Abstract
Objective
To assess the In vivo antioxidFant and hepatoprotective activity of methanolic extract of Daucus carota (D. carota) seeds in experimental animals.
Methods
Methanolic extracts of D. carota seeds is used for hepatoprotection assessment. Oxidative stress were induced in rats by thioacetamide 100 mg/kg s.c, in four groups of rats (two test, standard and toxic control). Two test groups received D. carota seeds extract (DCSE) at doses of 200 mg/kg and 400 mg/kg. Standard group received silymarin (25 mg/kg) and toxic control received only thioacetamide. Control group received only vehicle. On the 8th day animals were sacrificed and liver enzyme like serum glutamic pyruvic transaminase (SGPT), serum glutamic-oxaloacetic transaminase (SGOT) and alkaline phosphatase (ALP) were estimated in blood serum and antioxidant enzyme like superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GRD), glutathione peroxidase (GPX), glutathione-S-transferase (GST) and lipid peroxidation (LPO) were estimated in liver homogenate.
Results
A significant decrease in SGPT, SGOT and ALP levels was observed in all drug treated groups as compared to thioacetamide group (P < 0.001) and in case of antioxidant enzyme a significant (P < 0.001) increase in SOD, CAT, GRD, GPX and GST was observed in all drug treated groups as compared with thioacetamide group. But in case of LPO a significant (P < 0.001) reduction was observed as compared to toxic control group.
Conclusions
DCSE has contributed to the reduction of oxidative stress and the protection of liver in experimental rats.
Keywords: Daucus carota seeds extract, Thioacetamide, Silymarin, Hepatoprotection, Antioxidant, Biochemical parameters
1. Introduction
In oxidation process highly reactive and harmful chain reactions of oxygen species are generated, causing damage to living organism. The oxygen centered free radicals and other reactive oxygen species (ROS), which are continuously produced has resulted in cell death or tissue damage. This oxidative damage caused by free radical is related to pathogenesis of many chronic degenerative diseases like cancer, diabetes, neurodegenerative disease, atherosclerosis, cirrhosis, malaria and AIDS[1]. Reactive oxygen species including superoxide free radical, hydrogen peroxide, hydroxyl free radical and singlet oxygen play a key role in the oxidative damage of these diseases. This in turn resulted in DNA mutation, protein inactivation, rapid peroxidation and cell death[2].
Antioxidant is a molecule which terminate the chain reaction by removing free radical intermediates. Plants and animals maintain complex system of multiple type of antioxidant. The natural plant based antioxidants have played an important role in the maintenance of human health for the past three decades[3].
Daucus carota (D. carota) Linn commonly known as “Carrot” belongs to the Family Apiaceae (Umbelliferae) and is cultivated almost all over the world as a useful vegetable. The plant has undergone extensive phytochemical studies and a large number of active ingredients have been isolated. These include volatile oils, steroids, triterpenes, carbohydrates, glycerides, tannins, flavonoids, amino acid, carotene and hydro carotene[4]. Pharmacological studies showed that D. carota exhibit antifertility, hypoglycaemic, hepatoprotective and aphrodisiac activity. Recently two new guaiane-type sesquiterpenoids containing an interesting epoxy unit, daucuside and daucusol were isolated from the fruits of D. carota L and fresh juice extract of D. carota seeds is used for the treatment of leukemia[5],[6]. D. carota is also used as a noval model to evaluate the effect of light on carotenogenic gene expression and carrot seed oil exhibits both smooth-muscle relaxant and vasodilatory action in isolated animal organ studies[7],[8].
The objective of present study was to evaluate the hepatoprotective activity of methanolic extract of D. carota seeds extract in rats.
2. Materials and methods
2.1. Plant material
Carrot seeds were collected from Ooty, Tamilnadu. The plant was identified by a botanist, and voucher specimen was deposited in the department of Botany, Bharathiya University, Coimbatore. After authentication, seeds were cleaned and milled into coarse powder by a mechanical grinder.
