Abstract
Objective
To investigate the antidiabetic property of Merremia emarginata (M. emarginata) Burm. F. plant in streptozotocin induced diabetic rats.
Methods
The dose dependent effects of 28 days oral treatment with methanol extract (100, 200 and 400 mg/kg) from the plant of M. emarginata on blood glucose level, body weight, insulin, total hemoglobin, glycosylated haemoglobin (HbA1C), total protein, serum urea, serum creatinine and carbohydrate metabolizing enzymes were evaluated in streptozotocin induced diabetic rats. Histology of pancreas was also studied.
Results
A significant decrease in blood glucose, serum urea and serum creatinine and significant increase in body weight, insulin and protein level were observed in diabetic rats treated with M. emarginata. Treatment with M. emarginata resulted in a significant reduction of HbA1C and an increase in total hemoglobin level. The activities of carbohydrate metabolizing enzymes such as hexokinase were significantly increased whereas glucose-6-phosphatase, fructose-1, 6-bisphosphatase were significantly decreased by the administration of M. emarginata in diabetic rats. Histology of diabetic rats treated with M. emarginata showed the pancreatic β-cells regeneration.
Conclusions
These findings suggest that M. emarginata has potent antidiabetic activity in streptozotocin induced diabetic rats.
Keywords: Merremia emarginata, Diabetes mellitus, Carbohydrate metabolizing enzymes, Histology, Antidiabetic effect, Streptozotocin, Blood glucose, Insulin
1. Introduction
Diabetes mellitus is a chronic metabolic disease which may be suspected or recognized clinically by the onset of one or more of the characteristic symptoms such as polyuria, polydipsia, polyphagia and unsolved weight loss[1]–[3]. The high concentration of blood glucose and other biochemical abnormalities result from a deficiency of β-cells of the endocrine pancreas and/or from a sub sensitivity to insulin in target cells[4]–[6]. A worldwide survey reported that the estimated incidence of diabetes and projection for year 2030 is 350 million[7],[8]. The management of diabetes mellitus is considered a global problem and successful treatment is yet to be discovered[9]. Defects in carbohydrate machinery and consistent efforts of the physiological systems to correct the imbalance in carbohydrate metabolism pose an over exertion on the endocrine system leading to the deterioration of endocrine control[10]–[12]. Continuing deterioration of endocrine control exacerbates the metabolic disturbances by altering carbohydrate metabolic enzymes and leads primarily to hyperglycemia[13],[14].
Plants have always been an exemplary source of drugs and herbal drugs which have been investigated all over the world to treat diabetes[15],[16]. Till today more than 1 200 species of plants have been screened for activity on the basis of traditional knowledge[17]. However, the ultimate objective of their use is that they should interact directly with our body chemistry without side effects[18],[19]. Synthetic hypoglycemic agents that are capable of reducing blood sugar level possessed most worrying side effects. Most of the related side effects of synthetic drugs are metallic taste, gastro-intestinal discomfort and nausea. Therefore, finding other anti-diabetes agents, especially those made from natural sources is desired[20].
Merremia emarginata (M. emarginata) (Burm. F.) which belongs to Convolvulaceae family is a procumbent herb spreading up to 1 m and has yellow coloured flowers. The plant is widely distributed in India, Srilanka, Phillipines, Malaya, Tropical Africa and mainly grows in rainy and winter season. M. emarginata has been mentioned to be therapeutically used as deobstruent, diuretic, cough, headache, neuralgia and rheumatism. Methanol extract of this plant has been reported for antioxidant and antiobesity activities[21]. The plant is also traditionally used to treat diabetes in Srilanka and India[22].
The present study was undertaken to study the effect of methanol extract of M. emarginata plant on blood glucose, body weight, insulin, total hemoglobin, glycated hemoglobin (HbA1C), protein, serum urea, serum creatinine, carbohydrate metabolizing enzymes and histological studies in streptozotocin induced diabetic rats.
