Abstract
Objective
To evaluate the antioxidant potential of methanolic leaf extract of Indigofera cassioides (MEIC) using various in vitro antioxidant assay systems.
Methods
Antioxidant and free radical scavenging activity of MEIC was assayed by using different in vitro models like ABTS, DPPH, nitric oxide, superoxide, hydrogen peroxide and hydroxyl radical. Reductive ability of the extract was tested by the complex formation with potassium ferricyanide. Further total phenol and flavonoid contents of the crude extract were also determined. Rutin and ascorbic acid were used as standards.
Results
MEIC exhibited potent and concentration dependent free radical scavenging activity in all the tested models. Reductive ability was also found to increase with increase in MEIC concentration. Total phenol and flavonoid content determination showed that the extract is rich in phenols and flavonoids.
Conclusions
All the results of the in vitro antioxidant assays reveal potent antioxidant and free radical scavenging activity of the leaves of Indigofera cassioides, equivalent to that of standard ascorbic acid and rutin. This potent antioxidant activity may be attributed to its high phenolic and flavonoid contents.
Keywords: Indigofera cassioides, Antioxidant, Free radicals, Oxidative stress, Total reducing power, Free radical scavenging activity
1. Introduction
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced through oxidative process within the mammalian body. These radicals play an important role in oxidative stress related pathogenesis of various diseases. The human body possesses many defense mechanisms against oxidative stress, including antioxidant enzymes and non-enzymatic compounds. Free radicals and antioxidant should maintain equilibrium for the maintenance of tissue structure and function. Under some circumstances including exposure to some environmental pollutants such as smoke, pesticides and ultra violet radiations, the natural antioxidant mammalian mechanism becomes insufficient and the excessive generation of free radicals results in cellular stress that damages the structure and function of a cell membrane in a chain reaction leading to degenerative disorders like Alzheimer's diseases, cataracts, liver injury, cardiovascular diseases, neoplastic disorders, atherosclerosis, nephritis, diabetes, rheumatoid arthritis and inflammation process. Antioxidants mediate their protective effect by directly reacting with free radicals, quenching them and thereby prevent damage to cellular components, thus consequently hindering diseases[1]–[3].
Recently, interest has increased considerably in finding naturally occurring antioxidants to replace synthetic antioxidants, which are being restricted due to their carcinogenicity[4]. The antioxidant phytochemicals such as polypheols, flavonoids and related compounds found in medicinal plants have received increasing attention for their potential role in prevention of human diseases[5]. Many ethnomedicinal plants have been investigated and reported to have antioxidant and radical scavenging potential[6]–[8].
Indigofera cassioides (I. cassioides) Rottl. Ex. DC. (Syn: Indigofera pulchella Roxb.) belonging to the family Fabaceae, is a large shrub, distributed throughout the hills of India. The flowers and leaves of the plant are reported to be antiscorbutic, diuretic and alternative. A decoction of the root is given for cough and its powder is applied externally for chest pains. The leaves and roots are used for swelling of the stomach[9],[10]. The leaves are used by tribes and native medical practitioners to treat many kinds of diseases such as arthritis, inflammation, tumor and liver diseases. However, no phytochemical and pharmacological evaluation has been carried out on I. cassioides. Based on this evidence we have selected I. cassioides for the present study. The aim of the present study is to evaluate the antioxidant and radical scavenging property of the methanol extract of leaves of I. cassioides (MEIC) by using various in vitro antioxidant assay models along with its total phenol and flavonoid content.
2. Materials and methods
2.1. Chemicals
2,2′-azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt (ABTS) and 1,1-diphenyl-2-picryl-hydrazyl (DPPH) were obtained from Sigma Aldrich Co., St. Louis, USA. Rutin and p-nitroso dimethyl aniline (p-NDA) were obtained from Acros Organics, New Jersy, USA. Naphthyl ethylene diamine dihydrochloride (NEDD) was obtained from Roch-Light Ltd., Suffolk, UK. Ascorbic acid and nitro blue tetrazolium (NBT) were obtained from S.D. Fine Chem, Ltd., Biosar, India. 2-Deoxy-D-ribose was from Hi-Media Laboratories Ltd., Mumbai. All other chemicals used were of analytical grade.
