Abstract
Objective
To access the in vitro antibacterial activity of Hibiscus rosa-sinensis (H. rosa- sinensis) flower extract against human pathogens.
Methods
Antibacterial activity was evaluated by using disc and agar diffusion methods. The protein was run through poly acrylmide gel electrophoresis to view their protein profile.
Results
The results showed that the cold extraction illustrates a maximum zone of inhibition against Bacillus subtillis (B. subtillis), Escherichia coli (E. coli) viz., (17.00 ± 2.91), (14.50 ± 1.71) mm, followed by hot extraction against, E. coli, Salmonella sp. as (11.66 ± 3.14), (10.60 ± 3.09) mm. In methanol extraction showed a highest zone of inhibition recorded against B. subtillis, E. coli as (18.86 ± 0.18), (18.00 ± 1.63) mm pursued by ethanol extraction showed utmost zone of inhibition recorded against Salmonella sp. at (20.40 ± 1.54) mm. The crude protein from flower showed a maximum inhibitory zone observed against Salmonella sp., E. coli viz., (16.55 ± 1.16), (14.30 ± 2.86) mm. The flower material can be taken as an alternative source of antibacterial agent against the human pathogens.
Conclusions
The extracts of the H. rosa-sinensis are proved to have potential antibacterial activity, further studies are highly need for the drug development.
Keywords: in-vitro, Antibacterial activity, Hibiscus rosa- sinensis, Protein, Human pathogen
1. Introduction
Nature has been a source of medicinal agents for thousands of years and a striking number of modern drugs have been isolated from natural source, many based on their use in traditional medicines or phytomedicines. Over the years, World Health Organization (WHO) advocated traditional medicines as safe remedies for aliments of both microbial and non microbial origins[1]. Over 50% of all modern clinical drugs are of natural product origin and natural products play an important role in drug development programs in the pharmaceutical industry. Some antibiotics have become almost archaic because of drug resistant and consequently new drugs must be sought, for which herbal treatment is one possible way to treat diseases caused by multi drug resistant bacteria.
It is well known that plants, through lacking the typical immune response, have an in-built system for production against biotic and abiotic, stress conditions. Since plants have coevolved with pathogens, they understandably have also developed the chemical protection pathways against the parasitic organisms. Therefore, it is reasonable to expect a verity of plant compounds with specific as well as general antimicrobial activity and antibacterial potential. The plants Hibiscus rosa-sinensis (H. rosa- sinensis) belongs to the family Malvaceae. Traditionally the flowers can be used as anti asthmatic agents[2],[3]. Many chemical constituents such as cyanidin, quercetin, hentriacontane, calcium oxalate, thiamine, riboflavin, niacin and ascorbic acids have been isolated from this plant. Resistance towards reveling antibiotics having become widespread among bacteria and fungi, new class of antimicrobial substances are urgently required. There are several studies which reveal the presence of such compounds with antimicrobial properties in various plant parts[4],[5]. The petals have some protective mechanism against microbial attack in most of the plants. The H. rosa-sinensis flower petals of a large number of plant species growing in the vicinity of our environment were screened for their antibacterial activity.
The present study has been designed to determine the role of flower in H. rosa-sinensis extract in the in-vitro antibacterial activity against human pathogens viz., Gram positive bacteria [Staphylococcus aureus (S. aureus), Streptococcus, Bacillus subtillis (B. subtillis)] and Gram negative bacteria [Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa) and Salmonella sp.] and view their protein profile.
2. Materials and methods
2.1. Collection of samples
The flowers were initially separated from the main plants body and rinsed with distilled water and shade dried and then homogenized into fine powder and stored in air tight bottles.
2.2. Extraction of aqueous component
2.2.1. Cold extraction
A total of 10 g of dried flower was soaked in 50 mL of cold water in a conical flask for 24 h and then filtered off using sterile Whatman No. 1 filter paper into a sterile conical flask and evaporated by using solvent distillation apparatus. The extract was got with the help of muslin cloth and centrifuged at 10 000 rpm for 5 min. The supernatant was obtained and stored at 4 °C for further use[6].
2.2.2. Hot extraction
A total of 10 g of dried flower was soaked in 50 mL of hot water which was then boiled for 30 min and kept for 24 h undisturbed and then filtered through sterile filter paper, evaporated by using solvent distillation apparatus. The extract was got with the help of a muslin cloth, centrifuged at 10 000 rpm for 5 min and the supernatant was stored at 4 °C for further use[7].