2. Materials and methods
2.1. Preparation of plant extracts
The whole plant M. emarginata Burm. F. (Convolvulaceae) was collected from Trichy district, Tamil nadu, India, during November 2010. The plant was authenticated by taxonomist at the Department of Botany, Loyola College, Chennai, India. A voucher specimen was deposited at the herbarium of the college for future reference. The whole plant was washed with distilled water and shade dried for two weeks. After drying, the plant material was powdered. Powdered plant material (3 kg) was extracted in Soxhlet apparatus using 9 L of methanol at [(50–60) °C] and residues of the solvent were removed under reduced pressure. The yield (8.57% w/w) was dried in vacuum desicator and stored in a refrigerator at 4 °C for further use.
2.2. Animals
Male Wistar rats weighing (190–200 g) were obtained from the Central Animal House, Loyola College. The experimental protocol was approved by the Institutional Ethical Committee of Loyola College (Reg. No. 367/07/A/CPCSEA). They were housed in ventilated cages and fed with a normal pellet diet (Hindustan lever, Mumbai, India) and water ad libitum. The animals were maintained in accordance with the guidelines of the National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India.
2.3. Acute toxicity study
Healthy adult male Wistar rats were starved overnight, divided into five groups (n=6) and were orally fed with the M. emarginata in increasing dose levels of 100, 500, 1 000, 2 000 and 4 000 mg/kg. The animals were observed continuously for 2 h under behavioral, neurological, autonomic profiles. After a period of 24 and 72 h, they were observed for any lethality or death[3].
2.4. Induction of diabetes
After overnight fasting, diabetes was induced by intraperitoneal injection of streptozotocin (STZ) (Sigma, St. Louis, MO) dissolved in 0.1 M cold sodium citrate buffer, pH 4.5, at a dose of 55 mg/kg[23]. The control rats received the vehicle alone. The animals were allowed to drink 5% glucose solution overnight to overcome the drug-induced hypoglycemia. After 1 week time for the development of diabetes, the rats with moderate diabetes having glycosuria and hyperglycemia (blood glucose range of above 250 mg/dL) were considered as diabetic rats and used for the experiment.
2.5. Experimental design
The rats were divided into six groups of 6 animals in each group as follows: group I: normal control administered with 0.9% sodium chloride (NaCl); group II: STZ-induced diabetic control administered with 0.9% NaCl; group III: diabetic rats administered with M. emarginata (100 mg/kg); group IV: diabetic rats administered with M. emarginata (200 mg/kg); group V: diabetic rats administered with M. emarginata (400 mg/kg); group VI: diabetic rats administered with glibenclamide (2.5 mg/kg).
M. emarginata and glibenclamide were suspended in 0.9% NaCl in warm water as vehicle solution and administered orally for 28 days.
2.6. Biochemical estimations
The fasting blood glucose was measured on 0, 14, 21 and 28 days by GOD-POD estimation kit. Body weight and plasma insulin by enzyme linked immunosorbent assay (ELISA) kit were measured on day 0 and 28. After 28 days of treatment, the rats were fasted for 16 h. The animals were then sacrificed by cervical decapitation and blood was collected in the tubes containing potassium oxalate and sodium fluoride as anticoagulants for the estimations of total hemoglobin[24], HbA1C[25], protein[26], urea[27] and creatinine[28]. For enzyme assays, a portion of the liver tissue was dissected out, washed with ice-cold saline immediately, and kept at 4 °C. The liver tissue was homogenized in 0.1 M Tris-HCl (pH 7.4), and supernatant was quantified for hexokinase, glucose-6-phosphatase and fructose-1, 6-bisphosphatase spectrophotometrically according to the commercial instructions of the kits.
2.7. Histopathological study
A portion of pancreatic tissue was dissected out and fixed in 10% buffered neutral formal saline and processed. After fixation, tissues were embedded in paraffin. Fixed tissues were cut at 5 µm and stained with hematoxylin and eosin. The sections were examined under light microscope and photomicrographs were taken[29].
2.8. Statistical analysis
Results were presented as mean ± SEM. Statistical analysis of all the data obtained was evaluated using one-way ANOVA followed by Student's t-test (SPSS Program; Version 11.5). The differences were considered significant at P ≤ 0.05.