2.2. Plant material and extraction
Leaves of I. cassioides were collected from the Yercaud hills in the month of November 2008. The plant was authenticated by Dr. GVS Murthy, Joint Director, Botanical Survey of India, Coimbatore, Tamilnadu, India. A voucher specimen was preserved in our laboratory for future reference (Voucher No. P. Ch. IC 003/2008). The plant material was shade dried, pulverized and extracted (500 g) with 80% methanol at room temperature for 72 h. The extract was filtered and concentrated to dryness under reduced pressure and controlled temperature (40–50 °C) in a rotary evaporator. The extract was a dark brown solid weighing 41 g (yield, 8.2%) and was preserved in a vacuum desiccator at 4 °C until further use.
2.3. Preparation of test and standard solutions
The methanolic extract of I. cassioides and the standard antioxidants (ascorbic acid and rutin) were dissolved in distilled dimethyl sulfoxide (DMSO) separately and used for the in vitro antioxidant assays except the hydrogen peroxide method because it interferes with the method. For hydrogen peroxide method, the extract and the standards were dissolved in distilled methanol and used. The stock solutions were serially diluted with the respective solvents to obtain the lower concentrations.
2.4. Estimation of total phenol content
About 0.1 mL of the extract (10 µg/mL) was mixed with 0.5 mL of Folin-Ciocalteu reagent (diluted 1:10 ratio with distilled water) and 1.5 mL of sodium carbonate. The mixture was shaken thoroughly and made up to 10 mL with double distilled water. The mixture was allowed to stand for 2 h. The absorbance was measured at 750 nm using PerkinElmer Lambda 25 UV-Visible spectrophotometer. Using the gallic acid standard curve (2–10 µg/mL), the total phenol content was obtained. The total phenol content was expressed as gallic acid equivalent in mg/g of the extract[11].
2.5. Estimation of total flavonoid content
About 0.5 mL of the plant extract (100 µg/mL) was mixed with 1.5 mL of methanol (75% v/v), 0.1 mL of aluminium chloride (10% w/v), 0.1 mL of potassium acetate (1 M) and 2.8 mL of double distilled water. The reaction mixture was allowed to incubate for 30 min at room temperature before the absorbance was taken at 435 nm. Water (0.1 mL) was used to substitute aluminium chloride for blank. Rutin was used as a standard for the calibration curve. The result was expressed as rutin equivalent in mg/g of extract[12].
2.6. In vitro antioxidant activity
The methanol extract of I. cassioides was tested for its in vitro antioxidant activity using the standard methods. In all these methods, a particular concentration of the extract or standard solution was used which gave a final concentration of 1 000–15 625 µg/mL after all the reagents were added. Absorbance was measured against a blank solution containing the extract or standards, but without the reagents. A control test was performed without the extract or standards. Percentage scavenging and IC50 values ± SEM were calculated.
2.6.1. ABTS radical scavenging activity
In a final volume of 1 mL, the reaction mixture comprised 950 µL of ABTS+ solution and 50 µL of the plant extract at various concentrations. The reaction mixture was homogenized and incubated for 20 min. Absorbances of these solutions were measured spectrophotometrically at 734 nm[13].
2.6.2. DPPH radical scavenging activity
The DPPH assay method depends on the reduction of purple DPPH to a yellow colored diphenyl picrylhydrazine and the remaining DPPH which showed maximum absorption at 517 nm was measured. About 2 mL of various concentrations of the plant extract or standards were added to 2 mL of DPPH solution (0.1 mM, 2 mL). After 20 min of incubation at 37 °C in the dark, the absorbance was recorded at 517 nm[14].
