±1.17b | 28.58±0.54b | 332.62±3.61b | 5.31±0.17b |
a: P<0.001 diabetic control compared to control group; b: P<0.001 AETPB 100 and 200 mg/kg, glibenclamide 5 mg/kg compared to diabetic control group; c: P<0.01 AETPB 100 and 200 mg/kg compared to diabetic control group; d: P<0.05 AETPB 200 mg/kg compared to diabetic control group; ns: No significance; CMC: carboxymethylcellulose.
4. Discussion
STZ [2-deoxy-2-(3-methyl-3-nitrosoureido)-D-glucopyranose] is commonly used to induce experimental diabetes in animals[13]. STZ-induced diabetes may be due to vitiate glucose oxidation and reduction of insulin biosynthesis and secretion. The toxicity of STZ is due to DNA alkylation of its methyl nitrosourea moiety mainly at O6 position of guanine[14]. The transfer of methyl group from STZ to the DNA molecule causes damage which results in fragmentation of DNA and functional defects of the beta cells. Moreover, STZ is potential to act as an intracellular nitric oxide (NO) donor and generates reactive oxygen species (ROS). The synergistic action of both NO and ROS may also contribute to DNA fragmentation and other deleterious changes caused by STZ[15]. In our study, elevated blood glucose level and decreased insulin level were observed in STZ-induced diabetic rats and it may be due to above stated mechanism of STZ. Oral administration of AETPB 100, 200 mg/kg and glibenclamide to the diabetic rats significantly reduced blood glucose level from the first week to the fourth week compared to diabetic control rats. Also, the decreased insulin levels were noticed in diabetic rats compared to normal control rats which directly support and represent STZ-mediated beta cell destruction or damage. In diabetic rats, treatment of AETPB and glibenclamide increased the insulin level compared to diabetic control rats. Hence, the hypoglycemic activity of AETPB may be due to its protective action against STZ-mediated damage to the pancreatic beta cells and also possibly because of regeneration of damaged beta cell or increased insulin release or secretion.
Muscle wasting is an unintentional loss of body weight due to accelerated muscle proteolysis, resulting in loss of body cell mass. Insulin is an important regulator of protein synthesis and proteolysis in skeletal muscle. Insulin resistance or deficiency produces impaired muscle protein turnover and muscle wasting. The uncontrolled diabetes is associated with severe muscle wasting[16]. In this study, STZ-induced diabetic rats showed severe weight loss and decreased serum protein level compared to normal control rats. AETPB and glibenclamide administration in diabetic rats prevented the body weight loss and showed gaining in the body weight as well as increase in serum protein level compared to diabetic control rats. The above effect in diabetic rats may be due to its preventive action on pancreatic beta cells destruction by STZ which improves insulin levels, stabilization of blood glucose in diabetic rats and thereby inhibition of muscle wasting and increase in serum protein level were obtained.
HbA1c is the product of non-enzymatic reaction between glucose and free amino groups of Hb (glycosylation)[17]. It is a marker of evaluation of long-term glycemic control in diabetic patients and predicts risks for the development and/or progression of diabetic complications[18]. Previous studies were reported that 10% stable reduction in HbA1c determines a 35% risk reduction for retinopathy, a 25%–44% risk reduction for nephropathy and a 30% risk reduction for neuropathy[19]. Our study results showed that increased level of HbA1c and decreased Hb level were observed in diabetic rats compared to normal control rats which indicate the occurrence of glycosylation in diabetic rats due to hyperglycemia. Administration of AETPB and glibenclamide to the diabetic rats significantly reduced HbA1c levels and increased Hb levels compared to diabetic control rats. This action represents that AETPB has an ability to prevent the development of diabetes associated complications.
STZ utilizes low-affinity glucose transporter 2 in the plasma membrane and is selectively accumulated in pancreatic beta cells and also it damages other organs which can express this transporter, particularly kidney and liver[15]. In STZ-induced diabetic rats, elevated levels of SGPT and SGOT were observed and it may be due to STZ mediated liver damages which may cause leakage of above enzymes into the blood[20]. Kidney is playing a key role to remove the metabolic waste such as creatinine and urea from body, thereby helping to maintain body homeostasis of above substances. The persistent hyperglycemia, haemodynamic changes within the kidney tissue and free radical generation mediated stress in diabetes produce renal dysfunction which results in elevation of urea and creatinine levels in blood[21]–[23]. In the present study, elevated levels of SGOT, SGPT, urea and creatinine suggested the occurrence of liver and kidney damages after the administration of STZ to the rats compared to normal control rats. Administration of AETPB and glibenclamide to the diabetic rats significantly reduced the SGOT, SGPT, creatinine and urea levels which represent the preventive action of AETPB on liver and kidney damages in diabetic condition.