3. Results
The acute toxicity studies revealed the nontoxic nature of the M. emarginata. There was no lethality or any toxic reactions found at any of the doses selected until the end of the study period.
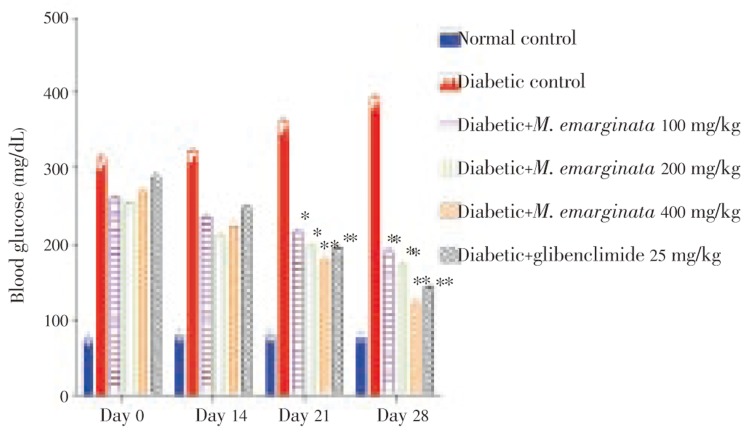
Figure 1 showed the level of blood glucose in normal and experimental animals on 0, 14, 21 and 28 days after drug treatment. There was a significant reduction in blood glucose level in M. emarginata and glibenclamide treated hyperglycemic animals as compared with diabetic animals.
Figure 1. Levels of glucose in normal and experimental rats after 0, 14, 21 and 28 days of treatment with M. emarginata.
Values indicate mean ± SEM for six animals; *P ≤ 0.05, **P ≤0.005, compared with corresponding control values.
Table 1 showed changes in body weight, the levels of plasma insulin, total haemoglobin, HbA1C of normal and experimental rats. There was a significant elevation in HbA1C, significant decrease in body weight, plasma insulin and total haemoglobin levels in diabetic rats. The administration of M. emarginata and glibenclamide to diabetic rats restored the changes in levels of body weight, plasma insulin, total hemoglobin and HbA1C to near normal.
Table 1. Levels of body weight, insulin, total hemoglobin, HbA1C, proteins, serum urea and serum creatinine of control and experimental rats after 28 days of treatment with M. emarginata (mean±SEM).
| Groups | Body weight (g) |
Insulin (U/L) |
Total hemoglobin (mg/dL) | HbA1C (% Hb) | Protein (g/dL) | Serum urea (mg/dL) | Serum creatinine (mg/dL) | ||
| Day 0 | Day 28 | Day 0 | Day 28 | ||||||
| Group I | 195.23±9.56 | 220.32±2.59 | 16.47±1.57 | 16.58±1.13 | 14.86±0.21 | 5.63±0.95 | 7.28±0.57 | 24.49±3.48 | 0.30±0.05 |
| Group II | 190.67±9.66 | 136.66±6.39 | 5.88±1.98 | 6.49±0.91 | 10.36±1.31 | 12.52±1.18 | 5.37±0.53 | 67.52±3.14 | 1.08±0.03 |
| Group III | 191.56±5.34 | 173.54±5.32* | 6.06±2.15 | 10.56±1.64* | 12.46±1.65 | 8.46±0.93 | 5.61±0.54 | 53.48±2.69* | 0.85±0.02** |
| Group IV | 191.56±4.67 | 184.21±4.03** | 6.87±1.84 | 12.57±1.17** | 13.39±1.49 | 7.44±0.93* | 6.47±0.59 | 49.54±2.53** | 0.57±0.03** |
| Group V | 190.36±6.22 | 188.46±3.01** | 6.91±1.38 | 14.53±1.12** | 14.34±1.15* | 6.50±0.84** | 7.31±0.77* | 37.36±3.13** | 0.41±0.03** |
| Group VI | 198.56±4.19 | 196.39±5.77** | 6.33±1.46 | 15.51±1.39** | 14.30±1.05* | 6.58±0.51** | 7.44±0.57* | 26.44±1.85** | 0.30±0.02** |
*P ≤ 0.05, **P ≤ 0.005 compared with diabetic control values.