2.3. Methanol and ethanol extractions
A total of 10 g of flower air dried powder was weighed and was placed in 100 mL of organic solvents (methanol and ethanol) in a conical flask and then kept in a rotary shaker at 190-220 rpm for 24 h. And then it was filtered with the help of muslin cloth and centrifuged at 10 000 rpm for 5 min. The supernatant was collected and the solvent was evaporated by solvent distillation apparatus to make the final volume of one-fourth of the original volume, giving a concentration of 40 mg/mL. It was stored at 40 °C in air tight bottles for further studies[8],[9].
2.4. Extraction of protein
One gram of dry weight of the flower was dissolved into 2 mL of phosphate buffer and mixed nicely. Centrifuged at 10 000 rpm for 15 min at 4 °C, supernatant was taken. Further, the equal volume of acetone was added and centrifuged at 10 000 rpm for 15 min at 4 °C. The pellet was taken and dissolved in phosphate buffer. This was taken as pure sample. The process was preceded in the same way as above for the extraction of crude sample which was dividing of acetone[10].
2.5. Protein profile
The estimated protein was resolved in poly acrylmide gel electrophoresis (PAGE) in which the sample was diluted with sample buffer in the ratio of 1:1 in 2× sample buffer at 100 volt.
2.6. Test microorganism for antibacterial assay
For the in vitro antibacterial assay the following human bacterial pathogens were studied such as S. aureus, Streptococcus, B. subtillis, E. coli, P. aeruginosa and Salmonella sp.
2.7. Culture preparation for antibacterial assay
The cultures were grown on nutrient agar at 37 °C for 18 h and the colonies were suspended in saline (0.85% Nacl) and its turbidity was adjusted to 0.5 Mac Farland standards (108 CFU/mL). This saline culture preparation was used to inoculate the plates[11].
2.8. Anti bacterial assay
2.8.1. Disc diffusion
In the agar disc diffusion method the test compounds, i.e. the flower aqueous and organic extract were introduced into a disc 0.5 mm (hi-media) and then allowed to dry. Thus the disc was completely saturated with the test compound at concentration of 40 mg/mL. Then these discs were placed directly on the surface of Muller Hinton agar plates, swabbed with the test organism and the plates were incubated at 37 °C for 24 h.
2.8.2. Agar well diffusion method
Muller Hinton agar plates were prepared and wells of 5 mm were cut and swabbed with different cultures. The cut wells were then filled with 50 µL of both aqueous and solvent extracts of flowers and leaves separately and the plates were kept for incubation at 37 °C for 24 h[12].
2.9. Statistical analysis
The results were analyzed by using standard deviation (SD) statistical method[8].
3. Results
3.1. Aqueous extraction
The results clearly showed that cold extractions of flower inhibited B. subtillis, E. coli with (17.00 ± 2.91), (14.50 ± 1.71) mm, respectively. Hot extraction showed an antibacteria activity against E. coli, Salmonella sp. at (11.66 ± 3.14), (10.60 ± 3.09) mm. The hot and cold extractions had very low inhibition effects against B. subtillis, P. aeruginosa at (1.00 ± 0.81), (0.00 ± 0.00) mm and Staphylococcus sp., Salmonella sp. (8.00 ± 1.63), (8.76 ± 2.71) mm (Table 1).
Table 1. Antibacterial activity of cold and hot aqueous extract of H. rosa-sinensis in agar and disc diffusion method (Mean ± SD) (mm).
| Test organisms | Agar diffusion method |
Disc diffusion method |
||
| Cold aqueous extract | Hot aqueous extract | Cold aqueous extract (mm) | Hot aqueous extract | |
| S. aureus | 11.43 ± 2.85 | 0.00 ± 0.00 | 8.00 ± 1.63 | 1.71 ± 1.47 |
| Streptococcus sp. | 13.80 ± 3.23 | 5.44 ± 2.74 | 11.77 ± 4.22 | 3.50 ± 3.45 |
| B. subtillis | 17.00 ± 2.94 | 1.66 ± 2.35 | 9.00 ± 2.16 | 1.00 ± 0.81 |
| E. coli | 14.50 ± 1.71 | 11.60 ± 3.14 | 13.66 ± 3.85 | 9.00 ± 2.44 |
| Salmonella sp. | 13.15 ± 1.71 | 10.66 ± 3.09 | 8.76 ± 2.71 | 10.00 ± 2.44 |
| P. aeruginosa | 14.00 ± 3.26 | 0.00 ± 0.00 | 13.56 ± 2.36 | 2.88 ± 1.50 |
3.2. Solvent extraction
Methanol extraction showed a highest zone of inhibition recorded against B. subtillis, E. coli as (18.86 ± 0.18), (18.00 ± 1.63) mm followed by ethanol extraction showed maximum zone of inhibition recorded against Salmonella sp. with (20.4 ± 1.54) mm (Table 2).