Hyperglycemia is an important contributor for the cardiovascular diseases (CVD) risk. The animal studies revealed that hyperglycemia produces glycation and peroxidation of proteins which cause damage to the arterial walls[24]. The prevalence of all forms of CVD is 2–8 folds higher in diabetic person compared to non-diabetic person. The accelerated coronary heart disease (CHD) has emerged as a leading cause of morbidity and mortality in diabetic patients in the worldwide[25]. Both type 1 and 2 diabetes are independent risk factors for CHD[26]. The vascular diseases occurred in diabetes due to disturbance in lipoprotein metabolism which causes acceleration of atherosclerosis[27]. In diabetic condition, increased levels of TC, TG and reduced level of HDL along with altered composition of LDL particles were commonly reported[28]. In the present study, administration of STZ showed alteration of normal lipid profiles such as increased TC, TG, LDL and VLDL levels as well as decreased HDL level compared to normal control rats. These altered lipid profiles were reversed to near normal level after the treatment of both doses of AETPB and glibenclamide in STZ-induced diabetic rats. This lipid lowering action may be due to proper stabilization of glucose level and increase in insulin level after the administration of AETPB which may normalize the disturbed lipid metabolism in diabetic rats. Therefore, hypolipidemic effect of AETPB in diabetic rats supports its ability to prevent the CVD diseases associated with diabetes.
It is well established that free radicals derived from oxygen have been implicated in the pathophysiology of diabetes mellitus and other diseases[29]. A number of studies revealed that oxidative stress plays a major role in the development, progression of diabetes and its related complications[30]–[39]. In diabetic state, free radical generation may occur via increased glycolysis, intercellular activation of polyol pathway, auto-oxidation of glucose and non-enzymatic protein glycation[40]. Moreover, drastic reduction of in vivo antioxidant enzymes level in various tissues was reported in diabetic condition[41]. In our study, decreased levels of liver and kidney SOD, CAT, GPx, GSH as well as increased level of TBARS were observed in STZ-induced diabetic rats compared to normal control rats. The reduction of above enzymes directly reflects the oxidative stress in diabetic rats and these enzyme level changes may be due to generation of free radicals by polyol pathway, auto-oxidation of glucose, glycosylation in hyperglycemic condition as well as STZ mediated generation of ROS by its NO donor property to the intracellular molecules. In the present study, increased SOD, CAT, GSH and GPx levels as well as reduced TBARS level were noticed in diabetic rats after the administration of both doses of AETPB and glibenclamide in liver and kidney. The above action represents the antioxidant property of AETPB in diabetic condition and hence, AETPB possesses a potential to reduce or prevent the diabetic micro and macrovascular complications. The presence of tannins and phenolic compounds in AETPB may be responsible for the hypoglycemic, hypolipidemic and antioxidant activities in diabetic rats.
Our study data confirm that AETPB possesses blood glucose lowering action in diabetic condition. Moreover, it has hypolipidemic and antioxidant activities in diabetic state, therefore it has an ability to prevent diabetic complications. Hence, above findings have given scientific evidence to the traditional use of T. paniculata bark in the treatment of diabetes.
Acknowledgments
I thank Dr. Nalla G Palanisami, Chairman and Dr. Thavamani D Palanisami, Managing Trustee for their constant support throughout the research. I thank Dr. Sangeetha Metha, MD (Pathologist), Kovai Medical Centre and Hospital, Coimbatore for the guidance in histopathology investigation. Also, I thank Dr. S Mohandass, Head, Department of Biochemistry, Dr. NGP Arts and Science, Coimbatore for his guidance on biochemical parameter estimation and valuable suggestion during the research. I thank Canara Bank, Zamin Uthukuli for the financial supports (EL.No. 133765125313) and its branch manager, Mr. Jabaraj David for the constant encouragement.
I thank Mr. Thangaraj Panneerselvam (Forest ranger), Mr. Natarajan (Forest guard), Mr. Dohni (Forest watcher) and Mr. Velliangiri (Anti-poaching watcher) for the given support during plant material collection. Also, I thank Professor K Asokkumar for the manuscript proof and English verification.