The levels of plasma protein, serum urea and creatinine in control and experimental rats were also shown in Table 1. A significant increase in urea, creatinine, and a concomitant decrease in the levels of protein were observed in the diabetic rats which were reverted to near normal by the administration of M. emarginata and glibenclamide.
Table 2 showed the activities of hexokinase, glucose-6-phosphatase, and fructose-1, 6-bisphosphatase in the control and experimental rats. The activity of hepatic hexokinase decreased, whereas the activities of glucose-6-phosphatase and fructose-1,6-bisphosphatase increased in STZ treated diabetic rats which were reverted to near-normal by the administration of M. emarginata and glibenclamide.
Table 2. Activities of hepatic hexokinase, glucose-6-phosphatase and fructose-1, 6-biphosphatase of control and experimental groups of rats after 28 days of treatment with M. emarginata (mean±SEM).
| Groups | Hexokinase (U/g protein) | Glucose-6-phosphatase (U/g protein) | Fructose- 1,6-biphoshphatase (U/g protein) |
| Normal control | 275.83±3.12 | 0.11±0.01 | 475.33±3.06 |
| Diabetic control | 154.16±2.66 | 0.18±0.02 | 784.16±2.58 |
| Diabetic + M. emarginata 100 mg/kg | 180.16±3.00** | 0.15±0.01** | 722.33±2.13** |
| Diabetic + M. emarginata 200 mg/kg | 214.16±2.83** | 0.13±0.02** | 654.16±1.55** |
| Diabetic + M. emarginata 400 mg/kg | 241.16±2.90** | 0.12±0.02** | 560.66±2.67** |
| Diabetic + glibenclimide 2.5 mg/kg | 255.83±3.12** | 0.11±0.02** | 520.65±2.89** |
**P ≤ 0.005, compared with diabetic control values.
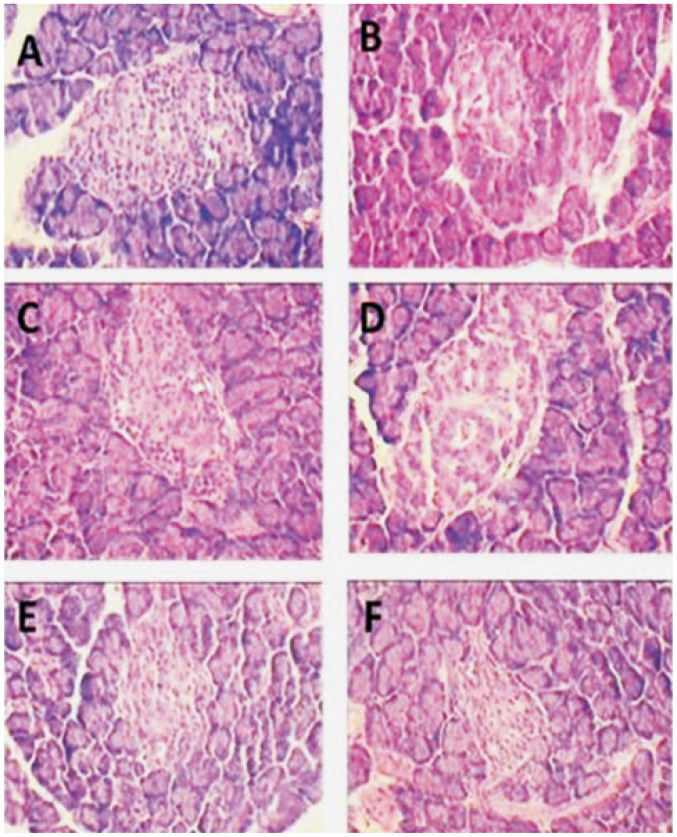
Figure 2 depicted the histopathological observations of M. emarginata (100, 200 and 400 mg/kg) treated for 28 days in STZ induced diabetic rat pancreas of different groups. Figure 2A showed normal islets. Figure 2B showed shrunken islets and the presence of pancreatic acini in diabetic rat. Figure 2C and 2D showed expansion and dilation of islet cells after treatment of diabetic rats with M. emarginata (100 and 200 mg/kg), respectively. Figure 2E showed moderate expansion of islets and hyperplastic islet after treatment of diabetic rats with M. emarginata (400 mg/kg). Figure 2F showed moderate expansion of islets and hyperplastic islet after treatment with glibenclamide.
