Abstract
Objective
To elucidate the ameliorative effect of hydroalcoholic extract of leaves of Hibiscus rosa sinensis (HRS) in acetic acid induced experimental colitis in male wistar rats.
Methods
The animals were administered with 2 mL acetic acid (4%) via intra rectal. The animals were divided into various treatment groups (n=6). Prednisolone was used as standard drug and HRS was administered at a dose of 50, 100 and 200 mg/kg p.o. The control group of animals received 1 mL of vehicle (distilled water). Ulcer area, ulcer index, spleen weight, colon weight to length ratio, macroscopic score, haematological parameters, colonic superoxide dismutase (SOD), glutathione (GSH), myeloperoxidase (MPO), malondialdehyde (MDA), tumor necrosis factor-α (TNF-α), nitric oxide (NO) and histological changes were recorded after the treatment regimen of 11 days.
Results
Intrarectal instillation of acetic acid caused enhanced ulcer area, ulcer index, spleen weight, colon weight to length ratio, colonic MPO, MDA, NO and TNF-α It caused significant decreased level of SOD and GSH. Pretreatment with HRS for 7 days exhibited significant effect in lowering of oxidative stress, colonic NO, TNF-α and elevation of SOD and GSH at a dose of 100 and 200 mg/kg in acetic acid induced colitis.
Conclusions
The present investigation demonstrates HRS is of potent therapeutic value in the amelioration of experimental colitis in laboratory animals by inhibiting the proinflammatory mediator like NO and TNF-α.
Keywords: Acetic acid, Hibiscus rosa sinensis, Inflammatory bowel disease, Inflammatory cytokine, Nitrite/nitrate, Oxidative stress, Ulcerative colitis, Prednisolone
1. Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of intestine which mainly includes ulcerative colitis (UC) and Crohn's disease (CD). An array of etiopathogenic factors contribute to the development of this disease. Genetic modulation, infective agents, immunological disturbance, smoking, microorganism have been implicated to orchestrate mucosal inflammation, hemorrhage and development of strictures in the colon[1].
A plethora of free radicals contribute, aggravate and precipitate pathobiological changes in the colonic mucosa and have been proposed as therapeutic targets. Localised inflammation, neutrophil infiltration and vicious cascade of generation of inflammatory mediators have been elucidated to damage the colonic mucosa[2].
In humans IBD affects people between 15 to 35 years old and continues for lifetime. In laboratory animals similar experimental conditions are produced by rectal administration of acetic acid which mimics the human condition. Acetic acid induced IBD is a reproducible laboratory animal model and is useful for screening of drugs for ulcerative colitis[1].
The present treatment regimen includes salicylate, corticosteroids, antimicrobials and immunosuppressive agents such as azathioprine and mercaptopurine, etc. which possess a wide range of side effects which could be eliminated in herbal drug treatment. Polyphenols, flavonoids have been shown to alleviate chronic inflammation in experimental model of IBD. Flavonoids could be used to treat IBD because firstly, the inflammation mediatory enzymes are inhibited by the compounds which are present in it; secondly, they cause in vitro down regulation of a number of cells of the immune system and thirdly, they exhibit potent antioxidative/radical scavenging effects[3].
Hibiscus rosa sinensis (HRS) has been evaluated for an array of diseases like heart disorders, tumors, convulsion, diabetes, inflammation, oxidative stress, diarrhoea, ulcer by various authors[4]–[8]. In the Indian traditional system of medicine, it is claimed to possess therapeutic utility in IBD. The objective of present investigation was to unravel the therapeutic potential of hydroalcoholic extract of leaves of HRS to treat experimental IBD in laboratory animals.
2. Materials and methods
2.1. Collection of plant material
The leaves of HRS were collected from rural areas of Pune District, Maharashtra in the month of February 2010. Authentication of plant was carried out by P.G. Diwakar, Joint Director, Botanical Survey of India, Pune. The voucher specimen number was BSC/WRC/Tech./2009/33.
2.2. Preparation of extract
The powdered plant material of 1 000 g was macerated 6 L of water: alcohol (7:3) for about 48 hours with occasional shaking. Macerate was decanted and filtered through cloth and then through filter paper to obtain a clear extract. This process was repeated with the same volume of hydroalcoholic mixture. Macerates were pooled and collected in trays and evaporated to dryness at 30–35 °C. The yield was found to be equal to 65 g (6.5%). Solution of HRS hydroalcoholic extract was prepared by using distilled water as vehicle.
2.3. Preliminary phytochemical screening
The preliminary phytochemical screening of the above extract of HRS was carried out according to the previously mentioned methods[9]. Phytochemical analysis of the extract was performed for the identification of phytochemicals like alkaloid, flavonoids, steroid and phenols etc.
2.4. Animals
Healthy adult male swiss albino mice (20–30 g) and male wistar rats (230–250 g) were obtained from the National Toxicological Centre, Pune, India. The animals were housed in groups of 6 in solid bottom polypropylene cages. They were maintained at (24 ± 1) °C, with relative humidity of 45–55% and 12:12 h dark/light cycle. The animals were acclimatized for a period of two weeks and were kept under pathogen free conditions. The animals had free access to standard pellet chow (Chakan Oil Mills, Sangli) throughout the experimental protocol, with the exception of overnight fasting before induction of experimental colitis. The animals had access to filtered water. The pharmacology and acute toxicity protocols were approved by the Institutional Animal Ethics Committee (IAEC) of Poona College of Pharmacy, Pune (CPCSEA/06/2010).