2.2. Preparation of seeds extract
Powdered seeds (2 kg) were extracted with 95 % methanol using a Soxhlet apparatus. The methanolic extract was filtered and concentrated by distillation process. A brownish green colored residue was obtained (yield 6.79 % w/w) and was kept in a desiccators. This methanolic extract of D. carota seeds extract (DCSE) was used for further experimants.
2.3. Experimental animals
Healthy, adult male rats of Wister strain, weighing (180 ± 5) g were obtained from animal house, IRT Perundurai medical college, Erode, Tamilnadu, India. The animals were kept in a well-ventilated room and they were exposed to 12 hours day and 12 hours night cycle with a temperature between (20 ± 2) °C. The animals were housed in spacious, hygienic polypropylene cages during the course of the experiment. The animals were fed with water and mice pellet feed (M/s. industan Lever Ltd., Mumbai) ad libitum. All the experimental procedures and protocols used in this study were reviewed by institutional animal ethics committee (NCP/IAEC/PG-05/2009) and were in accordance with the guidelines of the CPCSEA.
2.4. Drugs and chemicals
Silymarin (Silybin 140) was purched from Microlabs Limited, Goa and Thioacetamide from Lova Laboratories Pvt. Ltd., Mumbai. All others chemicals used in this study were of analytical grade.
2.5. Experimental design
Rats were randomly divided into five groups of six animals each and each group was kept in a separate cage. All the groups were treated orally for 7 days[9].
Group I served as normal control and was treated with vehicle (0.5% carboxyl methyl cellulose). Group II served as toxin control and treated with vehicle (thioacetamide 100 mg/kg, s.c.). Group III served as standard and was treated with silymarin 25 mg/kg. Group IV was treated with 200 mg/kg DCSE by suspending in 0.5% carboxyl methyl cellulose. Group V was treated with 400 mg/kg DCSE by suspending in 0.5% carboxyl methyl cellulose.
On the 6th day, rats of group II, III, IV and V were treated with a single dose of thioacetamide (100 mg/kg, s.c.) as 2 % w/v solution in double distilled water.
2.6. Biochemical estimation
On the 8th day, the rats were anesthetized with light ether anesthesia and blood samplings were performed by cardiac puncher. The collected blood samples were allowed to clot for 45 min at room temperature. Serum was separated by centrifugation at 4 000 rpm for 20 min. Serum enzymes like serum glutamic pyruvic transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT) and alkaline phosphatase (ALP) values were evaluated[10],[11].
For the determination of antioxidant enzymes, liver was dissected out, washed in the ice-cold saline, and homogenate was prepared in 0.1 M Tris–HCl buffer (pH 7.4). The homogenate was centrifuged and supernatant was used for the assay of antioxidant enzymes, namely glutathione peroxidase (GPX)[12], glutathione S-transferase (GST)[13], glutathione reductase (GRD)[14], Superoxide dismutase (SOD[15], catalase (CAT)[16], and lipid peroxidation (LPO)[17] are estimated.
2.7. Histopathological studies
At the end of 8th day study. The animals were sacrificed with excess dose of anesthetic ether. Animals were dissected; liver was collected and weighed. Then the organs were fixed in 10% buffered neutral formalin. The tissues were embedded in liquid paraffin, cut into 5-6 µm and stained with hematoxylin and eosin stain for histopathological findings.
2.8. Statistical analysis
The collected data were subjected to appropriate statistical test like one-way ANOVA (Analysis of variance), followed by an appropriate turkey test. P values of less than 0.01 were considered as significant. The analysis was carried out using Graph pad prism software of Version 4.
3. Results
Biochemical parameters like SGPT, SGOT and ALP activity was significantly (P< 0.001) increased in thioacetamide treated group when compared to control. The DCSE dose at 200 mg/kg and 400 mg/kg dose significantly decrease the level (P< 0.001) in blood serum as compared to thioacetamide treated animals. Silymarin treated group showed significant decrease (P< 0.001) when compared to thioacetamide treated animals. The dose of 400 mg/kg treated group was found to be more effective similar to silymarin treated group (Table 1).
