Abstract
Objective
To study detailed pharmacognostic profile of leaves and stem of Careya arborea (C. arborea) Roxb. (Lecthyidaceae), an important medicinal plant in the Indian system of medicine.
Methods
Leaf and stem samples of C. arborea were studied by macroscopical, microscopical, physicochemical, phytochemical, fluorescence analysis of powder of the plant and other methods for standardization recommended by WHO.
Results
Macroscopically, the leaves are simple, broadly obovate in shape, acuminate apex with crenate, dentate margin, petioles (0.1–1.8 cm) long. Microscopically, the leaf showed the presence of median large size vascular bundle covered with fibrous bundle sheath, arrangement of xylem in cup shape and presence of cortical vascular bundle, patches of sclerenchyma, phloem fibers in groups and brown pigment containing cells in stem are some of the diagnostic features noted from anatomical study. Powder microscopy of leaf revealed the presence of parenchyma cells, xylem with pitted vessels and epidermis with anisocytic stomata. The investigations also included leaf surface data; quantitative leaf microscopy and fluorescence analysis. Physiochemical parameters such as loss on drying, swelling index, extractive values and ash values were also determined and results showed that total ash of the stem bark was about two times higher than leaf and water soluble extractive value of leaf and stem bark was two times higher than alcohol soluble extractive value. Preliminary phytochemical screening showed the presence of triterpenoids, saponins, tannins and flavonoids.
Conclusions
The results of the study can serve as a valuable source of information and provide suitable standards for identification of this plant material in future investigations and applications.
Keywords: Careya arborea, Microscopy, Macroscopy, Pharmacognosy, Stomata, Xylem, Phloem, Physicochemical
1. Introduction
Careya arborea (C. arborea) Roxb. (Lecthyidaceae) is commonly known as Wild Guava in English and Kumbhi in Hindi. It is a medium sized deciduous tree and widely available in India, Sri Lanka, Malay and Peninsula. The plant can be identified by its thick dark grey bark, large showy flowers and the leaves which turn red in winter. It flowers during March-April. Stem bark of C. arborea is traditionally used in the treatment of tumours, bronchitis, skin disease, epileptic fits, astringents, antidote to snake-venom, abscesses, boil and ulcer[1]. Fruits are used as decoction to promote digestion. Leaves and flowers are used in the form of paste to cure several skin diseases. It is also used as remedy for diarrhea, dysentery with bloody stools and ear pain. Leaf paste and pulp used as poultice rapidly heals ulcers and root is used for the treatment of tuberculosis and skeletal fractures[2],[3]. C. arborea is reported to possess in vitro cytotoxic activity[4], antitumor effect[5],[6], N-nitrosodiethylamine induced hepatocarcinogenesis[7], CNS depressant[8], anticoagulant[9] and antioxidant activity[10]. Leaf extract is used as an indicator in acid base titration[11]. Qualitative chemical tests revealed the presence of terpenoids, flavonoids, alkaloids, saponins and tannins in the stem bark of C. arborea Roxb[12]. However, available literature revealed that no pharmacognostic study has been carried out on the plant except on stem bark; hence the present investigation was under taken. The object of present study is to evaluate various pharmacognostical parameters such as macroscopic, microscopy, physicochemical, fluorescence and phytochemical studies of the plant.
2. Materials and methods
2.1. Plant material
C. arborea plant was collected from the Bauli jungle, Rewa district, India in the month of March. The plant was identified by Dr. Tarique Hussain, Taxonomist, National Botanical Research Institute, Lucknow, India and voucher specimen of the plant (No. UIOP/M-1103) was deposited at the herbarium section of departmental museum for future reference.
2.2. Pharmacognostic study
Fresh leaves and stem were taken for morphological and histological studies. Coarse powder (60 #) was used to study microscopical characters, physicochemical parameters and phytochemical investigation. For the microscopical studies, transverse sections of leaves and stem were prepared and stained as per standard procedure[13]–[15]. The powder microscopy was performed according to the method of Khandelwal[15].