2.6.3. Nitric oxide radical inhibition assay
Nitric oxide was generated from sodium nitroprusside and measured by the Griess reaction. Sodium nitroprosside in aqueous solution at physiological pH spontaneously generates nitric oxide which interacts with oxygen to produce nitric ions that can be estimated by using Griess reagent. Scavengers of nitric oxide compete with oxygen leading to reduced production of nitric oxide. The reaction mixture (6 mL) contained sodium nitroprusside (10 mM, 4 mL), phosphate buffer saline (PBS, pH 7.4, 1 mL) and extract or standard (1 mL) in DMSO at various concentrations and it was incubated at 25 °C for 150 min. After incubation, 0.5 mL of the reaction mixture containing nitrite ion was removed, sulphanilic acid reagent was added (0.33% w/v, 1 mL), mixed well and allowed to stand for 5 min for completion of diazotisation. Then, 1 mL of NEDD was added, mixed and allowed to stand for 30 min in diffused light. A pink colored chromophore was formed. The absorbance was measured at 540 nm[15].
2.6.4. Superoxide radical scavenging activity by alkaline DMSO method
In this method, superoxide radical is generated by the addition of sodium hydroxide to air saturated DMSO. The generated superoxide remains stable in solution and reduces nitroblue tetrazolium (NBT) into formazan dye at room temperature which can be measured at 560 nm. Briefly, 0.1 mL of NBT (1 mg/mL) was added to the reaction mixture containing 1 mL of alkaline DMSO (1 mL DMSO containing 5 mM NaOH in 0.1 mL water) and 0.3 mL of the extract in freshly distilled DMSO at various concentrations, to give a final volume of 1.4 mL. The absorbance was measured at 560 nm[15].
2.6.5. Hydrogen peroxide radical scavenging method
In this method, when a scavenger is incubated with hydrogen peroxide, the decay or loss of hydrogen peroxide can be measured spectrophotometrically at 230 nm. A volume of 2 mL of hydrogen peroxide (20 mM) in phosphate buffer saline was added to 1 mL of various concentrations of extract or standard in methanol. After 10 min the absorbance was measured at 230 nm[15].
2.6.6. Hydroxyl radical scavenging activity
2.6.6.1. p-NDA method
Various concentrations of the extract or standards in 0.5 mL of distilled DMSO were added to a solution mixture containing 0.5 mL of ferric chloride (0.1 mM), 0.5 mL of ethylene diamine tetraacetic acid (EDTA) (0.1 mM), 0.5 mL of ascorbic acid (0.1 mM), 0.5 mL of hydrogen peroxide (2 mM) and 0.5 mL of p-NDA (0.01 mM) in phosphate buffer (pH 7.4, 20 mM) to produce a final volume of 3 mL. Absorbance was measured spectrophotometrically at 440 nm[15].
2.6.6.2. Deoxyribose method
A volume of 0.2 mL of various concentrations of extract or standards in freshly distilled DMSO were added to the reaction mixture containing deoxyribose (0.2 mL, 3 mM), ferric chloride (0.2 mL, 0.1 mM), EDTA (0.2 mL, 0.1 mM), ascorbic acid (0.2 mL, 0.1 mM) and hydrogen peroxide (0.2 mL, 2 mM) in phosphate buffer (pH 7.4, 20 mM) to give a total volume of 1.2 mL. The solutions were then incubated for 30 min at 37 °C. After incubation, ice-cold trichloroacetic acid (0.2 mL, 15% w/v) and thiobarbituric acid (0.2 mL, 1% w/v), in 0.25 N HCl were added. The reaction mixture was kept in a boiling water bath for 30 min, cooled and the absorbance was measured at 532 nm[15].
2.6.7. Total iron reducing power assay
A volume of 1 mL of the plant extract was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The reaction mixture was incubated at 50 °C for 20 min. After incubation, 2.5 mL of 10% trichloroacetic acid was added and the reaction mixture was centrifuged at 1 000 rpm for 10 min. The upper 2.5 mL layer was mixed with 2.5 mL of deionized water and 0.5 mL of ferric chloride and thoroughly mixed. The absorbance was measured spectrophotometrically at 700 nm. A higher absorbance indicates a higher reducing power[16].