Table 1. Estimation of rat's blood serum profile of DCSE on thioacetamide-intoxicated rats (Mean ± SEM).
| Group (n=6) | SGPT (U/L) | SGOT (U/L) | ALP (U/L) |
| Normal control | 84.72 ± 6.18 | 153.97 ± 14.98 | 507.02 ± 14.98 |
| Toxic control (thioacetamide, 100 mg/kg, s.c) | 396.67 ± 8.15*** | 623.70 ± 19.91*** | 735.60 ± 29.40*** |
| Standard (silymarin, 25 mg/kg) | 91.05 ± 1.91*** | 246.77 ± 23.12*** | 556.01 ± 14.13*** |
| DCSE (200 mg/kg) | 151.67 ± 6.98*** | 433.70 ± 19.91*** | 662.70 ± 20.48*** |
| DCSE (400 mg/kg) | 95.22 ± 2.46*** | 317.3 ± 20.12*** | 600.07 ± 6.92*** |
***P < 0.001, a treated group animals compared with control.
Enzymatic antioxidants like SOD, CAT, GRD, GPX and GST activity in liver was found significantly reduced (P< 0.001) in thioacetamide treated animals when compared to control. 200 mg/kg and 400 mg/kg DCSE treated group significantly increased (P< 0.001) the level of enzymatic antioxidants when compared to thioacetamide treated animals. Silymarin treated animals also increases more significantly(P< 0.001) the level of enzymatic antioxidants in the liver homogenate when compared with thioacetamide treated animals. The dose of 400 mg/kg treated group was found to be more effective similar to silymarin treated group (Table 2).
Table 2. Chemically intoxicated and its associated antioxidant activity of methanolic extract of D. carota seeds (DCSE) (Mean ± SEM).
| Group (n=6) | SOD (u/mg protein) | CAT (u/mg protein) | GRD (u/mg protein) | GPX (u/mg protein) | GST (u/mg protein) | LPO (nmol MDA/mg liver protein) |
| Control | 9.06 ± 0.06 | 55.93 ± 1.57 | 3.26 ± 0.08 | 11.26 ± 0.06 | 6.34 ± 0.13 | 7.70 ± 0.08 |
| Toxic control (thioacetamide, 100 mg/kg, s.c) | 4.79 ± 0.09*** | 31.04 ± 0.87*** | 1.91 ± 0.02*** | 5.90 ± 0.11*** | 3.50 ± 0.23*** | 16.81 ± 0.30*** |
| Standard (silymarin, 25mg/kg) | 8.65 ± 0.12*** | 55.36 ± 1.64*** | 3.28 ± 0.06*** | 9.85 ± 0.45*** | 5.69 ± 0.04*** | 10.86 ± 0.09*** |
| DCSE (200mg/kg) | 5.15 ± 0.09*** | 35.39 ± 1.60*** | 2.25 ± 0.04*** | 6.99 ± 0.058*** | 3.48 ± 0.19*** | 8.29 ± 0.08*** |
| DCSE (400mg/kg) | 7.18 ± 0.13*** | 42.79 ± 1.78*** | 2.70 ± 0.06*** | 9.20 ± 0.03*** | 5.07 ± 0.05*** | 7.26 ± 0.12*** |
***P < 0.001, a treated group animals compared with control.
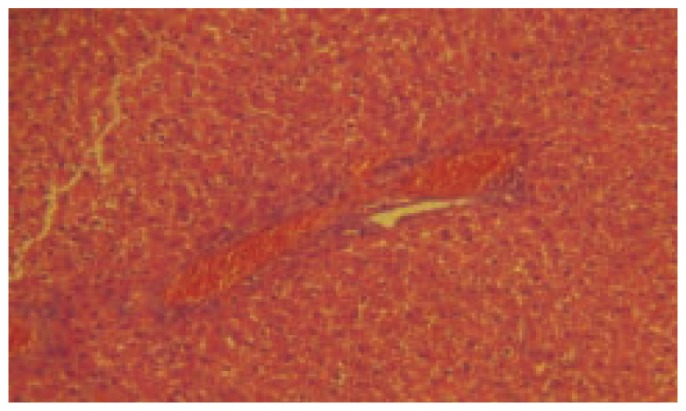
LPO activity was significantly increased (P< 0.001) in thioacetamide treated group when compared to control. The dose of 200 mg/kg and 400 mg/kg treated group was found significantly decreased (P< 0.001) the level of LPO in liver homogenate when compared to thioacetamide treated animals. Silymarin treated group was found to be more significant (P< 0.001) when compared to thioacetamide treated animals. The results of histopathology of livers are shown in Figure 1–5.