Table 2. Antibacterial activity of solvent extraction in H. rosa-sinensis in agar and disc diffusion method (Mean ± SD) (mm).
| Test organisms | Agar diffusion method |
Disc diffusion method |
||
| Methanol extract | Ethanol extract | Methanol extract | Ethanol extract | |
| S. aureus | 0.00 ± 0.00 | 10.00 ± 1.84 | 0.66 ± 0.94 | 13.27 ± 0.75 |
| Streptococcus sp. | 14.18 ± 2.80 | 10.16 ± 1.64 | 16.26 ± 1.67 | 12.66 ± 1.24 |
| B. subtillis | 18.86 ± 0.18 | 12.80 ± 2.75 | 16.50 ± 1.22 | 12.41 ± 1.62 |
| E. coli | 17.16 ± 1.64 | 0.33 ± 0.47 | 18.00 ± 1.63 | 0.00 ± 0.00 |
| Salmonella sp. | 0.00 ± 0.00 | 15.30 ± 2.35 | 0.00 ± 0.00 | 20.40 ± 1.54 |
| P. aeruginosa | 0.00 ± 0.00 | 16.30 ± 0.94 | 0.00 ± 0.00 | 15.58 ± 0.54 |
3.3. Protein assay
The crude protein from flower shows a maximum inhibitory zone observed against Salmonella sp, E. coli viz., (16.55 ± 1.16) and (14.30 ± 2.86) mm. Pure protein showed zone of inhibition against Staphylococcus sp, E. coli such as (11.4 ± 1.74), (12.25 ± 0.97) mm (Table 3).
Table 3. Antibacterial activity of protein in H. rosa-sinensis in agar and disc diffusion method (Mean ± SD) (mm).
| Test organisms | Agar diffusion method |
Disc diffusion method |
||
| Crude protein | Pure protein | Crude protein | Pure protein | |
| S. aureus | 0.00 ± 0.00 | 11.00 ± 2.94 | 1.00 ± 0.81 | 11.40 ± 1.74 |
| Streptococcus sp. | 12.04 ± 0.86 | 1.76 ± 0.88 | 13.06 ± 0.89 | 2.80 ± 0.57 |
| B. subtillis | 0.00 ± 0.00 | 1.00 ± 1.41 | 1.06 ± 0.09 | 4.16 ± 0.60 |
| E. coli | 13.70 ± 0.87 | 12.25 ± 0.97 | 14.30 ± 2.86 | 11.00 ± 1.63 |
| Salmonella sp. | 15.21 ± 2.28 | 1.86 ± 0.65 | 16.55 ± 1.16 | 4.26 ± 0.73 |
| P. aeruginosa | 11.50 ± 2.28 | 1.86 ± 0.65 | 13.01 ± 1.63 | 2.44 ± 1.02 |
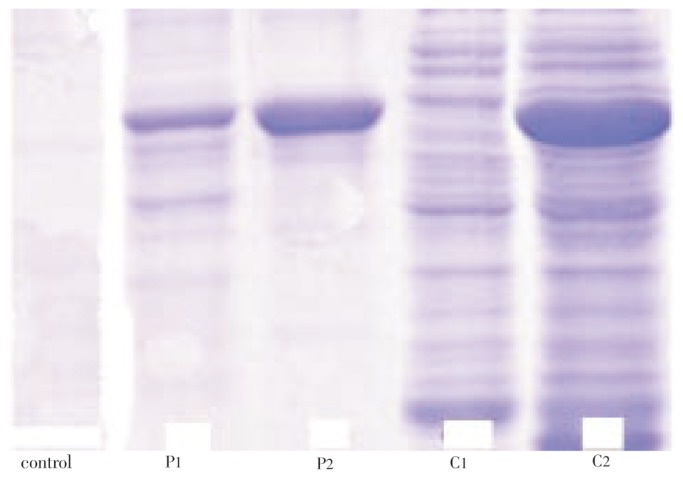
3.4. Protein profile
The crude and pure H. rosa-sinensis flower proteins were run in PAGE. The different band patterns are showed in Figure 1. The gel shows a different band separation, the crude protein shows a thick band and more bands were observed.