2.3. Physicochemical and phytochemical analysis
Physicochemical values such as percentage of ash values and extractive values were determined according to the well established official method and procedure[16],[17]. Preliminary screening was carried out using the standard procedure described by Khandelwal[15].
2.4. Florescence analysis
Powdered leaf and bark material were treated with various chemical reagents and exposed to visible, ultraviolet light (Short UV) to study their fluorescence behavior[18],[19].
3. Results
3.1. Macroscopic characteristics
Macroscopically, the fresh leaf of C. arborea is 15 to 22 cm long, 7 to 12 cm wide and petiole 0.1 to 1.8 cm in length, simple, glabrous, broadly obovate in shape, acuminate apex with crenate, dentate margin and green in color (Figure 1). Flower yellowish white, ill smelling, sessile; fruits large, round, green and fleshy; seed embedded in the fleshy pulp of the fruit. Bark dark grey exfoliating in thin strips.
Figure 1. Macroscopic characteristics of C. arborea Roxb.
3.2. Microscopical characteristics
3.2.1. Leaf microscopy
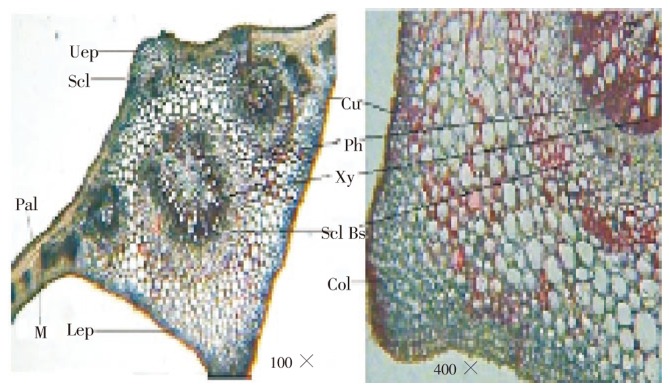
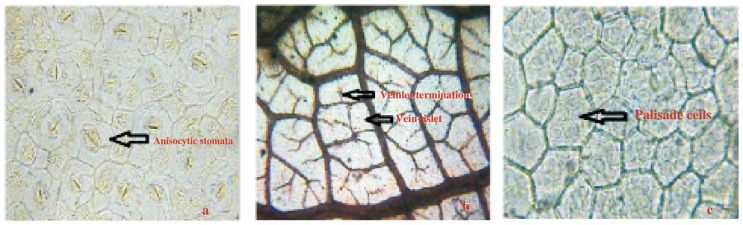
TS of leaf passing through midrib region shows slight upper notch and large notch at lower surface (Figure 2). Upper and lower surface of the leaf consists of rectangular thin walled epidermis, covered with thick cuticle followed by collenchymatous ground tissue; palisade cells reached up to the upper notched region. Palisade cell is single layered; midrib region show one median large size vascular bundle and two lateral vascular bundle. Vascular bundles are covered with fibrous bundle sheath which is very broad on lower side and 1 to 2 layers broad towards upper side. One group of sclerenchyma is present at upper notched side above the median vascular bundle. Xylem is arranged in cup shaped and surrounded by phloem facing toward the lower side. Xylem consists of vessels, tracheids, fibers and xylem parenchyma; inside the cup; cells are parenchymatous. Lateral vascular bundle also shows sclerenchymatous bundle sheath which encircles the vascular bundle. Sclerenchymatous bundle sheath is broad on both surfaces and only 1 or 2 layered on lateral side. TS passing through lamina region showed single layered palisade cells followed by several layers of spongy mesophyll embedded with lateral vascular bundles. C. arborea leaf surface shows the anisocytic stomata (Figure 3a) which is characteristic of Family Lecthyidaceae. Leaf surface also shows the presence of veins, vein islets, vein terminations (Figure 3b) and palisade cells (Figure 3c). Leaf constants such as stomatal number, stomatal index, palisade ratio, vein-islet number and veinlet terminations number were measured. The results are shown in Table 1.