2.7. Statistical analysis
GraphPad PRISM software (Version 4.03) was used for calculating IC50 values. The results were expressed as mean ± SEM and all the experiments were done in triplicates.
3. Results
3.1. Total phenol content and total flavonoid content
The total phenol content and total flavonoid content of the extract were found to be (387.00±24.17 mg) of gallic acid equivalent/g of extract and (231.65±3.54 mg) of rutin equivalent/g of extract, respectively.
3.2. ABTS and DPPH radical scavenging activity
ABTS and DPPH radical scavenging activity of MEIC was shown in Table 1. The extract exhibited potent radical scavenging activity against both the methods in concentration dependent manner. The IC50 value of the extract was comparable to the standards used.
Table 1. ABTS radical, DPPH radical, nitric oxide radical, superoxide radical, hydrogen peroxide radical, and hydroxyl radical scavenging activity of MEIC (mean±SEM).
| Drug/Standard | IC50 (µg/mL)* |
||||||
| ABTS radical scavenging assay | DPPH radical scavenging activity | Nitric oxide radical scavenging assay | Superoxide radical scavenging assay | Hydrogen peroxide radical scavenging method | Hydroxyl radical scavenging activity |
||
| p-NDA method | Deoxyribose method | ||||||
| MEIC | 28.11±0.51 | 13.97±0.80 | 70.45±1.46 | 232.00±7.78 | 129.41±2.82 | 126.13±5.83 | 50.27±0.61 |
| Ascorbic acid | 27.61±1.04 | 9.03±0.09 | 129.06±5.54 | >1 000 | 31.41±1.90 | >1 000 | – |
| Rutin | 12.37±0.24 | 9.65±0.19 | 54.27±1.00 | >1 000 | 127.63±3.20 | >1 000 | 32.82±0.60 |
*: Mean of three determinations.
3.3. Nitric oxide scavenging assay
MEIC showed a strong nitric oxide scavenging activity which was comparable to the standards ascorbic acid and rutin. The IC50 value of MEIC was less than ascorbic acid and near to rutin. IC50 values of extract and standards were presented in Table 1.
3.4. Superoxide and hydrogen peroxide radical scavenging activity
Superoxide radical scavenging activity of MEIC was assessed by alkaline DMSO method. The plant extract strongly inhibited the superoxide radical generation. In hydrogen peroxide radical scavenging assay, the extract was found to be equipotent with rutin but less potent when compared to ascorbic acid. The values were tabulated in Table 1.
3.5. Hydroxyl radical scavenging assay
Hydroxyl radical scavenging activity of MEIC was measured by p-NDA method and deoxyribose method. In p-NDA method, the extract showed very potent activity when compared to standards used. In deoxyribose method, the inhibitory concentration was comparable to that of rutin. The IC50 values were presented in Table 1.
3.6. Total iron reducing power assay
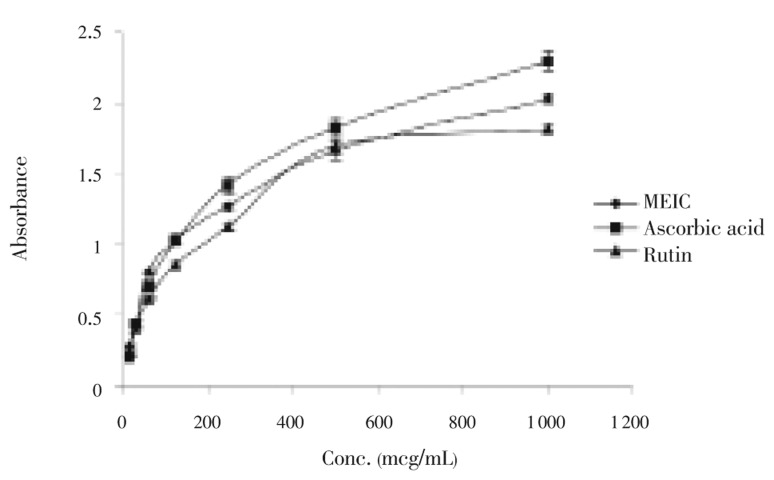
Figure 1 showed the reductive ability of MEIC compared to ascorbic acid and rutin. Absorbance of the solution was increased when the concentration increased. A higher absorbance indicates a higher reducing power. The total reducing power of MEIC was higher than rutin and less than ascorbic acid.