Figure 2. Histopathological observations of M. emarginata and glibenclamide treated pancreas in streptozotocin-induced diabetic rats (H&E, 400×).
A: Normal control (presence of normal pancreatic islet cells); B: Diabetic control (expansion and dilated islet cells); C: Diabetic + M. emarginata 100 mg/kg (mild expansion and absence of dilations); D: Diabetic + M. emarginata 200 mg/kg (moderate expansion of pancreatic islets, showing prominent hyper plastic islet); E: Diabetic + M. emarginata 400 mg/kg (absence of dilation and prominent hyperplastic of islets); F: Diabetic + glibenclamide 2.5 mg/kg (absence of dilation and prominent hyperplastic of islets).
4. Discussion
STZ induced diabetic rats are one of the animal models of type 1 diabetes mellitus[30]. It is well known for its selective pancreatic islet beta cell cytotoxicity and has been extensively used to induce type 1 diabetes in experimental rat model. Glibenclamide is often used as a standard antidiabetic drug in STZ induced diabetes to compare the efficacy of variety of hypoglycemic drugs[31].
This study showed that M. emarginata produced a marked decrease in blood glucose at 100, 200, and 400 mg/kg in diabetic rats after 28 days of treatment. The antidiabetic effect of M. emarginata may be due to increased release of insulin from the existing β-cells of pancreas. Our findings are in agreement with those reported by Pareek et al[32]. Further, the antihyperglycemic activity of M. emarginata was associated with an increase in plasma insulin level, suggesting an insulinogenic activity of the plant extracts. The observed increase in the level of plasma insulin indicates that M. emarginata stimulates insulin secretion from the remnant beta cells or from regenerated beta cells. In this context, a number of other plants have also been reported to exert hypoglycemic activity through insulin release stimulatory effect[33],[34]. Decreased body weight observed in diabetic control rats in comparison to normal rats indicates that loss of body weight is a result of excessive breakdown of tissue proteins[35]–[41]. Treatment with M. emarginata improved body weight to a certain extent, indicating that control over muscle wasting resulted from glycemic control. This suggests the hypoglycemic effect of M. emarginata in diabetic rats.
Increased non enzymatic glycosylation is one of the possible mechanism linking hyperglycemia and vascular complications of diabetes. During diabetes, the excess glucose present in the blood reacts with hemoglobin to form HbA1C[42]. In the present study, the diabetic rats had shown higher level of HbA1C compared with those in normal rats, indicating their poor glycemic control. M. emarginata treated diabetic rats significantly decreased the level of HbA1C and increased total hemoglobin which might be the result of an improvement in the glucose metabolism.
Kondeti et al[42] have reported that STZ induced diabetic rats account for the observed decrease in the total protein content. Increased urea production in diabetes might be due to enhanced catabolism of both liver and plasma proteins[43]. M. emarginata treatment has appreciably normalized the content of protein and urea. In response to STZ treatment, creatinine was increased in the serum, suggesting an impairment of kidney functions[43]. M. emarginata showed a clear improvement in kidney functions, perhaps due to the antioxidant properties.
Hexokinase is the rate-limiting glycolytic enzyme that is severely impaired during diabetes. The activities of both glucose-6-phosphatase and fructose-1, 6-bisphosphatase are increased in the liver during the diabetic condition[43]. Treatment with M. emarginata has appreciably normalized the activity of these enzymes. In the diabetic rats, treatment with M. emarginata resulted in normalizing the pancreatic histoarchitecture quite appreciably. An increase in the number of beta cells in the islets showed that they were regenerated. Also, the increase in secretory granules in the cells indicates that the cells were stimulated for insulin synthesis. A decrease in the number of secretory granules, nuclear shrinkage and pycnosis, swelling of mitochondria, hypertrophied cytoplasmic organelles such as Golgi and endoplasmic reticulum have been observed in the beta cells of STZ induced diabetic rats.