2.5. Drugs and chemicals
Prednisolone was obtained as a gift sample from Symed Pharmaceuticals Pvt. Ltd., Hyderabad. Acetic acid, anaesthetic ether, ethanol, formalin, 1,1,3,3-Tetraethoxypropane, crystalline beef liver catalase, reduced glutathione (GSH), 5,5′-dithiobis (2-nitrobenzoic acid), bovine serum albumin, thiobarbituric acid, tris buffer, sucrose, trichloroacetic acid, citric acid monohydrate, sodium nitrate, copper sulphate, sodium potassium tartarate, ethylene diamine tetra acetic acid disodium salt, Folin's phenol reagent, sodium hydroxide, sodium carbonate, magnesium chloride, sodium carbonate, sodium bicarbonate, potassium chloride, calcium chloride, disodium hydrogen orthophosphate, potassium dihydrogen orthophosphate, carbon tetrachloride, chloroform, ether, hydrochloric acid and concentrated sulphuric acid were purchased from S.D. Fine Chemicals, Mumbai, India. Sulphanilamides, naphthalamine diamine HCl, phosphoric acid were obtained from LobaChemi Pvt. Ltd., Mumbai, India. TNF-α ELISA kit was obtained from Thermo Scientific, USA.
2.6. Acute toxicity testing
Acute oral toxicity in swiss albino mice was performed according to OECD guidelines using AOT 425 software.
2.7. Dosages of HRS extract and standard drugs used
The freshly prepared aqueous solution of HRS was administered to animals orally for 7 days in three different dosages (50, 100 and 200 mg/kg). On the 8th day, the colitis was induced by intrarectal administration of acetic acid. The drug treatment was continued even after administration of acetic acid. Prednisolone was used as standard drug, but it was not given as pre-treatment. Prednisolone was administered at a dose of 2 mg/kg/day orally in rats as suspension in 0.5% of sodium CMC.
2.8. Induction of colitis
Colonic inflammation was induced in fasted rats following the method of Mascolo et al[10]. The study comprised of six groups of six animals each as follows:
Group I: Normal animals (received 2 mg/kg/day of distilled water); Group II: Acetic acid control animals (received 2 mL of 4% acetic acid solution intrarectally on the 8th day); Group III: HRS (50 mg/kg) treated animals (received 7 days pretreatment with 50 mg/kg of HRS, p.o. and 2 mL of 4% acetic acid solution, intrarectally on the 8th day. Drug treatment was continued till the 11th day); Group IV: HRS (100 mg/kg) treated animals (received 7 days pretreatment with 100 mg/kg of HRS, p.o. and 2 mL of 4% acetic acid solution, intrarectally on the 8th day. Drug treatment was continued till 11th day); Group V: HRS (200 mg/kg) treated animals (received 7 days pretreatment with 200 mg/kg of HRS, p.o. and 2 mL of 4% acetic acid solution, intrarectally on the 8th day. Drug treatment was continued till the 11th day); Group VI: Prednisolone treated group, which received prednisolone (2 mg/kg, p.o., for 3 days) and acetic acid (2 mL of 4% solution, once, intrarectally). Prednisolone and acetic acid acetic acid treatment was started on the same day.
On the 11th day blood was withdraw by retroorbital puncture and then animals were sacrificed by cervical dislocation and colons were collected and the spleen from each animal was also weighed. Portions of colonic specimens were kept in 10% formalin for histopathological studies.
2.9. Evaluation of the disease
The disease induced in experimental animals was evaluated based on its macroscopic characteristics. Evaluation pattern for macroscopic characteristics, reported by Morris et al[11] was used after some modifications.
2.9.1. Determination of ulcer index
The evaluation of ulcer index was performed according to Dengiz et al[12].
2.9.2. Determination of haematological parameters
Haematological parameters were determined using an automated haematological analyzer (Sysmex KX-21) with specific software for rat blood samples[13]. The parameters analyzed were white blood cell (WBC) number, red blood cell (RBC) number, haemoglobin (Hb) concentration, hematocrit (HCT) and platelet (PLT) count.
2.9.3. Biochemical assays
Samples from the colon were stored immediately at −80°C till analysis. Tissue samples were homogenized in 10 mmol Tris–HCl buffer (pH 7.1) and the homogenate was used for the measurement of myeloperoxidase (MPO), malondialdehyde (MDA), GSH, superoxide dismutase (SOD), nitric oxide (NO) and TNF-α in colon tissue.
2.9.3.1. Determination of colonic SOD contents
The mucosal pathological alteration occurs due to the overproduction of ROS. Colonic SOD assay were determined as previously described by Misera and Fridovich[14]. SOD activity was expressed as U/mg protein.
2.9.3.2. Determination of colonic GSH contents
The colonic GSH assay was performed according to method previously describe by Moron et al[15]. The amount of reduced GSH was expressed as µg of GSH / mg protein.
2.9.3.3. Determination of colonic MPO contents
The colonic MPO assay was assessed as a marker of neutrophil infiltration according to the method described by Krawisz et al[16]. MPO activity was defined as the quantity of enzyme degrading 1µmol of peroxide per min at 25 °C and was expressed in units per gram (U/gm) of wet scrapings.
2.9.3.4. Determination of colonic MDA contents
MDA levels in the colon tissue were determined by the method of Slater and Sawyer[17]. The values were expressed as nanomoles of MDA/mg protein.
2.9.3.5. Determination of colonic nitrite/nitrate level
Colonic NO level was estimated as nitrite and nitrate by the acidic Griess reaction after reduction of nitrate to nitrite by vanadium trichloride according to the method described by Miranda et al[18]. The Griess reaction relies on a simple colorimetric reaction between nitrite, sulfonamide and N-(1- naphthyl) ethylenediamine to produce a pink azo-product with maximum absorbance at 543 nm. The concentrations were determined using a standard curve of sodium nitrate and the results were expressed as µg/mg of wet tissue.