Figure 1. Total iron reducing power assay of MEIC.
Values were average of three determinations. Data were expressed as mean±SEM.
4. Discussion
Free radicals are chemical species which contains one or more unpaired electrons. They are highly unstable and cause damage to other molecules by extracting electrons from them in order to attain stability. They are formed inside the system, and are highly reactive and potentially damaging transient chemical species. These radicals are continuously produced in the human body because they are essential for detoxification, chemical signaling, energy supply and immune function. Free radicals are regulated by endogenous antioxidant enzyme system, but due to over production of free radicals by exposure to environmental oxidant substances such as cigarette smoking, UV radiation, etc or a failure in antioxidant defense mechanism or damage to cell structures, the risk increases for many diseases such as Alzheimer's disease, mild congestive impairment, Parkinson's disease, cardiovascular disorders, liver diseases, ulcerative colitis, inflammation and cancer[6]. Interestingly, the body possesses defense mechanisms against free radical induced oxidative stress, which involve preventive and repair mechanisms, i.e. physical defense and antioxidant defense. Enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), etc and non-enzymatic antioxidants such as carotenoids, ascorbic acid, phenolic compounds, flavonoids, etc act by one or more mechanisms like reducing activity, free radical scavenging, potential complexing of pro-oxidant metals and quenching of singlet oxygen. It is possible to reduce the risks of chronic diseases and prevent the disease progression by either enhancing the body's natural antioxidant defense or supplementing with proven antioxidants. For this reason, discovery of natural antioxidants is a major thrust area[17]. The antioxidant activity of the methanol extract of I. cassioides was investigated against various in vitro models. Since, free radicals are of different chemical entities, it is essential to test the extract against many free radicals to prove its antioxidant activity. Hence, a large number of in vitro methods were used for the screening. IC50 values obtained were compared with the standards used.
ABTS radical scavenging activity is relatively recent one, which involves a more drastic radical, chemically produced and is often used for screening complex antioxidant mixtures such as plant extracts, beverages and biological fluids. The ability in both the organic and aqueous media and the stability in a wide pH range raised the interest in the use of ABTS+ for the estimation of antioxidant activity[13]. The extract showed potent antioxidant activity in ABTS method which is comparable to the standard used. Here, the extract's radical scavenging activity showed a direct role of its phenolic compounds in free radical scavenging.
The DPPH is a stable free radical, which has been widely accepted as a tool for estimating free radical scavenging activities of antioxidants. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule[18]. The reduction capability of DPPH radical is determined by the decrease in absorbance at 517 nm induced by antioxidants. The experimental data of the extract revealed that the extract is likely to have the effects of scavenging free radicals. From the result we observe a dose dependent relationship in the DPPH radical scavenging activity. The involvement of free radicals, especially their increased production, appears to be a feature of most of the human diseases including cardiovascular diseases and cancer. It has been found that cysteine, glutathione, ascorbic acid, tocopherols, flavonoids, tannins and aromatic amines reduce and decolorize the DPPH by their hydrogen donating ability. Flavonoids and phenolic compounds of MEIC are possibly involved in its antiradical activity[19].
Superoxide radical is known to be a very harmful species to cellular components as a precursor of more reactive species[19]. The superoxide radical is known to be produced in vivo and can result in the formation of hydrogen peroxide via dismutation reaction. The extract is found to be an efficient scavenger of superoxide radical generated in alkaline DMSO system. The result clearly indicates that the plant extract has a noticeable effect as scavenging superoxide radical.