Figure 2. T.S. of C. arborea leaf.
Uep: Upper epidermis; Lep: Lower epidermis; Cu: Cuticle; Pal: Palisade cell; Scl: Sclerenchyma; Col: Collenchyma; M: Mesophyll; Scl BS: Sclerenchymatous bundle sheath; Ph: Phloem; Xy: Xylem.
Figure 3. Leaf surface of C. arborea.
a: Stomata; b: Vein-islet and veinlet termination; c: Palisade cells.
Table 1. Leaf constants (at 100X).
| S. No. | Parameters | Value (in 1 mm2 area) |
| 1 | Stomata number, upper surface | 28.00 |
| 2 | Stomata number, lower surface | 188.00 |
| 3 | Stomatal index, upper surface | 8.26 |
| 4 | Stomatal index, lower surface | 31.18 |
| 5 | Vein-islet number | 6.00–8.00 |
| 6 | Veinlet termination number | 7.00–9.00 |
| 7 | Palisade ratio | 6.00–8.00 (per cell) |
3.2.2. Stem microscopy
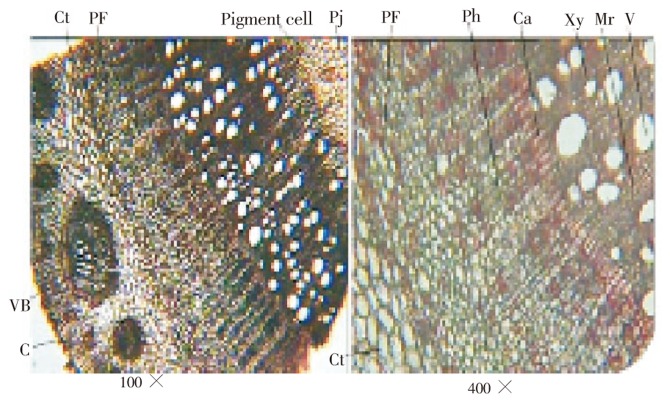
TS of stem shows 3 to 4 layered outer most cork; cork cambium is 1 to 2 layered but not continuous; cortex collencymatous is embedded with cortical vascular bundles (Figure 4). Cortical vascular bundles of various shape and size are present and surrounded by sclerenchymatous bundle sheath. Amphicribal vascular bundles are present. Most of cortical cells are pitted; endodermis is not distinct; pericycle is present in patches of sclerenchyma. Phloem is very broad, consisting of phloem fibers in groups and in concentric bands, sieve tubes, companion cells and phloem parenchyma followed by vascular cambium 4 to 5 layered and 4 to 5 cells broad in continuous layers. Xylem is present in form of continuous ring and consists of vessels, tracheids, fibers and xylem parenchyma; medullary rays 1 to 2 cells broad and radiating; vessels are mostly solitary towards the centre and in group of 2 to 4 towards the periphery. Central portion is occupied by collenchymatous pith; most of the pith cells are pitted, some cells are filled with brown content.
Figure 4. T.S. of C. arborea stem.
C: Cork; Ct: Cortex; VB: Vascular bundle; PF: Pericyclic fiber; Ph: Phloem; Ca: Cambium; Xy: Xylem; Mr: Medullary rays; V: Vessels; Pi: Pith.
3.3. Powder microscopic characteristics
3.3.1. Leaf
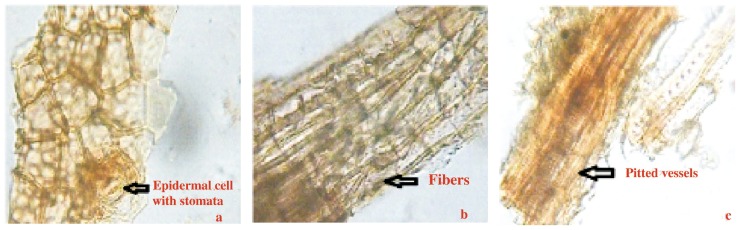
The powder plant material is greenish in color, showing fragments of parenchyma, palisade cells, fragments of epidermal cells along with stomata (Figure 5a), lignifiedfied fibers (Figure 5b) and vessels having simple pits (Figure 5c).