2.9.3.6. Determination of colonic TNF-α levels
Colonic samples were immediately weighed, minced on an ice-cold plate, suspended in a tube with 10 mmol/L sodium phosphate buffer (pH 7.4) (1:5 w/v). The tubes were placed in a shaking water bath (37 °C) for 20 min and centrifuged at 9 000 g for 30 s at 4 °C; the supernatant was frozen at −80 °C until assay. TNF-αwas quantified by enzyme-linked immunoabsorbent assay and the results were expressed as picograms/mg of wet tissue.
2.9.4. Evaluation based on microscopical (histological) characters
To process for microscopic studies, 5 µm thick paraffin sections were stained in haematoxylin and eosin (H&E). The stained sections were examined for any inflammatory changes like infiltration of the cells, necrotic foci, damage to tissue structures like peyer's patches, damage to nucleus.
2.10. Data and statistical analysis
All the results were expressed as Mean ± SEM. Data analysis was performed using GraphPad Prism 5.0 software (GraphPad, San Diego, CA). Statistical comparisons were made between drug-treated groups and colitis control animals. Data of biochemical parameters were analyzed using one-way ANOVA; Dunnett's multiple range test was applied for post hoc analysis. A value of P < 0.05 was considered to be statistically significant.
3. Results
3.1. Acute toxicity testing
Acute toxicity studies of the hydroalcoholic extract of HRS showed no signs and symptoms such as restlessness, respiratory distress, diarrhea, convulsions and coma and it was found safe up to 5 000 mg/kg.
3.2. Preliminary phytochemical screening
The hydroalcoholic extracts of HRS leafs was screened for various chemical tests as per the reported methods[9] and was found to contain alkaloids, steroids, flavonoids and polyphenols
3.3. Acetic acid-induced colitis
Intrarectal instillation of acetic acid (4%) caused inflammatory reaction in the colon. The inflammation occurred in rectum and distal colon portion. HRS treated group showed mild lesions, regeneration and inflammatory reaction.The prednisolone treated group showed suppressed inflammatory reaction.
3.3.1. Effect of HRS on colon weight to length ratio
The ratio of colon weight/length was found to be increased (0.180 ± 0.004) significantly in acetic acid control group as compared to normal group (0.070 ± 0.008). Pre-treatment of HRS (50, 100 and 200 mg/kg, p.o.) for 7 days decreased the colon weight/length ratio (0.150 ± 0.010, 0.140 ± 0.010 and 0.090 ± 0.007, respectively), and was found to be significant (P < 0.05, P < 0.01 and P < 0.001, respectively) as compared to acetic acid control group in dose dependant manner (Table 1).
Table 1. Effect of HRS on colon weight/ length ratio, spleen weight, macroscopic score, ulcer area and ulcer index of rat in acetic acid induced IBD (Mean ± SEM).
| Groups (n=6) | Colon weight to length ratio | Spleen weight (g) | Macroscopic score | Ulcer area (mm2) | Ulcer index |
| Normal | 0.070 ± 0.008 | 1.040 ± 0.088 | --- | --- | --- |
| Acetic acid control | 0.180 ± 0.004 | 2.060 ± 0.084 | 8.83 ± 0.40 | 41.67 ± 1.96 | 66.04 ± 4.66 |
| Prednisolone (2 mg/kg) | 0.100 ± 0.009*** | 1.260 ± 0.040*** | 2.50 ± 0.42*** | 10.00 ± 1.23*** | 15.65 ± 2.31*** |
| HRS (50 mg/kg) | 0.150 ± 0.010* | 1.740 ± 0.063** | 8.83 ± 0.47 | 29.67 ± 1.40 * | 51.19 ± 2.88* |
| HRS (100 mg/kg) | 0.140 ± 0.010** | 1.560 ± 0.050*** | 7.16 ± 0.40* | 26.83 ± 1.57** | 45.84 ± 1.62** |
| HRS (200 mg/kg) | 0.090 ± 0.007*** | 1.280 ± 0.035*** | 3.66 ± 0.49*** | 20.67 ± 2.40*** | 27.64 ± 2.45*** |
Data are analyzed by One Way ANOVA followed by Dunnett's test. *P < 0.05, **P < 0.01, ***P < 0.001 as compared to acetic acid control group.
3.3.2. Effect of HRS on spleen weight
In the present study, rats with acetic acid induced colitis exhibited splenic enlargement (2.060 ± 0.084) g as compared with normal (1.040 ± 0.088) g. Pre-treatment of HRS (50, 100 and 200 mg/kg, p.o.) for 7 days inhibiting spleen enlargement [(1.740 ± 0.063), (1.56 ± 0.05) and (1.28 ± 0.035) g, respectively] was found to be significant (P < 0.01 and P < 0.001, respectively) as compared to acetic acid control group (Table 1).
3.3.3. Effect of HRS on macroscopic scores
The colons of the rats were examined macroscopically for signs of hemorrhage and ulceration by an independent blinded observer, unaware of treatment schedule, using a previously established scoring system[11]. After 11 days, colons from acetic acid administered rats displayed considerable damage. Acetic acid control rats had a mean macroscopic score (8.83 ± 0.40). Rats pretreated with the HRS (100 and 200 mg/kg, p.o.) had significantly improved macroscopic scores (7.16 ± 0.40 and 3.66 ± 0.49) (P < 0.05 and P < 0.001 respectively) compared with colitis control rats, as did prednisolone pretreated rats (2 mg/kg/day) (2.50 ± 0.42) (P < 0.001). In the normal control group there was no visible damage (Table 1).