Figure 1. Histopathology of control group-treated with carboxy methylated cellulose only (40×).
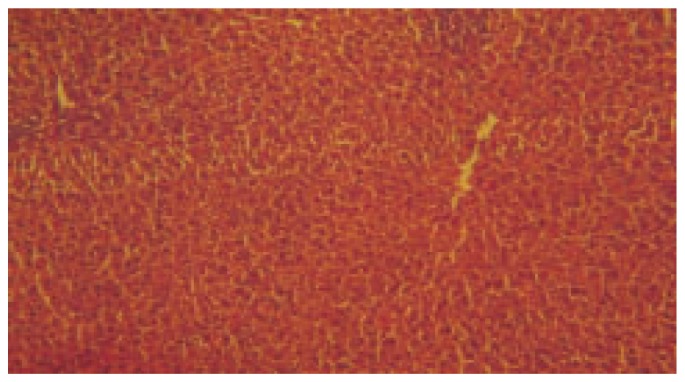
Figure 5. Histopathology of DCSE (400 mg/kg) treated group-treated with thioacetamide along with DCSE (40×).
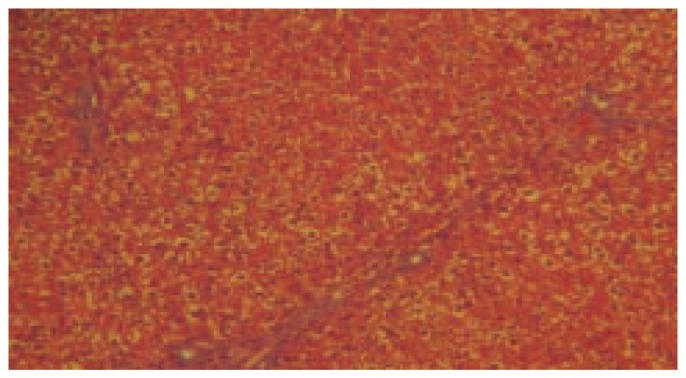
Figure 2. Histopathology of toxic control group-treated with thioacetamide (40×).
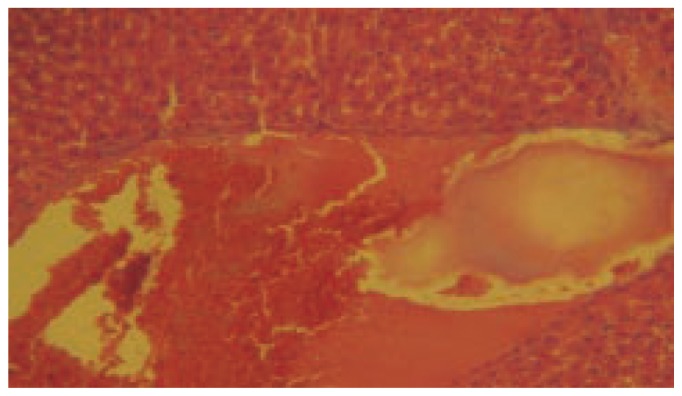
Figure 3. Histopathology of Standrad: treated with Thioacetamide along with Silymarin (40×).
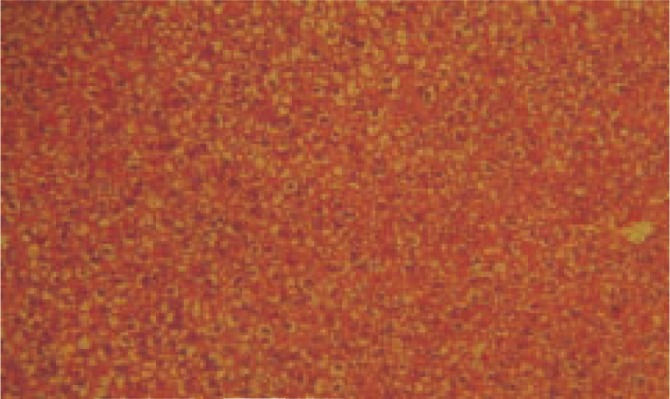
Figure 4. Histopathology of DCSE (200 mg/kg) treated group-treated with thioacetamide along with DCSE (40×).
