Abstract
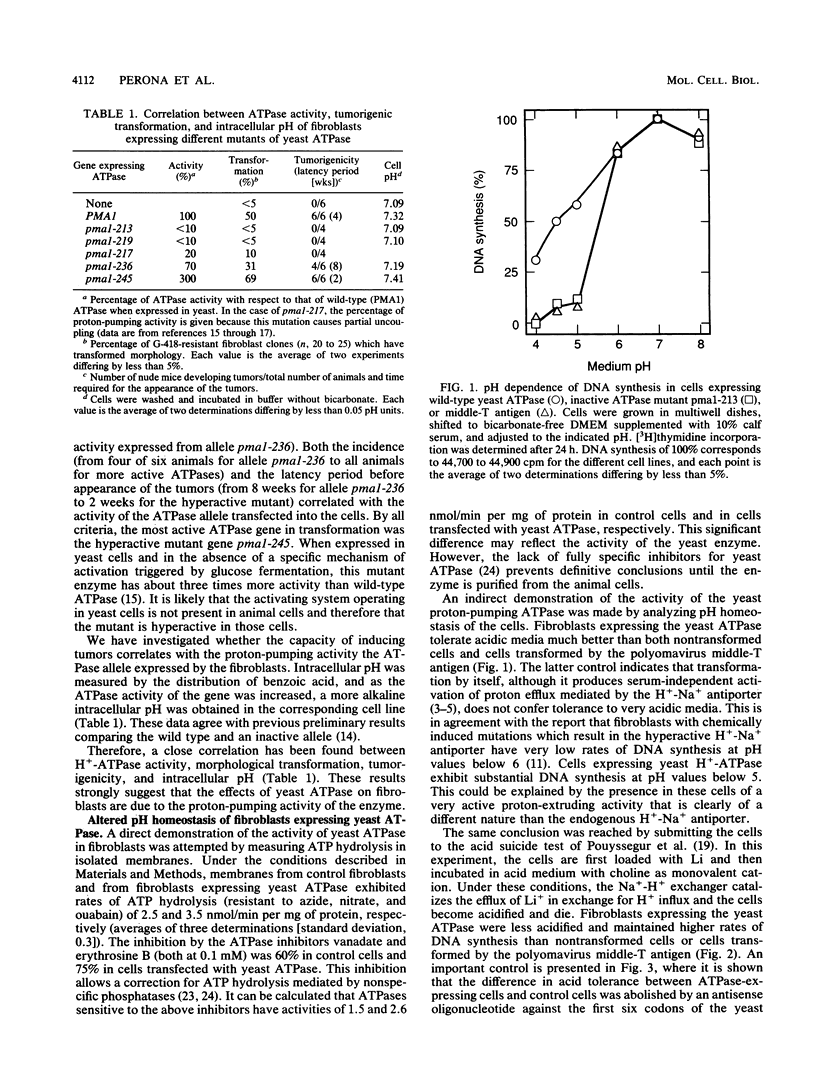
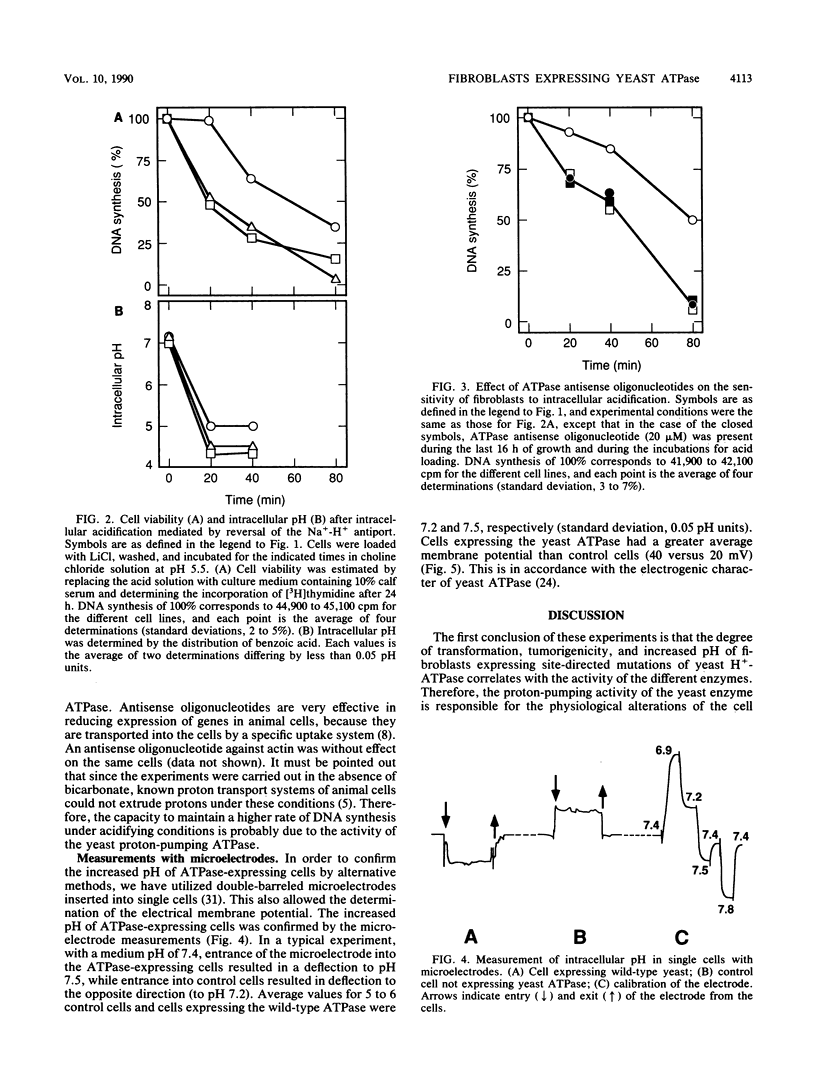
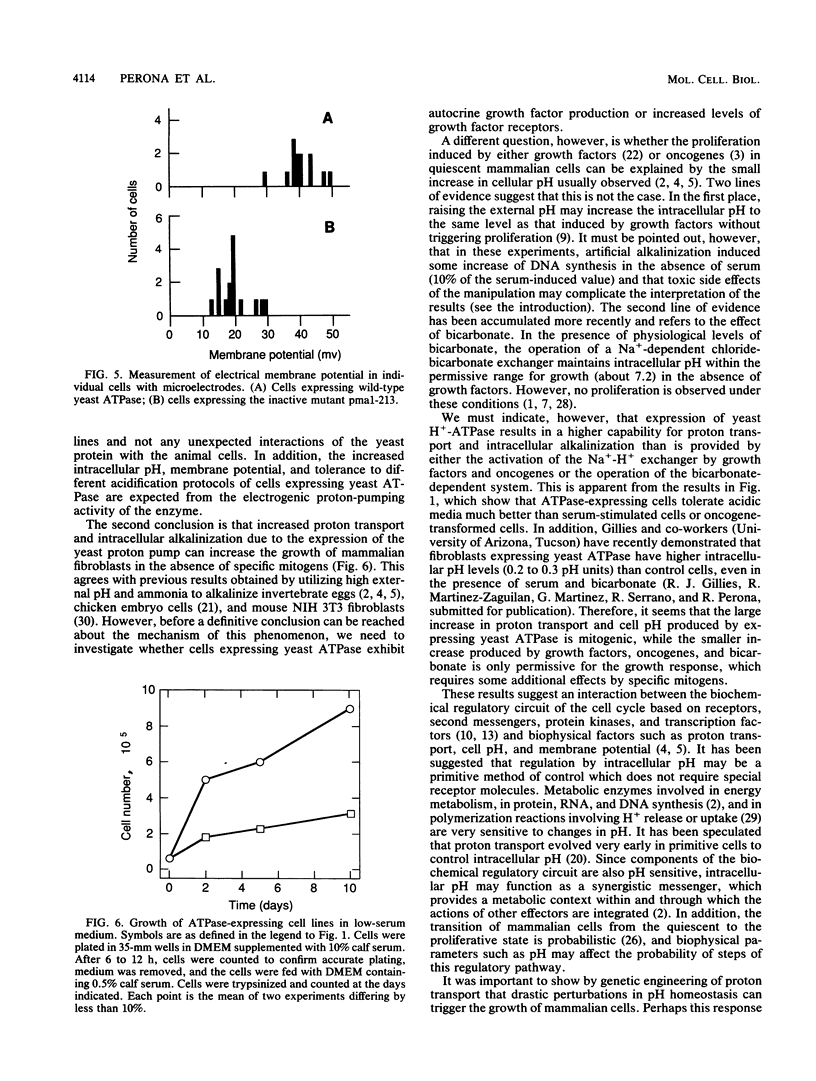
Mouse fibroblasts expressing a yeast proton-pumping ATPase show tumorigenic transformation (R. Perona, and R. Serrano, Nature (London) 334:438-440, 1988). By expressing site-directed mutations of the yeast ATPase with different levels of activity, a close correlation has been found between enzyme activity, tumorigenic transformation, and intracellular pH measured by weak-acid distribution. Fibroblasts expressing the yeast proton-pumping ATPase showed increased capability to grow at acidic pH and to resist lethal acidification mediated by reversal of the Na(+)-H+ antiporter. Measurements with microelectrodes in individual cells demonstrated electrical hyperpolarization and confirmed the increased pH of cells expressing yeast ATPase. These results indicate that the yeast enzyme expressed in mouse fibroblasts has electrogenic proton-pumping activity and that this activity deregulates fibroblast growth. This suggests a connection between the biophysical phenomena of proton transport, intracellular pH, and membrane potential and the biochemical regulatory circuits based on protein kinases and transcription factors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bierman A. J., Cragoe E. J., Jr, de Laat S. W., Moolenaar W. H. Bicarbonate determines cytoplasmic pH and suppresses mitogen-induced alkalinization in fibroblastic cells. J Biol Chem. 1988 Oct 25;263(30):15253–15256. [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984 Apr;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Doppler W., Jaggi R., Groner B. Induction of v-mos and activated Ha-ras oncogene expression in quiescent NIH 3T3 cells causes intracellular alkalinisation and cell-cycle progression. Gene. 1987;54(1):147–153. doi: 10.1016/0378-1119(87)90357-x. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rotin D., Mason M. J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta. 1989 Jan 18;988(1):73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Harguindey S., Anton Aparicio L. M., Martin Algarra S. Integrated etiopathogenesis of cancer of mucosal surfaces with emphasis on the digestive