3.3.4. Effect of HRS on ulcer area
Rectal administration of 4% acetic acid produced ulcers of colon in acetic acid control and all drug treated animals. The mean ulcer area of acetic acid control group was (41.67 ± 1.96) mm2, showing high ulcerogenic effect of acetic acid. Pre-treatment of HRS (50, 100 and 200 mg/kg, p.o.) for 7 days decreased the ulcer area of colon [(29.67 ± 1.40), (26.83 ± 1.57) and (20.67 ± 2.40) mm2, respectively] (P < 0.05, P < 0.01 and P<0.001 respectively) as compared to acetic acid control group in dose dependant manner (Table 1).
3.3.5. Effect of HRS on ulcer index
The mean ulcer index of acetic acid control group was (66.04 ± 4.66) showing high ulcerogenic effect of acetic acid. Pre-treatment of HRS (50, 100 and 200 mg/kg, p.o.) for 7 days decreased the ulcer index of colon (51.19 ± 2.88, 45.84 ± 1.62 and 27.64 ± 2.45, respectively) (P < 0.05, P < 0.01 and P < 0.001, respectively) as compared to acetic acid control group in dose dependant manner (Table 1).
3.3.6. Effect of HRS on hematology
Table 2 summarizes the effect of HRS on haematological parameters of acetic acid control and experimental animals. The acetic acid control rats showed a significant decrease in the haematological parameters in WBC count, RBC count, Hb, HCT and PLT count as compared to normal rats. These reductions were significantly attenuated in the HRS pre-treated group (200 mg /kg, p.o.) (P < 0.001). No effect was observed in the rest of the haematological parameters analyzed.
Table 2. Effect of HRS on haematological parameters of rat in acetic acid induced IBD (n=6) (Mean ± SEM).
| Parameters | Normal | Acetic acid control | Prednisolone (2 mg/kg) | HRS |
||
| 50 mg/kg | 100 mg/kg | 200 mg/kg | ||||
| WBC ( X103 /µL) | 22.76 ± 0.76 | 9.76 ± 1.18 | 20.16 ± 1.05*** | 12.11 ± 0.72 | 14.15 ± 0.75*** | 18.60 ± 0.64*** |
| RBC ( X106 /µL) | 13.38 ± 1.07 | 5.08 ± 0.55 | 12.13 ± 0.73*** | 7.51 ± 0.46 | 10.08 ± 0.70*** | 11.40 ± 0.27*** |
| HGB (g /dL) | 15.96 ± 0.93 | 9.84 ± 0.69 | 14.03 ± 0.36*** | 10.13 ± 0.78 | 11.57 ± 0.75 | 13.08 ± 0.63* |
| HCT (%) | 49.58 ± 1.59 | 34.61 ± 1.33 | 46.22 ± 0.90*** | 38.66 ± 1.83 | 40.12 ± 0.89 | 42.11 ± 1.45*** |
| PLT ( X105 /µL) | 11.24 ± 0.55 | 5.19 ± 0.45 | 10.51 ± 0.41*** | 6.91 ± 0.42 | 8.21 ± 0.25* | 9.73 ± 0.33*** |
Data are analyzed by One Way ANOVA followed by Dunnett's test. *P < 0.05, **P < 0.01, ***P < 0.001 as compared to acetic acid control group.
3.3.7. Effect of HRS on colonic SOD concentrations
Induction of colitis produced a significant decrease in colonic SOD content [(2.46 ± 0.51) U/mg of protein)] as compared with the normal group [(12.27 ± 0.79) U/mg of protein)]. HRS (100 and 200 mg/kg, p.o.) pre-treatment for 7 days significantly increased SOD content [(5.74 ± 0.53) and (8.58 ± 0.55) U/mg of protein] as compared with acetic acid control group (P < 0.01 and P < 0.001 respectively). Prednisolone also protected against SOD depletion induced by acetic acid [(9.72 ± 0.60) U/mg of protein, P < 0.001] (Table 3).
Table 3. Effect of HRS on various antioxidant parameter of rat colon in acetic acid induced IBD (n=6) (Mean ± SEM).
| Parameter | Normal | Acetic acid control | Prednisolone (2 mg/kg) | HRS |
||
| 50 mg/kg | 100 mg/kg | 200 mg/kg | ||||
| SOD (U/mg protein) | 12.27 ± 0.79 | 2.46 ± 0.51 | 9.72 ± 0.60*** | 3.580 ± 0.57 | 5.74 ± 0.53** | 8.58 ± 0.55*** |
| Reduced GSH (µg of GSH/ mg protein) | 26.89 ± 1.85 | 16.22 ± 1.35 | 24.50 ± 1.54*** | 15.51 ± 0.79 | 19.06 ± 1.16 | 24.88 ± 1.52*** |
| Lipid peroxidation (nmoles of / mg protein) | 24.27 ± 2.71 | 75.9 ± 5.72 | 32.83 ± 5.88*** | 69.22 ± 7.33 | 56.58 ± 4.29 | 43.62 ± 4.38*** |
| MPO (U/mg) | 4.39 ± 0.36 | 21.24 ± 0.52 | 6.77 ± 0.39*** | 20.86 ± 0.78 | 18.55 ± 0.47 | 10.58 ± 0.41*** |
Data are analyzed by One Way ANOVA followed by Dunnett's test. *P < 0.05, **P < 0.01, ***P < 0.001 as compared to acetic acid control group.
3.3.8. Effect of HRS on colonic GSH concentrations
Induction of colitis produced a significant decrease in colonic GSH content [(16.22 ± 1.35) µg/ mg of protein] as compared with the normal group [(26.89 ± 1.85) µg/mg of protein]. HRS (200 mg/kg, p.o.) pre-treatment for 7 days significantly increased GSH content as compared with acetic acid control group [(24.88 ± 1.52) µg/ mg of protein, P < 0.001] but with no dose-dependency. Prednisolone also protected against GSH depletion induced by acetic acid [(24.50 ± 1.54) µg/ mg of protein, P < 0.001] (Table 3).