Footnotes
Foundation Project: This work was financially supported by Canara Bank, Zamin Uthukuli (grant No. 133765125313).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Davis S. Insulin, oral hypoglycemic agents and the pharmacology of the endocrine pancreas. In: Brunton L, Lazo J, Parker K, editors. Goodman and Gilman's the pharmacological basis of therapeutics. New York: McGraw Hill; 2006. pp. 1613–1646. [Google Scholar]
- 2.Howard BV. Lipoprotein metabolism in diabetes mellitus. J Lipid Res. 1987;28:613–628. [PubMed] [Google Scholar]
- 3.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee P, Maiti K, Mukherjee K, Houghton PJ. Leads from Indian medicinal plants with hypoglycemic potentials. J Ethnopharmacol. 2006;106:1–28. doi: 10.1016/j.jep.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari A, Rao J. Diabetes mellitus and multiple therapeutic approaches of phyochemicals: present status and future prospects. Curr Sci. 2002;83:30–38. [Google Scholar]
- 6.Varier PS. Indian medicinal plants compendium of 500 species. Hyderabad: Orient Longman Ltd; 1995. [Google Scholar]
- 7.Khandelwal KR. Practical pharmacognosy. Pune: Nirali Prakashan; 2005. [Google Scholar]
- 8.OECD . OECD guidelines for testing of chemicals 423: acute oral toxicity-acute toxic class method. Paris: OECD; 2001. [Google Scholar]
- 9.Ramachandran S, Asokkumar K, Uma Maheswari M, Ravi TK, Sivashanmugam AT, Saravanan S, et al. Investigation of antidiabetic, antihyperlipidemic, and in vivo antioxidant properties of Sphaeranthus indicus Linn. in type 1 diabetic rats: an identification of possible biomarkers. Evid Based Complement Alternat Med. 2011;2011:571721. doi: 10.1155/2011/571721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanko Y, Yerima M, Mahdi MA, Yaro AH, Musa KY, Mohammed A. Hypoglycemic activity of methanolic stem bark of Adansonnia digitata extract on blood glucose levels of streptozotocin-induced diabetic Wistar rats. Int J Appl Res Nat Prod. 2008;1:32–36. [Google Scholar]
- 11.Chandramohan G, Ignacimuthu S, Pugalendi KV. A novel compound from Caearia esculenta (Roxb.) root and its effect on carbohydrate metabolism in streptozotocin-diabetic rats. Eur J Pharmacol. 2008;590:437–443. doi: 10.1016/j.ejphar.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 12.Murugan P, Pari L. Antioxidant effect of tetrahydrocurcumin in streptozotocin-nicotinamide induced diabetic rats. Life Sci. 2006;79:1720–1728. doi: 10.1016/j.lfs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Al-Attar AM, Zari TA. Modulatory effect of ginger and clove oil on physiological response in streptozotocin-induced diabetic rats. Int J Pharmacol. 2007;3:34–40. [Google Scholar]
- 14.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:536–546. [PubMed] [Google Scholar]
- 15.Lenzen S. The mechanism of alloxan and streptozotocin induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 16.Castaneda C. Muscle wasting and protein metabolism. J Anim Sci. 2002;80(E Suppl 2):E98–E105. [Google Scholar]
- 17.Mohammadi J, Naik PR. Evaluation of hypoglycemic effect of Morus alba in an animal model. Indian J Pharmacol. 2008;40:15–18. doi: 10.4103/0253-7613.40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tembhurne SV, Sakarkar DM. Protective effect of Murraya koenigii (L) leaves extract in streptozotocin induced diabetics rats involving possible antioxidant mechanism. J Med Plant Res. 2010;4:2418–2423. [Google Scholar]
- 19.Calisti L, Tognetti S. Measure of glycosylated haemoglobin. Acta Biomed. 2005;76(Suppl 3):59–62. [PubMed] [Google Scholar]
- 20.Jasmine R, Daisy P. Hypoglycemic and hepatoprotective activity of Eugenia jumbolana in streptozotocin-induced diabetic rats. Int J Biol Chem. 2007;1:117–121. [Google Scholar]
- 21.Prabhu KS, Lobo R, Shirwaikar A. Antidiabetic properties of the alcoholic extract of Sphaeranthus indicus in streptozotocin-nicotinamide induced diabetic rats. J Pharm Pharmacol. 2007;60:909–916. doi: 10.1211/jpp.60.7.0013. [DOI] [PubMed] [Google Scholar]
- 22.Shokeen P, Anand P, Murali YK, Tandon V. Antidiabetic activity of 50% ethanolic extract of Ricinus communis and its purified fractions. Food Chem Toxicol. 2008;46:3458–3466. doi: 10.1016/j.fct.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Aurell M, Bjorck S. Determination of progressive renal disease in diabetes mellitus. Kidney Int. 1992;41:38–42. [PubMed] [Google Scholar]