tract: an appraisal. J Biol Response Mod. 1989 Feb;8(1):1–10. [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Role of a Na+-dependent Cl-/HCO3- exchange in regulation of intracellular pH in fibroblasts. J Biol Chem. 1985 Apr 25;260(8):4877–4883. [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Defize L. H., De Laat S. W. Ionic signalling by growth factor receptors. J Exp Biol. 1986 Sep;124:359–373. doi: 10.1242/jeb.124.1.359. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Dominoes and clocks: the union of two views of the cell cycle. Science. 1989 Nov 3;246(4930):614–621. doi: 10.1126/science.2683077. [DOI] [PubMed] [Google Scholar]
- Ober S. S., Pardee A. B. Intracellular pH is increased after transformation of Chinese hamster embryo fibroblasts. Proc Natl Acad Sci U S A. 1987 May;84(9):2766–2770. doi: 10.1073/pnas.84.9.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhaensli R. D., Hilton-Jones D., Bore P. J., Hands L. J., Rampling R. P., Radda G. K. Biochemical investigation of human tumours in vivo with phosphorus-31 magnetic resonance spectroscopy. Lancet. 1986 Jul 5;2(8497):8–11. doi: 10.1016/s0140-6736(86)92558-4. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Perona R., Serrano R. Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nature. 1988 Aug 4;334(6181):438–440. doi: 10.1038/334438a0. [DOI] [PubMed] [Google Scholar]
- Portillo F., Serrano R. Dissection of functional domains of the yeast proton-pumping ATPase by directed mutagenesis. EMBO J. 1988 Jun;7(6):1793–1798. doi: 10.1002/j.1460-2075.1988.tb03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo F., Serrano R. Growth control strength and active site of yeast plasma membrane ATPase studied by site-directed mutagenesis. Eur J Biochem. 1989 Dec 22;186(3):501–507. doi: 10.1111/j.1432-1033.1989.tb15235.x. [DOI] [PubMed] [Google Scholar]
- Portillo F., de Larrinoa I. F., Serrano R. Deletion analysis of yeast plasma membrane H+-ATPase and identification of a regulatory domain at the carboxyl-terminus. FEBS Lett. 1989 Apr 24;247(2):381–385. doi: 10.1016/0014-5793(89)81375-4. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., L'Allemain G., Paris S. Cytoplasmic pH, a key determinant of growth factor-induced DNA synthesis in quiescent fibroblasts. FEBS Lett. 1985 Oct 7;190(1):115–119. doi: 10.1016/0014-5793(85)80439-7. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Sardet C., Franchi A., L'Allemain G., Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J. A., Smith F. A. The evolution of chemiosmotic energy coupling. J Theor Biol. 1976 Apr;57(2):301–312. doi: 10.1016/0022-5193(76)90003-5. [DOI] [PubMed] [Google Scholar]
- Rubin H. pH, serum and Zn++ in the regulation of DNA synthesis in cultures of chick embryo cells. J Cell Physiol. 1973 Oct;82(2):231–238. doi: 10.1002/jcp.1040820211. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rozengurt E. Na+/H+ antiport in Swiss 3T3 cells: mitogenic stimulation leads to cytoplasmic alkalinization. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7778–7782. doi: 10.1073/pnas.79.24.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. H+-ATPase from plasma membranes of Saccharomyces cerevisiae and Avena sativa roots: purification and reconstitution. Methods Enzymol. 1988;157:533–544. doi: 10.1016/0076-6879(88)57102-1. [DOI] [PubMed] [Google Scholar]
- Serrano R., Kielland-Brandt M. C., Fink G. R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986 Feb 20;319(6055):689–693. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- Shields R. Transition probability and the origin of variation in the cell cycle. Nature. 1977 Jun 23;267(5613):704–707. doi: 10.1038/267704a0. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., Cantley L. C. Mitogens and ion fluxes. Annu Rev Physiol. 1988;50:207–223. doi: 10.1146/annurev.ph.50.030188.001231. [DOI] [PubMed] [Google Scholar]
- Szwergold B. S., Brown T. R., Freed J. J. Bicarbonate abolishes intracellular alkalinization in mitogen-stimulated 3T3 cells. J Cell Physiol. 1989 Feb;138(2):227–235. doi: 10.1002/jcp.1041380203. [DOI] [PubMed] [Google Scholar]
- Williams R. J. Ion pumps and cell shapes. Trends Biochem Sci. 1988 Jul;13(7):249–249. [PubMed] [Google Scholar]
- Zetterberg A., Engström W. Mitogenic effect of alkaline pH on quiescent, serum-starved cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4334–4338. doi: 10.1073/pnas.78.7.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]