3.3.9. Effect of HRS on colonic MDA concentration
Colonic lipid peroxides/MDA concentration in acetic acid control group increased [(75.9 ± 5.72) nmole/mg of protein] in comparison to the normal group [(24.27 ± 2.71) nmole/mg of protein] (Table 3). The 7 days pre-treatment of rats with HRS (200 mg/ kg p.o.) produced a marked significant decrease in lipid peroxides concentration [(43.62 ± 4.38 nmole/mg of protein] (P < 0.001). Prednisolone also provided protection against the elevation in lipid peroxides concentration induced by acetic acid treatment [(32.83 ± 5.88) nmole/mg of protein] (P < 0.001) (Table 3).
3.3.10. Effect of HRS on colonic MPO concentrations
The colitis caused by acetic acid was associated with an increase in MPO activity at (21.24 ± 0.52) U/mg. In the groups pre- treated with HRS (100 and 200 mg/kg, p.o.) for 7 days, the MPO activity of colonic mucosal scrapings were significantly decreased [(18.55 ± 0.47) and (10.58 ± 0.41) U/mg respectively] (P < 0.01, P < 0.001), as compared with acetic acid control group (Table 3).
3.3.11. Effect of HRS on colonic nitrite/nitrate concentration
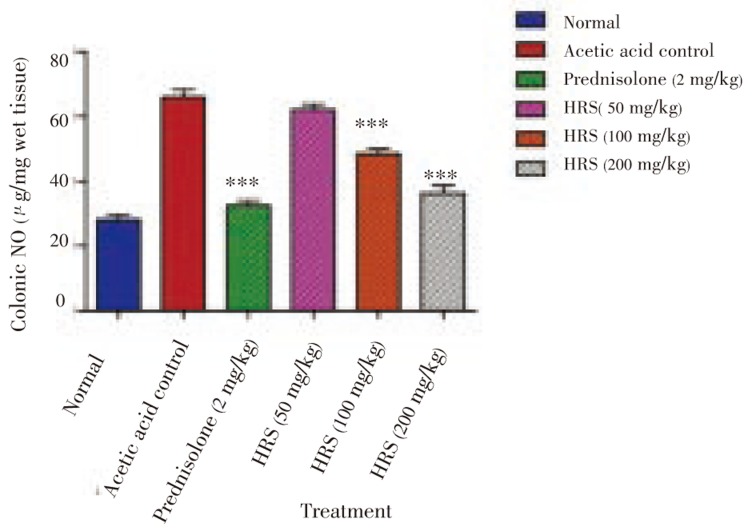
The results of biochemical parameters i.e. colonic nitrile/nitrate level for every group are gathered in Figure 1. Acetic acid-induced colitis resulted in increased colonic nitrite/ nitrate level in comparison with normal animals [(66.05 ± 2.20)µg/mg vs. (28.02 ± 1.59)µg/mg). The 7 days pre-treatment of rats with HRS (100 and 200 mg/kg p.o.) produces a significant reduction in colonic nitrite/ nitrate level compared to acetic acid-induced colitis group [(48.48 ± 2.24) µg/mg and (36.32 ± 2.32) µg/mg respectively, P < 0.001].
Figure 1. Effect of HRS on colonic TNF-α level of rats in acetic acid induced IBD. Data are expressed as mean ± S.E.M. from six rats and analyze by One Way ANOVA followed by Dunnett's test. *P < 0.05, **P < 0.01, ***P < 0.001 as compared to acetic acid control group.
3.3.12. Effect of HRS on colonic TNF-α concentration
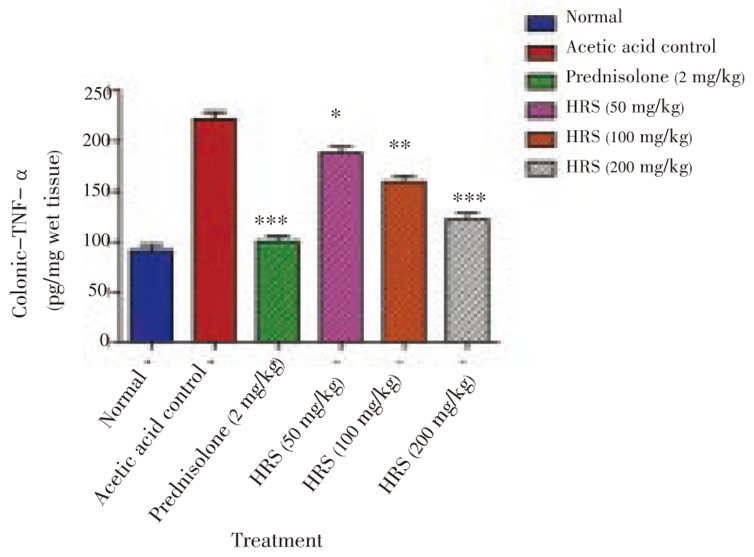
Colonic TNF-α level in acetic acid control group was significantly higher than the corresponding value in the normal group [(221.8 ± 7.16) pg/mg vs. (91.19 ± 6.04) pg/mg] (Figure 2). This increase in TNF-α was significantly attenuated by the 7 days pretreatment with HRS (50, 100 and 200 mg/kg p.o.) as compared to acetic acid-induced colitis group [(188.4 ± 5.77), (160.0 ± 5.40) and (122.5 ± 5.66) pg/mg respectively) (P < 0.05, P < 0.01, P < 0.001).