Figure 5. Powder characteristics of C. arborea leaf.
3.3.2. Stem
The stem shows fragments of cork cells, fibers, and parenchymatous cells.
3.4. Preliminary phytochemical screening
Preliminary phytochemical screening of leaf mainly revealed the presence of triterpenoids, saponins, tannins and flavonoids.
3.5. Physicochemical parameter
Physicochemical analysis of leaf and stem bark powder viz. foreign matter, loss on drying, swelling index, ash value and extractive value are presented in Table 2. The fluorescence analysis of C. arborea leaf and stem bark under day light and UV (Short, 254 nm) light is recorded in Table 3.
Table 2. Physico-chemical parameters.
| Physico-chemical constant | Leaf | Stem bark |
| Foreign matter (% w/w) | 0.20 | 0.85 |
| Loss on drying (% w/w) | 3.20 | 6.20 |
| Total ash (% w/w) | 6.00 | 11.2 |
| Water soluble ash (% w/w) | 2.20 | 1.80 |
| Acid insoluble ash (% w/w) | 1.40 | 0.80 |
| Water soluble (% w/w) | 18.40 | 14.8 |
| Alcohol soluble (% w/w) | 8.20 | 7.40 |
| Swelling index (mL) | 4.70 | 3.73 |
Table 3. Fluorescence analysis of leaf and stem bark powder of C. arborea.
| Treatment | Day light |
UV light (Short, 254 nm) |
||
| Leaf | Stem bark | Leaf | Stem bark | |
| Powder (P) as such | Pale green | Buff | Green | Grayish green |
| P + 1N NaOH in Methanol | Citrine green | Buff | Green | Herbage green |
| P + 1N HCl | Pale green | Brown | Jelly green | Green |
| P + 1N NaOH in water | Honey | Rust | Green | Herbage green |
| P + HNO3 (1:1) | Yellowish brown | Green | Brown | Fluorescent green |
| P + H2SO4 (1:1) | Green | Green | Brown | Fluorescent green |
4. Discussion
Ethnomedically, the leaves and stem bark of this plant were used by local people in the treatment of various disease conditions without standardization. The standardization of a crude drug is an integral part for establishing its correct identity. Before any crude drug can be included in an herbal pharmacopoeia, pharmacognostic parameters and standards must be established. Microscopic method is one of the simplest and cheapest methods to start with for establishing the correct identity of the source materials[20]–[24]. The pharmacognostic standards for leaves and stem of C. arborea are carried out for the first time in this study. The macroscopical characters of the leaf and stem can serve as diagnostic parameters. Microscopical studies indicated the presence of median large size vascular bundle and cup shaped xylem in leaf. Presence of cortical vascular bundle, patches of pericyclic fibers and brown pigment containing cells are the characteristics of the plant. Ash values and extractive values can be used as reliable aid for detecting adulteration. These studies help in the identification of the plant materials[25]. Percentage extractives and ash analysis were carried out and results showed that total ash of stem bark is about two times higher than leaf and water soluble extractive value of leaf and stem bark was two times higher than alcohol soluble extractive value. Ash values of drug give an idea of earthy matter or the inorganic composition and other impurities present along with drug. Extractive values are primarily useful for the determination of exhausted and adulterated drugs. Extractive values are also useful to evaluate the chemical constituents present in the crude drug and also help in estimation of specific constituents soluble in particular solvents[26],[27]. The fluorescent analysis under day light and UV light by treatment with different chemical reagents showed different color. Results of fluorescent analysis of the leaves and stem bark showed pale green color for leaf and buff color for stem bark powder as such in day light, green color for leaf and grayish green color for stem bark powder as such in UV light, green color for leaf and herbage green color for stem bark powder mounted in 1 N NaOH in methanol, as such in UV light while citrine green color for leaf and buff color for stem bark as such in day light. This analysis suggests that, leaves and stem bark extract of C. arborea probably contain active agent(s) and this provides the basis for their folkloric use as a cure for some human ailments.