4. Discussion
Thioacetamide is a potent hepatotoxin and carcinogen for rats. It is also known to produce marked liver damage in exposed animals. Toxicity experienced by liver during thioacetamide poisoning results from the production of metabolite, thioacetamide S- oxide, which is direct hepatotoxin. It has also been observed that thioacetamide causes changes in nucleolus and increased synthesis of guanine and cytosine rich RNA, with concomitant decrease in ribosomal RNA in the cytoplasm[18]–[27].
In the present study pretreatment with extract was found to significantly reverse the thioacetamide rise in the biochemical parameters like SGPT, SGOT and ALP level, thereby demonstrating the membrane stabilizing activity of the extract. The activities of SGPT and SGOT were almost brought down to normal suggesting the membrane stabilizing effect of the extract. The difference between group II and group V was found to be more statistically significant. The level of ALP, which was elevated was also brought down in the rats pretreated with the extract followed by thioacetamide.
In case of antioxidant enzyme, DCSE was found to significantly increase the level of SOD, CAT, GRD, GPX and GST. The level of LPO which was elevated due to thioacetamide also came to normal.
DCSE has contributed to the reduction of oxidative stress and showed hepatoprotective activity in experimental rats.
Acknowledgments
We are grateful to Chandra Diagnostic Lab, Salem for the contribution in this study and thanks also goes to management of Nandha college of Pharmacy, Erode, for providing all the facilities to make this work a success. We are also grateful to AICTE for providing us grant under JRF scheme.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Nagler R, Reznick A, Shafir Y, Shehadeh N. Free radical related effects and antioxidants in saliva and serum of adolescents with Type 1 diabetes metllitus. Arch Oral Biol. 2006;40:156. doi: 10.1016/j.archoralbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Vertuani S, Angusti A, Manfredini S. The antioxidant and proantioxidants network: An overview. Curr Pharm Des. 2004;10:1677–1694. doi: 10.2174/1381612043384655. [DOI] [PubMed] [Google Scholar]
- 3.Devasagayam TPA, Tilak JC, Boloor KK. Review: free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 4.Vasudevan M, Gunnam KK, Parle M. Antinociceptive and anti- inflammatory properties of Daucus carota seeds extract. J Health Sci. 2006;52:598–606. [Google Scholar]
- 5.Fu HW, Zhang L, Yi T, Feng YL, Tian JK. Two new guaiane-type sesquiterpenoids from the fruits of Daucus carota L. Fitoterapia. 2010;81:443–446. doi: 10.1016/j.fitote.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Rana Z, Malcolm R, Christine L, Le Maitre. Bioactive chemicals from carrot (Daucus carota) juice extracts for the treatment of leukemia. J Med Food. 2011;14:1–10. doi: 10.1089/jmf.2010.0284. [DOI] [PubMed] [Google Scholar]
- 7.Stange C, Fuentes P, Handford M, Pizarro L. Daucus carota as a noval model to evaluate the effect of light on carotenogenic gene expression. Boil Res. 2008;41:289–301. [PubMed] [Google Scholar]
- 8.Gilani AH, Shaheen E, Saeed SA, Bibi S, Irfanullah, Sadiq M, et al. Hypotensive action of coumarin glycosides from Daucus carota. Phytomed. 2000;7:423–426. doi: 10.1016/s0944-7113(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 9.Arjuman A, Nair V, Gopalakrishna HN, Nandini M. Evaluation of the antioxidant potential of NR-ANX-C (a polyherbal formulation) and its individual constituents in reversing haloperodal- induced catalepay in mice. Indian J Pharmacol. 2007;39(3):151–154. [Google Scholar]