Figure 2. Effect of HRS on colonic nitrite/nitrate level of rats in acetic acid induced IBD. Data are expressed as mean ± S.E.M. from six rats and analyze by One Way ANOVA followed by Dunnett's test. *P < 0.05, **P < 0.01, ***P < 0.001 as compared to acetic acid control group.
3.3.13. Histopathological results
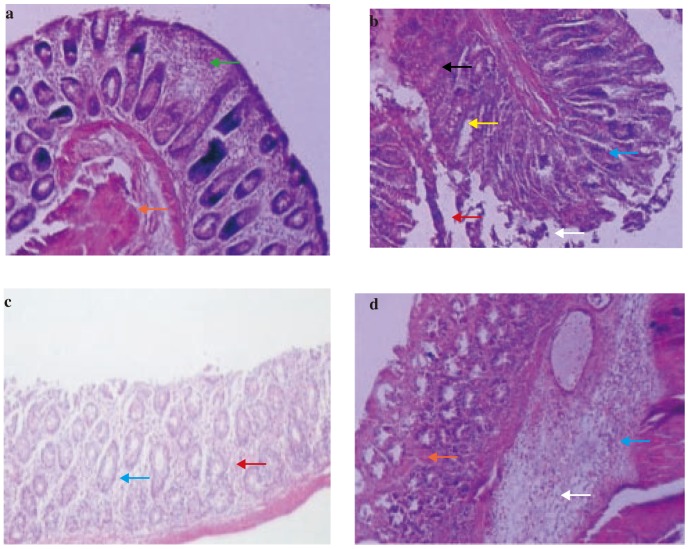
We examined the H & E stained sections of ulcerated areas of the colons of the rats for signs of colitis. The histopathological features of untreated animals included edema in submucosa, cellular infiltration, haemorrhages, necrosis, congested capillaries in lamina propria, dense inflammation of lymphocytes, complete lost goblet cells (Figure 3b). The polymorphonuclear neutrophil infiltration was found in affected tissues. As compared to acetic acid control group pretreatment of rats with HRS (200 mg/kg) (Figure 3d) showed significant decrease in ulceration, hyperemia, necrosis, edema, cellular in filtration, hemorrhages and goblet cell hyperplasia. However, treatment with prednisolone (Figure 3c) still showed more significant decreased in all these pathological parameters as compared to HRS. The 7 days pretreatment of rats with HRS or treatment with prednisolone significantly attenuated the extent and severity of the histological signs of cell damage. In case of normal rats colon there were no inflammatory cells in the lamina propria and the epithelium remained intact (Figure 3a) (Table 4).
Figure 3. Photomicrographs of sections of colons from rats stained with H&E.
Colon microscopic image of (a) Normal rat with intact epithelial (orange arrow) and mucosal layer (green arrow); (b) Acetic acid induced colitis rat with extensive damage including edema in submucosa (white arrow) and cellular infiltration (blue arrow), hemorrhages (red arrow), necrosis (yellow arrow) and ulceration (black arrow); (c) Prednisolone (2 mg/kg, p.o.) treated rat with infiltration (blue arrow) and hemorrhages (red arrow); (d) HRS (200 mg/kg p.o.) 7 days pretreated rat with edema in submucosa (white arrow), cellular infiltration (blue arrow) and hemorrhages (red arrow). Images (× 100 magnification) are typical and representative of each study group.
Table 4. Effect of HRS on pathological changes of rat colon in acetic acid induced IBD.
| Groups | Ulceration | Hyperemia | Necrosis | Edema | Cellular in filtration | Goblet cell hyperplasia |
| Normal | 0 | + | 0 | + | 0 | 0 |
| Acetic acid control | +++ | +++ | ++++ | +++ | ++++ | ++ |
| Prednisolone (2 mg/kg) | + | ++ | + | ++ | ++ | + |
| HRS (200 mg/kg) | + | ++ | ++ | ++ | ++ | + |
0: No abnormality detected; +: Damage/ active changes up to less than 25 %; ++: Damage/ active changes up to less than 50 %; +++: Damage/ active changes up to less 75 %; ++++: Damage/ active changes up to more than 75 %.
4. Discussion
Acetic acid induced ulcerative colitis in laboratory rats has been used to screen various drugs[19],[20]. Severe inflammation, oxidative stress, neutrophil infiltration and release of various mediators of inflammation are the hall marks of this animal model[21]. The present investigation exhibits the role of HRS in halting and reversing an array of macroscopical and biochemical aberrations caused by instillation of 4% acetic acid into the colonic mucosa.
The wet weight of colon is regarded as a reproducible parameter indicating the degree of inflammation in colon. The elevation in the colon weight and colon weight to length ratio was inhibited by HRS depicting its healing property. Spleen is an indispensible part of immune system and reticuloendothelial system which has been found to be atrophied in chronic colitis[22]. The reduction in the splenic enlargement by the HRS proved the ability of HRS to modulate the immune system.
The gross morphological lesions characterized by ulcer and necrotic area of various sizes, were healed depicting protection of microflora from the corrosive effect of acetic acid by HRS. Ulcer area and ulcer index were quantitatively determined reflecting the protective action of HRS. Clinical manifestation of IBD includes exacerbated hematological imbalance leading to unexplained diarrhoea and malena[13]. This feature was reversed in HRS treated animals showing its therapeutic potential. Hematology provides an insight into the disease state of colitis. The various components of blood were disproportionately altered in the vehicle treated group whereas their ratios were unchanged in HRS treated animals.