Figure 1. Protein profile from H. rosa-sinensis flower.
P-1 and P-2: pure protein; C-1 and C-2: Crude protein
4. Discussion
The knowledge of medicinal property of plants has been accumulated in the course of many centuries. The local inhabitants have inherited rich traditional knowledge on the use of many plants or plant parts for treatment of common disease. Medicinal plants provide accessible and culturally relevant sources of primary health care. The remedies based on these plants often have minimal side effect[13]. The bioactive substances in plants are produced as secondary metabolites, which may not only be developmental stage specific but also organ and tissue specific. While plant leaf, stem and root extracts have been widely evaluated for bioactive compounds, screening of plant flower has not been extensive. Secondary metabolites belonging to polyketide and nonribosomal peptide families constitute a major class of natural products with diverse biological functions and they have a variety of pharmaceutically important properties. Experimental studies have shown that the biosynthetic machanism for polyketide and nonribosomal peptides involves multi-functional megasynthases[14].
The antibacterial activities of H. rosa-sinensis flower petals were carried out. Most of the extract shows an antibacterial activity against the human pathogens such as E. coli, B. subtillis, P. aeruginosa, S. aureus, Streptococcus sp. Salmonella sp. All the extracts of flower have shown the activity. Investigations were carried out of plant materials as alternative sources of antibacterial agents. It has become more common over the past few years, due to the increased rate of development of antibiotic resistance organism. The inhibition of bacterial growth in-vitro by the extracts of flower could be due to the presence of some active compounds in the extracts. These active compounds may act alone or in combination to inhibit bacterial growth. The crude extracts containing multiple organic components including flavonoids, tannins, alkaloids, triterpenoids, all of which are known to have antibacterial affects. Flower extract contain phenolics compounds like tannins that are very good antimicrobial agent[15]. Thus it may be summarized that the class of natural compounds must exhibit the antibacterial activity. The metabolites have been shown to be responsible for various therapeutic activities of medicinal plants[16]. Flavonoids especially are known to be effective antimicrobial agent against a wide array of microorganisms. The activity is attributed to their ability to complex with extra cellular and soluble proteins and with bacterial cell wall[17]. There are several reports published on antibacterial activity of different herbal extracts[18]–[25]. It supports the earlier investigation that the tannins isolated from the flower possess remarkable toxic activity against bacteria and may assume pharmacological importance[26]–[38]. Many antimicrobial screening studies use a relatively small number of microorganisms for testing. It is possible that these plant materials contain antibacterial compounds against pathogenic bacteria other than those tested in this study. In addition, the lack of activity may be because of degradation of active chemicals during the drying process, the extraction process.
In the present investigation flower extracts from H. rosa-sinensis were screened for antibacterial activity against human pathogenic bacterial strains. Most of the extracts have shown antibacterial activity against these pathogens. E. coli are common member of the normal flora of large intestine. It is predominant facultative organism in the gastrointestinal tract and colonizes the tract within hours or few days. It is responsible for causing diarrhea which is characterized by rapid onset of watery non bloody fluid. Pseudomonas sp. is the epitome of an opportunistic pathogen to human. It causes urinary tract infection, respiratory system infection, dermatitis soft tissue infection, gastrointestinal infection and a variety of systemic infection. S. aureus is a facultative anaerobe that grows by aerobic respiration or by fermentation which yields lactic acid. These are pathogenic to human beings. They cause a wild range of superlative infection as well as food poisoning and toxic shock syndrome. Salmonella sp. includes a large number of pathogens of human beings as well as mammals. These are pathogenic when acquired by oral route. Broadly they may cause enteric fever, septicemia and enteritis. The enteric fever and septicemia are caused by thousand of Salmonella. Thus the plant extracts can be used as an important antibiotic to cure above mentioned disorders caused by the different strains of bacteria. The present studies conclude these extract could inhibit human pathogens growth[39]–[41]. The results are encouraging but precise assessment is utterly necessary before being situate in practice as well as the most active extracts can be subjected to isolation of the therapeutic antimicrobials and undergo secondary pharmacological evaluation.