In conclusion, these parameters which are being reported for the first time, could be useful in setting some diagnostic indices for the identification and preparation of a monograph of the C. arborea plant.
Acknowledgments
Authors thank, Vice Chancellor, C.S.J.M. University, Kanpur, for providing facilities to conduct this research work and Dr. Sayyada Khatoon, Scientist E1, NBRI, in identifying the microscopical features. Authors also acknowledge Dr. S. N. Mishra and Mr. B. L. Pandey, Rewa, (M.P.) in collection of the plant material.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Nadkararni KM. Medicinal plants of India. Dehradun: Reprint Prakashan; 2004. p. 87. [Google Scholar]
- 2.Kirtikar KR, Basu BD. Indian medicinal plants. 2nd ed. Dehradun, India: Bishen Singh Mahendra Pal Singh; 1975. pp. 894–895. [Google Scholar]
- 3.Kumar Satish BN, Swami Vrushabendra BM, Kumar GK, Gobinda B. Review on Careya arborea Roxb. Int J Res In Ayurveda Pharm. 2010;1(2):306–315. [Google Scholar]
- 4.Senthil Kumar N, Badami S, Cherian MM, Hariharapura RC. Potent in vitro cytotoxic and antioxidant activity of Careya arborea bark extract. Phytother Res. 2007;21(5):492–495. doi: 10.1002/ptr.2118. [DOI] [PubMed] [Google Scholar]
- 5.Natesan S, Badami S, Dongre SH, Godavarthi A. Antitumor activity and antioxidant status of the methanol extract of Careya arborea bark against Dalton's lymphoma ascites induced ascetic and solid tumor in mice. J Pharmacol Sci. 2007;103:12–23. doi: 10.1254/jphs.fp0060907. [DOI] [PubMed] [Google Scholar]
- 6.Sambath Kumar R, Sivakumar T, Mazumder UK, Malaya Gupta. Antitumor effect of Careya arborea against Ehrlic h ascites carcinoma with reference to lipid peroxidation and enzymatic and non enzymatic antioxidant system in Swiss albino mice. Int J Oriental Pharm Exp Med. 2008;8:154–163. [Google Scholar]
- 7.Sambath Kumar R. The antioxidant defense system induced by methanol extract of Careya arborea on N-nitrosodiethylamine-induced hepatocarcinogenesis. J Comple Integr Med. 2008;5(1):10. [Google Scholar]
- 8.Kumar RS, Sundram RS, Shiv Kumar P, Nethaji R, Senthil V, Murthy NV, et al. CNS activity of the methanol extract of Careya arborea in experimental animal model. Bangladesh J Pharmacol. 2008;3:36–43. [Google Scholar]
- 9.Varadharajan SD, Josey C, Kuppusamy AK, Andichettiar T, Muthuswamy UM, Sivashan M, et al. Anticoagulant activity of methanolic extract of Careya arborea Roxb. Bark. Int J Pharm Sci Bio. 2010;1(2):93–95. [Google Scholar]
- 10.Wadkar KA, Magdum CS. Evaluation of total phenolic content, flavonoid content and antioxidant activity of stem bark of Careya arborea Roxb. Res J Pharmacogn Phytochem. 2010;2(1):49–51. [Google Scholar]
- 11.Wadkar KA, Magdum CS, Kondawar MS. Use of Careya arborea Roxb. leaf extract as an indicator in acid base titration. Res J Pharm Technol. 2008;1(4):535–536. [Google Scholar]
- 12.Wadkar KA, Magdum CS. Pharmacognostic profiles of bark of Careya arborea roxb. J Pharmacogn Phytother. 2009;1(5):64–66. [Google Scholar]