Hydrogen peroxide itself is not very reactive, but sometimes it is toxic to cell because it may give rise to hydroxyl radical in the cells. Therefore, removing of hydrogen peroxide is very important for antioxidant defense in cell system. Polyphenols have also been shown to protect mammalian cells from damage induced by hydrogen peroxide, especially compounds with the orthohydroxy phenolic compounds like quercetin, gallic acid, caffeic acid and catechin[20]. Therefore, the phenolic compounds of the I. cassioides extract may probably be involved in scavenging hydrogen peroxide.
Nitric oxide formed during their reduction with oxygen or with superoxide, such as NO2, N2O4, N3O4 is very reactive. These radicals are responsible for altering the structure and functional behavior of many cellular components. The MEIC showed better activity in competing with oxygen to react with nitric oxide and thus inhibited the generation of anions and the activity is comparable to the standards used. The plant secondary metabolites may have the property to counteract the effect of nitric oxide formation and in turn may be considerably interested in preventing the ill effects of excessive nitric oxide generation in the human body. Further the scavenging activity may also help to arrest the chains or reactions initiated by excess generation of nitric oxide that are dangerous to the human health. Nitric oxide is also implicated for inflammation, cancer and other pathological conditions[21].
Among the oxygen radicals, hydroxyl radical is the most reactive and induces severe damage to adjacent biomolecules[22]. In the present study, the hydroxyl radical scavenging activity of MEIC was assessed by the inhibition of p-NDA bleaching method and deoxyribose degradation method. In p-NDA method, the hydroxyl radical is generated through Fenton reaction. In this reaction, iron-EDTA complex reacts with hydrogen peroxide in presence of ascorbic acid to generate hydroxyl radical which can bleach p-NDA specifically. The extract shows potent scavenging activity by inhibition of bleaching of p-NDA. In deoxyribose method, the sugar is degraded on exposure to hydroxyl radical generated by Fenton reaction. The resulting complex mixture of products is heated under acid condition; malondialdehyde (MDA) is formed and detected by its ability to react with thiobarbituric acid to form a pink chromogen. In the deoxyribose method, the plant extract shows potent hydroxyl radical scavenging activity which can be comparable to the standards used. The scavenging activity may be due to the presence of various phytochemicals including polyphenols and flavonoids in MEIC.
In the measurement of the reducing ability, it has been investigated from the Fe3+ – Fe2+ transformation. Fe3+ reduction is often used as an indicator of electron donating activity, which is an important mechanism of phenolic antioxidant action and can be strongly correlated with other antioxidant properties[23]. The reducing properties of the plant extracts are generally associated with the presence of reductones, which have been shown to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom[24]–[29]. Reductones are also reported to react with certain precursors of peroxide, thus preventing peroxide formation. The data obtained in the present study suggest that it is likely to contribute significantly towards the observed antioxidant effects. However, the antioxidant activity has been attributed by various mechanisms, like prevention of chain initiation, binding of transition metal ion catalysts, prevention of continued hydrogen abstraction, reductive capacity, radical scavenging activity and decomposition of peroxides. Like the antioxidant activity, the reducing power of the extract increases with increasing concentration.
The systemic literature collection, pertaining to this investigation indicates that the plant phenolics constitute one of the major groups of compounds acting as primary antioxidants or free radical scavengers. Therefore, it is necessary to determine the total amount of phenols and flavonoids in the plant extract chosen for the study. Flavonoids are the most diverse and widespread group of natural compounds and are likely to be the most important natural phenolics. These compounds possess a broad spectrum of chemical and biological activities including radical scavenging activity. The contents of total phenols and flavonoids were estimated by the standard curves and expressed as gallic acid equivalents for total phenols and rutin equivalents for flavonoids. The extract contains more than 20% of total flavonoids and is rich in phenols. Phenolic compounds are considered to be the most important antioxidants of plant materials. They constitute one of the major groups of compounds acting as primary antioxidants or free radical terminators. Antioxidant activity of phenolic compounds is based on their ability to donate hydrogen atoms to free radicals. In addition, they possess ideal structural properties for free radical scavenging properties[30].