- 10.Manigauha A, Patel S, Ali H, Chandy A, Uma Maheshwari M. Study the effect of phytochemical constituents of Piper betel leaves extracts on liver disorders by in vivo model. J Pharm Res. 2009;2:353–356. [Google Scholar]
- 11.Manigaunha A, Ganesh N, Kharya MD. Hepatoprotection by Kaempferia galanga against carbon tetrachloride induced liver damage in rats. Indian Drugs. 2010;47:55–60. [Google Scholar]
- 12.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, et al. Selenium: Biochemical role as a component of glutathione peroxidase. Sci. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 13.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferase, the first enzymatic step in mercapturicacid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 14.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 15.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):130–132. [PubMed] [Google Scholar]
- 16.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 17.Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- 18.Mondal A, Maity TK, Pal D, Sannigrahi S, Singh J. Isolation and in vivo hepatoprotective activity of Melothria heterophylla (Lour.) Cogn. against chemically induced liver injuries in rats. Asian Pac J Trop Med. 2011;4(8):619–623. doi: 10.1016/S1995-7645(11)60159-4. [DOI] [PubMed] [Google Scholar]
- 19.Murugesan G, Sundaram R, Samuel JI. Hepatoprotective and antioxidant properties of marine halophyte Luminetzera racemosa bark extract in CCL4 induced hepatotoxicity. Asian Pac J Trop Med. 2011;4(6):462–465. doi: 10.1016/S1995-7645(11)60126-0. [DOI] [PubMed] [Google Scholar]
- 20.Eesha BR, Amberkar VM, Kumari KM, Sarath B, Vijay M, Lalit M, et al. Hepatoprotective activity of Terminalia paniculata against paracetamol induced hepatocellular damage in Wistar albino rats. Asian Pac J Trop Med. 2011;4(6):466–469. doi: 10.1016/S1995-7645(11)60127-2. [DOI] [PubMed] [Google Scholar]
- 21.Gupta RK, Hussain T, Panigrahi G, Das A, Singh GN, Sweety K, et al. Hepatoprotective effect of Solanum xanthocarpum fruit extract against CCl4 induced acute liver toxicity in experimental animals. Asian Pac J Trop Med. 2011;4(12):964–968. doi: 10.1016/S1995-7645(11)60227-7. [DOI] [PubMed] [Google Scholar]
- 22.Thirumalai T, David E, Therasa SV, Elumalai EK. Restorative effect of Eclipta alba in CCl4 induced hepatotoxicity in male albino rats. Asian Pac J Trop Med. 2011;4:304–307. [Google Scholar]
- 23.Nwaehujor CO, Udeh NE. Screening of ethyl acetate extract of Bridelia micrantha for hepatoprotective and anti-oxidant activities on Wistar rats. Asian Pac J Trop Med. 2011;4(10):796–798. doi: 10.1016/S1995-7645(11)60196-X. [DOI] [PubMed] [Google Scholar]
- 24.Nnodim J, Anyadoh SO, Nwosunjoku EC. The antioxidant status and lipid peroxidation product of newly diagnosed and 6 weeks follow–up patients with pulmonary tuberculosis in Owerri, Imo state, Nigeria. Asian Pac J Trop Dis. 2011;1(4):292–294. [Google Scholar]
- 25.Thirumalai T, David E, Therasa S Viviyan, Elumalai EK. Restorative effect of Eclipta alba in CCl4 induced hepatotoxicity in male albino rats. Asian Pac J Trop Dis. 2011;1(4):304–307. doi: 10.1016/S2221-1691(11)60052-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erukainure OL, Ajiboye JA, Adejobi RO, Okafor OY, Adenekan SO. Protective effect of pineapple (Ananas cosmosus) peel extract on alcohol-induced oxidative stress in brain tissues of male albino rats. Asian Pac J Trop Dis. 2011;1(1):5–9. [Google Scholar]
- 27.Fatih Aydın A, Kusku-Kiraz Z, Dogru-Abbasoglu S, Gulluoglu M, Uysal M, Kocak-Toker N. Effect of carnosine against thioacetamide induced liver cirrhosis in rat. Pept. 2010;31:67–71. doi: 10.1016/j.peptides.2009.11.028. [DOI] [PubMed] [Google Scholar]