In conclusion, the present study shows that the methanol extract of M. emarginata has potential antidiabetic action in STZ induced diabetic rats and the effect was found to be more similar to the reference drug glibenclamide.
Acknowledgments
The authors are thankful to Loyola College, Chennai, India for financial assistance.
Footnotes
Foundation Project: This work was financially supported by Loyola College, Chennai, India.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Eliza J, Daisy P, Ignacimuthu S, Duraipandiyan V. Normo-glycemic and hypolipidemic effects of costunolide isolated from Costus speciosus (Koen ex. Retz.)Sm. in streptozotocin-induced diabetic rats. Chem Biol Interact. 2009;179:329–334. doi: 10.1016/j.cbi.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 3.Xie JT, Wang A, Mehendale S, Wu J, Aung HH, Dey L, et al. Antidiabetic effects of Gymnema yunnanese extract. Pharmacol Res. 2003;47:323–329. doi: 10.1016/s1043-6618(02)00322-5. [DOI] [PubMed] [Google Scholar]
- 4.Wua C, Li Y, Chena Y, Laoa X, Shenga L, Daia R, et al. Hypoglycemic effect of Belamcanda chinensis leaf extract in normal and STZ-induced diabetic rats and its potential active faction. Phytomedicine. 2011;18:292–297. doi: 10.1016/j.phymed.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Annapurna A, Mahalakshmi KD, Krishna MK. Antidiabetic activity of a polyherbal preparation (tincture of panchparna) in normal and diabetic rats. Indian J Exp Biol. 2001;39:500–502. [PubMed] [Google Scholar]
- 6.Tfayli H, Bacha F, Gungor N, Arslanian S. Phenotypic type 2 diabetes in obese youth: insulin sensitivity and secretion in islet cell antibody-negative versus-positive patients. Diabetes. 2009;58:738–744. doi: 10.2337/db08-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiao W, Zhao C, Qin N, Zhai HY, Duan HQ. Identification of trans-tiliroside as active principle with anti-hyperglycemic, anti-hyperlipidemic and antioxidant effects from Potentilla chinesis. J Ethnopharmacol. 2011;135:515–521. doi: 10.1016/j.jep.2011.03.062. [DOI] [PubMed] [Google Scholar]
- 8.Sunil C, Ignacimuthu S, Agastian P. Antidiabetic effect of Symplocos cochinchinensis (Lour.) S. Moore. in type 2 diabetic rats. J Ethnopharmacol. 2010;134:298–304. doi: 10.1016/j.jep.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Dewanjee S, Das AK, Sahu R, Gangopadhyay M. Antidiabetic activity of Diospyros peregrina fruit: effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food Chem Toxicol. 2009;47:2679–2685. doi: 10.1016/j.fct.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 11.Cheplick S, Kwon YI, Bhowmik P, Shetty K. Phenolic linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertension. Bioresour Technol. 2010;101:404–413. doi: 10.1016/j.biortech.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 12.Ramana KV, Srivastava SK. Aldose reductase: a novel therapeutic target for inflammatory pathologies. Int J Biochem Cell B. 2010;42:17–20. doi: 10.1016/j.biocel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiwari AK, Madhusudana RJ. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci. 2002;83:30–38. [Google Scholar]
- 14.Fava S. Role of postprandial hyperglycemia in cardiovascular disease. Expert Rev Cardiovasc Ther. 2008;6:859–872. doi: 10.1586/14779072.6.6.859. [DOI] [PubMed] [Google Scholar]
- 15.Kaushik G, Satya S, Khandelwal RK, Naik SN. Commonly consumed Indian plant food materials in the management of diabetes mellitus. Diabetes Metab Syndr. 2010;4:21–40. [Google Scholar]