Preliminary phytochemical screening of MEIC showed the presence of alkaloids, glycosides, steroids, terpenoids, phenols, flavonoids, tannins and amino acids. Phenolic compounds such as catechins, epigallocatechine gallate, ferulic acid, proanthocyanidins, flavonoids and tannins are commonly found in both edible and medicinal plants and they have been reported to have various biological effects including antioxidant activity[17]. The antioxidant activity of phenolic compounds is mainly due to their redox properties, which can play an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen or decomposing peroxides[31]. The methanolic extract of I. cassioides showed strong antioxidant activity in various in vitro systems tested. The antioxidant effect of I. cassioides may be due to the phenolic compounds present in it. To our knowledge, this is the first report on the antioxidant potential of I. cassioides.
The results from various free radicals scavenging systems reveal that methanol extract of I. cassioides has significant antioxidant activity. The extract is found to have different levels of antioxidant activity in all the methods tested. IC50 values obtained were comparable to that of the standards used, i.e. ascorbic acid and rutin. According to this study, a significant antioxidant activity was found. Total phenol and flavonoid contents determination indicates the high content of phenols and flavonoids and these compounds could be major contributors to antioxidant activity. Further studies in our laboratory are in progress for the isolation and identification of phytochemical compounds and to ensure that the medicinal properties of the plant in vivo correlate with its antioxidant activity.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Tesler J. Free radicals and antioxidants in normal physiological functions and human diseases. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar V, Guha G, Kumar RA. Antioxidant and anti-neoplastic activities of Picorhiza kurroa extracts. Food Chem Toxicol. 2011;49:363–369. doi: 10.1016/j.fct.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Hameed ES. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem. 2009;114:1271–1277. [Google Scholar]
- 5.Upadhyay NK, Yogendrakumar MS, Gupta A. Antioxidant, cytoprotective and antibacterial effects of sea buckthorn (Hippophae rhamnoides L.) leaves. Food Chem Toxicol. 2010;48:3443–3448. doi: 10.1016/j.fct.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Rajkapoor B, Burkan ZE, Senthilkumar R. Oxidants and human diseases: role of antioxidant medicinal plants-a review. Pharmacologyonline. 2010;1:1117–1131. [Google Scholar]
- 7.Rajkumar V, Guha G, Kumar RA, Mathew L. Evaluation of antioxidant activities of Bergenia ciliate rhizome. Rec Nat Prod. 2010;4:38–48. [Google Scholar]
- 8.Guha G, Rajkumar V, Kumar RA, Mathew L. Aqueous extract of Phyllanthus amarus inhibits chromium(VI)-induced toxicity in MDA-MB-435S cells. Food Chem Toxicol. 2010;48:396–401. doi: 10.1016/j.fct.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Nadkarni AK. Indian materia medica. Bombay: Popular Prakasan Pvt. Ltd; 1993. p. 680. [Google Scholar]
- 10.Kirtikar KR, Basu BD. Indian medicinal plants. Dehradun: International Book Publishers; 1993. pp. 714–7155. [Google Scholar]
- 11.Kumaran A, Karunakaran RJ. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci Technol. 2007;40:344–352. [Google Scholar]
- 12.Hsu C. Antioxidant activity of extracts from Polygonum aviculare L. Biol Res. 2008;39:281–288. doi: 10.4067/s0716-97602006000200010. [DOI] [PubMed] [Google Scholar]