- 24.Marks JB, Raskin P. Cardiovascular risk in diabetes: a brief review. J Diabetes Complications. 2000;14:108–115. doi: 10.1016/s1056-8727(00)00065-9. [DOI] [PubMed] [Google Scholar]
- 25.Wu KK, Huan Y. Diabetic atherosclerosis mouse models. Atherosclerosis. 2007;191:241–249. doi: 10.1016/j.atherosclerosis.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 26.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 27.Maser RE, Wolfson SK, Ellis D, Stein EA, Drash AL, Becker DJ, et al. Cardiovascular disease and arterial calcification in insulin-dependent diabetes mellitus: interrelations and risk factor profiles. Pittsburgh epidemiology of diabetic complication study-V. Arterioscler Thromb. 1991;11:958–965. doi: 10.1161/01.atv.11.4.958. [DOI] [PubMed] [Google Scholar]
- 28.Howard BV, Robbins DC, Sievers ML, Lee ET, Rhoades D, Devereux RB, et al. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: the strong heart study. Arterioscler Thromb Vasc Biol. 2000;20:830–835. doi: 10.1161/01.atv.20.3.830. [DOI] [PubMed] [Google Scholar]
- 29.Kakkar R, Mantha SV, Radhi J, Prasad K, Kalra J. Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. Clin Sci (Lond) 1998;94:623–632. doi: 10.1042/cs0940623. [DOI] [PubMed] [Google Scholar]
- 30.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 31.Oyedemi SO, Afolayan AJ. Antibacterial and antioxidant activities of hydroalcoholic stem bark extract of Schotia latifolia Jacq. Asian Pac J Trop Med. 2011;4(12):952–958. doi: 10.1016/S1995-7645(11)60225-3. [DOI] [PubMed] [Google Scholar]
- 32.Sajeesh T, Arunachalam K, Parimelazhagan T. Antioxidant and antipyretic studies on Pothos scandens L. Asian Pac J Trop Med. 2011;4(11):889–899. doi: 10.1016/S1995-7645(11)60214-9. [DOI] [PubMed] [Google Scholar]
- 33.Kumar D, Kumar S, Kohli S, Arya R, Gupta J. Antidiabetic activity of methanolic bark extract of Albizia odoratissima Benth. in alloxan induced diabetic albino mice. Asian Pac J Trop Med. 2011;4(11):900–903. doi: 10.1016/S1995-7645(11)60215-0. [DOI] [PubMed] [Google Scholar]
- 34.Kumar R, Kumar DP, Prasad SK, Sairam K, Hemalatha S. Antidiabetic activity of alcoholic leaves extract of Alangium lamarckii Thwaites on streptozotocin-nicotinamide induced type 2 diabetic rats. Asian Pac J Trop Med. 2011;4(11):904–909. doi: 10.1016/S1995-7645(11)60216-2. [DOI] [PubMed] [Google Scholar]
- 35.Poongothai K, Ponmurugan P, Ahmed KSZ, Kumar BS, Sheriff SA. Antihyperglycemic and antioxidant effects of Solanum xanthocarpum leaves (field grown & in vitro raised) extracts on alloxan induced diabetic rats. Asian Pac J Trop Med. 2011;4(10):778–785. doi: 10.1016/S1995-7645(11)60193-4. [DOI] [PubMed] [Google Scholar]
- 36.Mishra SB, Verma A, Mukerjee A, Vijayakumar M. Anti-hyperglycemic activity of leaves extract of Hyptis suaveolens L. Poit in streptozotocin induced diabetic rats. Asian Pac J Trop Med. 2011;4(9):689–693. doi: 10.1016/S1995-7645(11)60175-2. [DOI] [PubMed] [Google Scholar]
- 37.Ramachandran S, Rajasekaran A, Kumar KTM. Antidiabetic, antihyperlipidemic and antioxidant potential of methanol extract of Tectona grandis flowers in streptozotocin induced diabetic rats. Asian Pac J Trop Med. 2011;4(8):624–631. doi: 10.1016/S1995-7645(11)60160-0. [DOI] [PubMed] [Google Scholar]
- 38.Kapoor M, Jasani N, Acharya N, Acharya S, Kumar V. Phytopharmacological evaluation and anti-asthmatic activity of Ficus religiosa leaves. Asian Pac J Trop Med. 2011;4(8):642–644. doi: 10.1016/S1995-7645(11)60163-6. [DOI] [PubMed] [Google Scholar]
- 39.Kumar S, Kumar V, Prakash O. Antidiabetic, hypolipidemic and histopathological analysis of Dillenia indica (L.) leaves extract on alloxan induced diabetic rats. Asian Pac J Trop Med. 2011;4(5):347–352. doi: 10.1016/S1995-7645(11)60101-6. [DOI] [PubMed] [Google Scholar]
- 40.Sharma N, Garg V, Paul A. Antihyperglycemic, antihyperlipidemic and antioxidative potential of Prosopis cineraria bark. Indian J Clin Biochem. 2010;25:193–200. doi: 10.1007/s12291-010-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed RG. The physiological and biochemical effect of diabetes on the balance between oxidative stress and antioxidant defense system. Med J Islam World Acad Sci. 2005;15:31–42. [Google Scholar]