- 16.Hnatyszyn O, Mino J, Ferraro G, Acevedo C. The hypoglycemic effect of Phyllanthus sellowianus fractions in streptozotocin-induced diabetic mice. Phytomedicine. 2002;9:556–559. doi: 10.1078/09447110260573209. [DOI] [PubMed] [Google Scholar]
- 17.Hillay JE, Tahraoui A, Israili ZH, Lyouui B. Hypolipidemic effects of acute and subchronic administration of an aqueous extract of Ajuga iva L. whole plant in normal and diabetic rats. J Ethnopharmacol. 2006;105:441–448. doi: 10.1016/j.jep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 18.May L, Lefkowitch J, Kram M, Rubin D. Mixed hepatocellular-cholestatic liver injury after pioglitazone therapy. Ann Intern Med. 2002;136:449–452. doi: 10.7326/0003-4819-136-6-200203190-00008. [DOI] [PubMed] [Google Scholar]
- 19.Bhatnagar D. Lipid-lowering drugs in the management of hyperlipidaemia. Pharmacol Ther. 1998;79:205–230. doi: 10.1016/s0163-7258(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 20.Vishwakarma SL, Rakesh S, Rajani M, Goyal RK. Evaluation of effect of aqueous extract of Enicostemma littorale Blume. in streptozotocin induced type 1 diabetic rats. Indian J Exp Biol. 2010;48:26–30. [PubMed] [Google Scholar]
- 21.Babu AV, Rao RSC, Kumar KG, Babu BH, Satyanarayana PVV. Biological activity of Merremia emarginata crude extracts in different solvents. Res J Med Plant. 2009;4:134–140. [Google Scholar]
- 22.Ediriweera S, Ratnasoorya D. A review on herbs used in used in treatment of diabetes mellitus by Sri Lankan ayurvedic and traditional physicians. Int Q J Res Ayurveda. 2009;30:373–391. [Google Scholar]
- 23.Aslan M, Orhan DD, Orhan N, Sezik E, Yesilada E. In vivo antidiabetic and antioxidant potential of Helichrysum plicatum ssp. plicatum capitulums in streptozotocin induced diabetic rats. J Ethnopharmacol. 2007;109:54–59. doi: 10.1016/j.jep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Drabkin DL, Austin JM. Spectrophotometric constants for common hemoglobin derivatives in human dog and rabbit blood. J Biol Chem. 1932;98:719–733. [Google Scholar]
- 25.Nayak SS, Pattabiraman TN. A new colorimetric method for the estimation of HbA1C. Clin Chim Acta. 1981;109:267–274. doi: 10.1016/0009-8981(81)90312-0. [DOI] [PubMed] [Google Scholar]
- 26.Lowry OH, Rosenbrough NJ, Farr AL. Protein measurement with Folin-phenol reagent. J Biol Chem. 1951;35:1141–1145. [PubMed] [Google Scholar]
- 27.Natelson S, Scott ML, Begga E. A rapid method for the estimation of urea in biological fluids by means of the reaction between diacetyl and urea. Am J Clin Pathol. 1951;21:275–281. doi: 10.1093/ajcp/21.3_ts.275. [DOI] [PubMed] [Google Scholar]
- 28.Jaffe M. Estimation of creatinine. Z Physiol Chem. 1886;10:391–400. [Google Scholar]
- 29.Selvan VT, Manikandan L, Kumar SGP, Suresh R, Kakoti BB, Gomathi P, et al. Antidiabetic and antioxidant effects of methanol extract of Artanema sesamoides in streptozotocin induced diabetic animals. Int J Appl Res Nat Prod. 2008;1:25–33. [Google Scholar]
- 30.Tomlison KC, Gardiner SM, Hebden RA. Functional consequences of streptozotocin induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacol Rev. 1992;44:103–150. [PubMed] [Google Scholar]