- 13.Huang MH, Huang SS, Wang BS, Wu CH, Sheu MJ, Hou WC, et al. Antioxidant and anti-inflammatory properties of Cardiospermum halicacabum and its reference compounds ex vivo and in vivo. J Ethnopharmacol. 2011;133:743–750. doi: 10.1016/j.jep.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Shirwaikar A, Shirwaikar A, Rajendran K, Punitha IS. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberin. Biol Pharm Bull. 2006;29:1906–1910. doi: 10.1248/bpb.29.1906. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan R, Chandrasekar MJN, Nanjan MJ, Suresh B. Antioxidant activity of Caesalpinia digyna root. J Ethonopharmacol. 2007;113:284–291. doi: 10.1016/j.jep.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhu KX, Lian CX, Guo XN, Peng W, Zhou HM. Antioxidant activities and total phenol contents of various extracts from defatted wheat germ. Food Chem. 2011;126:1122–1126. [Google Scholar]
- 17.Ali SS, Kasoju N, Luthra A, Singh A, Sharanabasava H, Sahu A, et al. Indian medicinal herbs as sources of antioxidants. Food Res Int. 2008;41:1–15. [Google Scholar]
- 18.Kalaivani T, Mathew L. Fee radical scavenging activity from leaves of Acacia nilotica (L.) Wild ex Delile, an Indian medicinal tree. Food Chem Toxicol. 2010;48:298–305. doi: 10.1016/j.fct.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th ed. Oxford: Oxford University Press; 2007. [Google Scholar]
- 20.Esmaeili MA, Sonboli A. Antioxidant, free radical scavenging activities of Salvia brachyantha and its protective effect against oxidative cardiac cell injury. Food Chem Toxicol. 2010;48:846–853. doi: 10.1016/j.fct.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Hofseth LJ. Nitric oxide as a target of complementary and alternative medicines to prevent and treat inflammation and cancer. Cancer Lett. 2008;268:10–30. doi: 10.1016/j.canlet.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awah FM, Uzoegwu PN, Oyugi JO, Rutherford J, Ifeonu P, Yao XJ, et al. Free radical scavenging activity and immunomodulatory effect of Stachytarpheta angustifolia leaf exract. Food Chem. 2010;119:1409–1416. [Google Scholar]
- 23.Dorman HJD, Peltoketo A, Hiltunen R, Tikkanen MJ. Characterization of the antioxidant properties of deodorized aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003;83:255–262. [Google Scholar]
- 24.Gordon MH. The mechanisms of antioxidant action in vitro. In: Hudson BJF, editor. Food antioxidants. London: Elsevier Applied Sceince; 1990. pp. 1–18. [Google Scholar]
- 25.Oyedemi SO, Afolayan AJ. Antibacterial and antioxidant activities of hydroalcoholic stem bark extract of Schotia latifolia Jacq. Asian Pac J Trop Med. 2011;4(12):952–958. doi: 10.1016/S1995-7645(11)60225-3. [DOI] [PubMed] [Google Scholar]
- 26.Sajeesh T, Arunachalam K, Parimelazhagan T. Antioxidant and antipyretic studies on Pothos scandens L. Asian Pac J Trop Med. 2011;4(11):889–899. doi: 10.1016/S1995-7645(11)60214-9. [DOI] [PubMed] [Google Scholar]
- 27.Poongothai K, Ponmurugan P, Ahmed KSZ, Kumar BS, Sheriff SA. Antihyperglycemic and antioxidant effects of Solanum xanthocarpum leaves (field grown & in vitro raised) extracts on alloxan induced diabetic rats. Asian Pac J Trop Med. 2011;4(10):778–785. doi: 10.1016/S1995-7645(11)60193-4. [DOI] [PubMed] [Google Scholar]
- 28.Nain P, Kumar A, Sharma S, Nain J. In vitro evaluation of antimicrobial and antioxidant activities of methanolic extract of Jasminum humile leaves. Asian Pac J Trop Med. 2011;4(10):804–807. doi: 10.1016/S1995-7645(11)60198-3. [DOI] [PubMed] [Google Scholar]
- 29.Adewusi EA, Steenkamp V. In vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from southern Africa. Asian Pac J Trop Med. 2011;4(10):829–835. doi: 10.1016/S1995-7645(11)60203-4. [DOI] [PubMed] [Google Scholar]
- 30.Sulaiman SF, Yusoff NAM, Eldeen IM, Seow EM, Sajak AAB, Supriatno OKL. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.) J Food Compost Anal. 2011;24:1–10. [Google Scholar]
- 31.Osawa T. Novel natural antioxidants for utilization in food and biological systems. In: Uritani I, Garcia VV, Mendoza EM, editors. Postharvest biochemistry of plant food materials in the tropics. Tokyo: Japan Scientific Societies Press; 1994. pp. 241–251. [Google Scholar]