- 13.Brain KR, Turner TD. Bristol: Wright-Scientechnica; 1975. The practical evaluation of phytopharmaceuticals; pp. 4–9. [Google Scholar]
- 14.Pandya DJ, Desai TR, Nadpara NP, Mehta HA, Modi AM. Pharmacognostic study and establishment of quality parameters of leaves of Bombax insigne Linn. Int J Pharmacogn Phytochem Res. 2010;2(3):1–5. [Google Scholar]
- 15.Khandelwal KR. Practical pharmacognosy. 19th ed. Pune: Nirali publication; 2008. pp. 149–164. [Google Scholar]
- 16.Ministry of Health and Welfare . Indian Pharmacopeia. 4th ed. New Delhi: Government of India, Ministry of Health and Welfare, Controller of Publications; 1996. pp. A53–A54. [Google Scholar]
- 17.WHO . Quality control methods for medicinal plant material. Geneva: WHO; 1992. pp. 22–34. [Google Scholar]
- 18.Edwin S, Joshi SB, Jain DC. Comparative pharmacognostic studies on root powder of Plumbago zeylanica and Plumbago rosea. Indian J Nat Prod. 2008;2:27–29. [Google Scholar]
- 19.Pratt RJ, Chase CR. Flourescence of powdered vegetable drug with particular reference to development of a system of identification. J Am Pharm Assoc. 1949;38:324–333. doi: 10.1002/jps.3030380612. [DOI] [PubMed] [Google Scholar]
- 20.Singh S, Machawal L, Chauhan MG. Pharmacognostic study of male leaves of Trichosanthes dioica Roxb. with special emphasis on microscopic technique. J Pharmacogn Phytother. 2010;2(5):71–75. [Google Scholar]
- 21.Kapoor M, Jasani N, Acharya N, Acharya S, Kumar V. Phytopharmacological evaluation and anti-asthmatic activity of Ficus religiosa leaves. Asian Pac J Trop Med. 2011;4(8):642–644. doi: 10.1016/S1995-7645(11)60163-6. [DOI] [PubMed] [Google Scholar]
- 22.Jothimani R, Karthikeyan K, Balasundaram J. Phytochemical and pharmacological evaluation of prop roots of Pandanus fascicularis Lam. Asian Pac J Trop Med. 2011;4(8):649–653. doi: 10.1016/S1995-7645(11)60165-X. [DOI] [PubMed] [Google Scholar]
- 23.Subramanion Jo Thy Lachumy, Sasidharan S, Sumathy V, Zuraini Z. Pharmacological activity, phytochemical analysis and toxicity of methanol extract of Etlingera elatior (torch ginger) flowers. Asian Pac J Trop Med. 2010;3(10):769–774. [Google Scholar]
- 24.Bragadeeswaran S, Thangaraj S, Rajiv CR, Balaji D. Pharmacological and biomedical properties of sea anemones Paracondactylis indicus, Paracondactylis sinensis, Heteractis magnifica and Stichodactyla haddoni from East coast of India. Asian Pac J Trop Med. 2011;4(9):722–726. doi: 10.1016/S1995-7645(11)60181-8. [DOI] [PubMed] [Google Scholar]
- 25.Nayak BS, Patel KN. Pharmacognostic studies of the Jatropha curcas leaves. Int J PharmTech Res. 2010;2(1):140–143. [Google Scholar]
- 26.Thomas S, Patil DA, Patil AG, Chandra N. Pharmacognostic evaluation and physicochemical analysis of Averrhoa carambola L. fruit. J Herb Toxicol. 2008;2(2):51–54. [Google Scholar]
- 27.Kumar S, Kumar V, Prakash OM. Pharmacognostic study and anti-inflammatory activity of Callistemon lanceolatus leaf. Asian Pac J Trop Biomed. 2011;1(3):177–181. doi: 10.1016/S2221-1691(11)60022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]