- 31.Fernandes N, Lagishetty CV, Panda VS, Naik SR. An experimental evaluation of the antidiabetic and antilipidemic properties of a standardized Momordica charantia fruit extract. BMC Complement Altern Med. 2007;7:29. doi: 10.1186/1472-6882-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pareek H, Sharma S, Khajja BS, Jain K, Jain GC. Evaluation of hypoglycemic and anti-hyperglycemic potential of Tridax procumbens (Linn.) BMC Complement Altern Med. 2009;9:48. doi: 10.1186/1472-6882-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiaa Q, Liub X, Wua X, Wanga R, Hua X, Lia Y, et al. Hypoglycemic activity of a polyphenolic oligomer-rich extract of Cinnamomum parthenoxyl bark in normal and streptozotocin induced diabetic rats. Phytomedicine. 2009;16:744–750. doi: 10.1016/j.phymed.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Gireesh G, Thomas SK, Joseph B, Paulose CS. Antihyperglycemic and insulin secretory activity of Costus pictus leaf extract in streptozotocin induced diabetic rats and in in vitro pancreatic islet culture. J Ethnopharmacol. 2009;123:470–474. doi: 10.1016/j.jep.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 35.Salahuddin M, Jalalpure SS. Antidiabetic activity of aqueous fruit extract of Cucumis trigonus Roxb. in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2010;127:565–567. doi: 10.1016/j.jep.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Oyedemi SO, Afolayan AJ. Antibacterial and antioxidant activities of hydroalcoholic stem bark extract of Schotia latifolia Jacq. Asian Pac J Trop Med. 2011;4(12):952–958. doi: 10.1016/S1995-7645(11)60225-3. [DOI] [PubMed] [Google Scholar]
- 37.Sajeesh T, Arunachalam K, Parimelazhagan T. Antioxidant and antipyretic studies on Pothos scandens L. Asian Pac J Trop Med. 2011;4(11):889–899. doi: 10.1016/S1995-7645(11)60214-9. [DOI] [PubMed] [Google Scholar]
- 38.Kumar D, Kumar S, Kohli S, Arya R, Gupta J. Antidiabetic activity of methanolic bark extract of Albizia odoratissima Benth. in alloxan induced diabetic albino mice. Asian Pac J Trop Med. 2011;4(11):900–903. doi: 10.1016/S1995-7645(11)60215-0. [DOI] [PubMed] [Google Scholar]
- 39.Kumar R, Kumar DP, Prasad SK, Sairam K, Hemalatha S. Antidiabetic activity of alcoholic leaves extract of Alangium lamarckii Thwaites on streptozotocin-nicotinamide induced type 2 diabetic rats. Asian Pac J Trop Med. 2011;4(11):904–909. doi: 10.1016/S1995-7645(11)60216-2. [DOI] [PubMed] [Google Scholar]
- 40.Poongothai K, Ponmurugan P, Ahmed KSZ, Kumar BS, Sheriff SA. Antihyperglycemic and antioxidant effects of Solanum xanthocarpum leaves (field grown & in vitro raised) extracts on alloxan induced diabetic rats. Asian Pac J Trop Med. 2011;4(10):778–785. doi: 10.1016/S1995-7645(11)60193-4. [DOI] [PubMed] [Google Scholar]
- 41.Mishra SB, Verma A, Mukerjee A, Vijayakumar M. Anti-hyperglycemic activity of leaves extract of Hyptis suaveolens L. Poit in streptozotocin induced diabetic rats. Asian Pac J Trop Med. 2011;4(9):689–693. doi: 10.1016/S1995-7645(11)60175-2. [DOI] [PubMed] [Google Scholar]
- 42.Kondeti VK, Badri KR, Maddirala DR, Thur SKM, Fatima SS, Kasetti RB, et al. Effect of Pterocarpus santalinus bark, on blood glucose, serum lipids, plasma insulin and hepatic carbohydrate metabolic enzymes in streptozotocin induced diabetic rats. Food Chem Toxicol. 2010;48:1281–1287. doi: 10.1016/j.fct.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Hassan HA, El-Agmy SM, Gaur RL, Fernando A, Raj MHG, Ouhtit A. In vivo evidence of hepato and reno-protective effects of garlic oil against sodium nitrite-induced oxidative stress. Int J Biol Sci. 2009;5:249–255. doi: 10.7150/ijbs.5.249. [DOI] [PMC free article] [PubMed] [Google Scholar]