Over production of ROS contributes to pathobiological alterations in the mucosa. The principle reacting oxygen metabolites altering the colonic milieu including SOD, GSH and MDA[23],[24]. The superoxide anions are transformed into secondary antioxidant H2O2 by SOD. GSH has a detoxifying effect on electrophiles by direct reaction with various intermediates mediated by GSH S-transferase. It is well studied that depletion of GSH leads to the cellular damages. Depleted GSH is characteristic feature of colonic injury[25]. HRS restores the reduced levels of SOD and GSH.
Intrarectal administration of acetic acid causes protonation and migration of acetic acid molecule into colonic microflora after internalization to produce protons leading to epithelial denudation[2]. This process instigates infiltration of neutrophils into colonic tissue. The surge of neutrophil infiltration into tissue is a direct evidence of pathogenecity. The level of MPO is a measure of neutrophil infiltration[16]. Neutrophils exhibits a decisive role in the pathogenesis of IBD by dent of increase in an array of reactive oxygen species and superoxide anions which inturn upregulate hydroxyradical and peroxide exacerbation leading to mucosal dysfunction and tissue necrosis. The enhanced lipid peroxidation provides an index of oxidative stress. Acetic acid is known to elevate levels of MDA to produce colitis. Our investigations demonstrate that HRS reduced MPO and MDA reflecting its inhibitory activity on formation of reactive oxygen species. Enhanced peroxidation of lipid in the colonic tissue is responsible for initiation of vicious cycle and generating oxidative metabolite leading to tissue damage which was reversed by HRS. It may be hypothesized that the phytoconstituents like mucilage, saponins, tannins and flavanoids present in HRS may have orchestrated the amelioration of IBD[8]. Alkaloids, steroids, flavonoids, polyphenols have been proven antioxidant, anti-inflammatory and immunomodulatory active principles. These phytoconstituent of HRS may have synergistically contributed to the attenuation of ulcerative colitis.
NO is an unconventional intracellular messenger playing a vital role in various pathological and physiological processes. NO reacts with reactive oxygen species and acts as an oxidant. But, this oxidation is not specific and do not affect any cell molecule. Increase in NO level is the result of tissue toxicity caused by damage of colonic mucosa[25],[26]. It also reacts with SOD and other oxidative biomarkers to precipitate tissue damage. The protective cellular enzymes such as SOD, GSH serve as primary scavenger of free radicals. Appearance of NO accompanied by generation and release of mediators such as proinflammatory cytokines like TNF-α, interleukin-1β and interleukin-6[27]. Pretreatment with HRS inhibited elevated level of NO.
Cytokines are cardinal biomarkers which are increased in colonic mucosa after induction of IBD. TNF-α is a major proinflammatory cytokines responsible for migration of NF-κB into the nucleus of the mucosal cells[28]. It instigates a cascade which involves overproduction of other proinflammatory cytokines and adhesion molecule leading to further damage of colonic mucosa[25],[29]. HRS administration reduced TNF-α, proving it's potential as antioxidant and NF-κB inhibitor. These investigations indicate that TNF-α in colonic tissues is suppressed by the administration of HRS.
Prednisolone is a proven drug for IBD[30],[23]. The same has been observed in our investigation. The present investigation shows that HRS (200 mg/kg) exhibited an ameliorative effect in experimental IBD but, prednisolone (2 mg/kg) was superior in alleviating IBD. Hence, HRS (200 mg/kg) is not as potent as prednisolone. However, the side effects of prednisolone like stress, blurred vision, anxiety, hepatic steatosis, depression and restlessness are well documented in literature[31],[32]. Hence, it can't be prescribed to geriatric patients. However, HRS did not exhibit such side effects and thus provides a ray of hope for these patients.
The present investigation provides pharmacological credence to the ethnobotanical claims of HRS mentioned in the traditional Indian system of medicine. However, fractionation of HRS needs to be carried out to determine the isolated bioactive moieties responsible for healing effect of colitis in laboratory animal.
It could be concluded from this study that HRS possess potent therapeutic potency in treatment of experimentally induced IBD in laboratory animals.
Acknowledgments
The authors would like acknowledge Dr. SS Kadam, Vice-Chancellor and Dr. KR Mahadik, Principal, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune, India, for providing necessary facilities to carry out the study. We are also thankful to the All India Council of Technical and Education (AICTE), India (Ref No. PG/GATE-SCM/2004-2005/G-39, Dated: 11/02/2011) for financial support by awarding GATE Scholarship to one of the author Mr. Kandhare Amit for the research work.
Footnotes
Foundation Project: Supported by the All India Council of Technical and Education (AICTE), India (Grant No. PG/GATE-SCM/2004-2005/G-39, Dated: 11/02/2011).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Baugmart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369(9573):1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 2.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: A review of medical therapy. World J Gastroenterol. 2008;14(3):354–377. doi: 10.3748/wjg.14.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahimi R, Mozaffari S, Abdollahi M. On the use of herbal medicines in management of inflammatory bowel diseases: A systematic review of animal and human studies. Dig Dis Sci. 2009;54:471–480. doi: 10.1007/s10620-008-0368-x. [DOI] [PubMed] [Google Scholar]
- 5.Serrame E, Lim SCY. Antitumour promoting activity of decoctions and expressed juices from Philippine medicinal plants. Philipine J Sci. 1995;124:275–281. [Google Scholar]
- 6.Kasture VS, Chopde CT, Deshmukh VK. Anticonvulsive activity of Albizzia lebbeck, Hibiscus rosa sinensis and Butea monosperma in experimental animals. J Ethnopharmacol. 2000;71:65–75. doi: 10.1016/s0378-8741(99)00192-0. [DOI] [PubMed] [Google Scholar]
- 7.Alam MM, Siddiqui MB, Hussain W. Treatment of diabetes through herbal drugs in rural India. Fitoterapia. 1990;61:240–242. [Google Scholar]
- 8.Anita Kumari Gnana AV, Palavesam A, Anbu Jeba SJ, Anandarajagopal K, Vignesh M, Parkavi J. Preliminary phytochemical and antiulcer studies of Hibiscus rosa sinensis Linn. root extracts. Int J Green Pharm. 2010;4(1):41–43. [Google Scholar]
- 9.Khandelwal KR. Practical pharmacognosy, technique and experiments. 8th ed. India, Pune: Nirali Prakashan; 2007. pp. 149–153. [Google Scholar]
- 10.Mascolo N, Izzo A, Autore G, Maiello F, Di Carlo G, Capasso F. Acetic acid-induced colitis in normal and essential fatty acid deficient rats. J Pharmacol Exp Ther. 1995;272:469–475. [PubMed] [Google Scholar]
- 11.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterol. 1989;96:795–803. [PubMed] [Google Scholar]
- 12.Dengiz GO, Gursan N. Effects of Momordica charantia L. (Cucurbitaceae) on indomethacin-induced ulcer model in rats. Turk J Gastroenterol. 2005;16(2):85–88. [PubMed] [Google Scholar]
- 13.Larrosa M, González-Sarrías A, Yáñez-Gascón MJ, Selma MV, Azorín-Ortuño M, Toti S, et al. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2009;21(8):717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Misera HP, Fridovich I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for SOD. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 15.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 16.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gasteroenterol. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 17.Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride and other halogenoalkanes or peroxidative reactions in rat liver fractions in vitro. Biochem J. 1971;123:805–814. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miranda K, Espy MG, Wink DA. A rapid and simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 19.Munoz FS, Lopez AD, Furusho JKY. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14(27):4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohsen M, Alireza G, Parvin M, Elham JS. Comparative study of Berberis vulgaris fruit extract and Berberine chloride effects on acetic acid induced colitis in rats. Iranian J Pharm Res. 2011;10(1):97–104. [PMC free article] [PubMed] [Google Scholar]
- 21.Thippeswamy BS, Mahendran S, Biradar MI, Raj P, Srivastava K, Badami S, et al. Protective effect of embelin against acetic acid induced ulcerative colitis in rats. Eur J Pharmacology. 2011;654(1):100–105. doi: 10.1016/j.ejphar.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Cho EJ, Shin JS, Noh YS, Cho YW, Hong SY, Park JH, et al. Anti-inflammatory effects of methanol extract of Patrinia scabiosaefolia in mice with ulcerative colitis. J Ethnopharmacol. 2010;78:120–127. doi: 10.1016/j.jep.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 23.Jagtap AG, Niphadkar PV, Phadke AS. Protective effect of aqueous extract of Bombax malabaricum DC on experimental models of inflammatory bowel disease in rats and mice. Indian J Exp Biol. 2011;49(5):343–351. [PubMed] [Google Scholar]
- 24.Abdolghaffari AH, Baghaei A, Moayer F, Esmaily H, Baeeri M, Monsef-Esfahani HR, et al. On the benefit of Teucrium in murine colitis through improvement of toxic inflammatory mediators. Hum Exp Toxicol. 2010;29:287–295. doi: 10.1177/0960327110361754. [DOI] [PubMed] [Google Scholar]
- 25.Hagar HH, Medany AE, Eter EE, Arafa M. Ameliorative effect of pyrrolidinedithiocarbamate on acetic acid-induced colitis in rats. Eur J Pharmacol. 2007;554:69–77. doi: 10.1016/j.ejphar.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 26.Pawar P, Gilda S, Sharma S, Jagtap S, Paradkar A, Mahadik K, et al. Rectal gel application of Withania somnifera root extract expounds anti-inflammatory and mucorestorative activity in TNBS-induced inflammatory bowel disease. BMC Complement Altern Med. 2011;11:34–42. doi: 10.1186/1472-6882-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amini-Shirazi N, Hoseini A, Ranjbar A, Mohammadirad A, Khoshakhlagh P, Yasa N, et al. Inhibition of tumor necrosis factor and nitrosative/oxidative stresses by Ziziphora clinopoides (Kahlioti); a molecular mechanism of protection against dextran sodium sulfate-induced colitis in mice. Toxicol Mech Methods. 2009;19:183–189. doi: 10.1080/15376510701533996. [DOI] [PubMed] [Google Scholar]
- 28.de Moreno de Leblanc A, Chaves S, Perdigon G. Effect of yoghurt on the cytokine profile using a murine model of intestinal inflammation. Eur J Inflamm. 2009;7(2):97–109. [Google Scholar]
- 29.Selvam R, Maheswari P, Kavitha P, Ravichandran M, Benedikt Sas, Ramchand CN. Effect of Bacillus subtilis PB6, a natural probiotic on colon mucosal inflammation and plasma cytokines levels in inflammatory bowel disease. Indian J Biochem Biophys. 2009;46:79–85. [PubMed] [Google Scholar]
- 30.Patel MA, Patel PK, Patel MB. Aqueous extract of Ficus bengalensis Linn. bark for Inflammatory bowel disease. J Young Pharm. 2010;2(2):130–136. doi: 10.4103/0975-1483.63149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pooneh S. New horizons in management of inflammatory bowel disease. Inter J Pharmacol. 2011;7:679–681. [Google Scholar]
- 32.Smith MA, Marinaki AM, Arenas M, Shobowale-Bakre M, Lewis CM, Ansari A, et al. Novel pharmacogenetic markers for treatment outcome in azathioprine-treated inflammatory bowel disease. Aliment Pharmacol Ther. 2009;30(4):375–384. doi: 10.1111/j.1365-2036.2009.04057.x. [DOI] [PubMed] [Google Scholar]