Acknowledgments
We would like to acknowledge Alagappa University, School of Marine Sciences, Thondi Campus and PSGR Krishnammal College for Women, Department of Plant Biology and Plant Biotechnology, Coimbatore, Tamil Nadu, India and Tamil Nadu State Council for Science and Technology (ES03) for pecuniary sustain.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.WHO . Geneva: WHO; 2011. The promotion and development of tradional medicine; p. 622. [Google Scholar]
- 2.Zhao J, Zhou L, Wang J, Shan T, Zhong L, Liu X, et al. Endophytic fungi for producing bioactive compounds originally from their host plants. Curr Res, Technol Educ Trop Appl Microbiol Microbial Biotechnol. 2010;1:567–576. [Google Scholar]
- 3.Sikarwar Mukesh S, Patil MB. Antihyperlipidemic effect of ethanolic extract of Hibiscus rosa sinensis flowers in hyperlipidemic rats. RGUHS J Pharm Sci. 2011;1:117–122. [Google Scholar]
- 4.Olukoya DK, Idika N, Odugbemi T. Antibacterial activity of some medicinal plant from Nigeria. J Ethnopharmacol. 1993;39:69–72. doi: 10.1016/0378-8741(93)90051-6. [DOI] [PubMed] [Google Scholar]
- 5.Sorachai K, Boonsom L, Saisunee L, Aphiwat T, Stephen G, Mary J. Antimalarial, anticancer, antimicrobial activities and chemical constituents of essential oil from the aerial parts of Cyperus kyllingia Endl Rec. Nat Prod. 2011;4:324–327. [Google Scholar]
- 6.Farombi EO. African indigenous plant with chemotherapeutic potential and biotechnological approval to the production of bioactive prophylactic agent. Afr Biotech. 2003;2:662–667. [Google Scholar]
- 7.Ekpendu TO, Akshomejce AA, Okogun J. Anti inflammatory antimicrobial activity. Lett Appl microbial. 1994;30:379–384. [Google Scholar]
- 8.Anu Kiruthika K, Amutha Jaisheeba A, Sornaraj R. Evaluation of the antimicrobial property of selected flower extracts when exposed in a hospital environment. Int J Pharm Tech Res. 2011;2:769–774. [Google Scholar]
- 9.Ikram M, Inamul H. Screening of medicinal plants for antimicrobial activities. Fitoterapia. 1984;55:62–64. [Google Scholar]
- 10.Sambrook J, Fritsch EF, Maniatis T. 2nd ed. New York: Cold Spring Harbor Laboratory, Cold Spring Harbor; 1989. Molecular cloning: A laboratory manual. [Google Scholar]
- 11.Artizzu N, Bonaiganorel, Cottiglia F, Loy G. Studies of the diuretic and antimicrobial activity of cynodon dactylon essential oil. Fitoterapia. 1995;66:174–175. [Google Scholar]
- 12.Kumar S, Narain S. Herbal remedies of wetlands macrophytes in India. Int J Pharm BioSci. 2010;1(2):1–12. [Google Scholar]
- 13.Swadha A, Debasisa M. USA: IGI Global; 2011. Computational methods for identification of novel secondary metabolite biosynthetic pathways by genome analysis. In: Handbook of research on computational and systems biology: Interdisciplinary applications; pp. 380–381. [Google Scholar]
- 14.Ravi U, Neha M. Antimicrobial activity of flower extracts of coli forms Sphaeranthus Indicus on coli forms. Asian J Exp Biol Sci. 2011;2(3):513–516. [Google Scholar]
- 15.Scalbert AC. Antimicrobial properties in tannins. Phytochem. 1991;30:3875–3883. [Google Scholar]
- 16.Padmaja M, Sravanthi M, Hemalatha KPJ. Evaluation of antioxidant activity of two Indian medicinal plants. J Phytol. 2011;3(3):86–91. [Google Scholar]
- 17.Ionela DC, Ion IB. Plant products as antimicrobial agents. Secţiunea Genetică Biologie Molecula. 2007;8:104–111. [Google Scholar]
- 18.Saravanan R, Dhachinamoorthi D, Senthilkumar K, Thamizhvanan K. Antimicrobial activity of various extracts from various parts of Calophyllum inophyllum L. J Appl Pharm Sci. 2011;1(3):102–106. [Google Scholar]
- 19.Morand C, Manach C, Crespy V, Remesy C. Respective bioavailability of quercetin aglycone and its glycosides in a rat model. Biofactors. 2000;12(4):169–174. doi: 10.1002/biof.5520120127. [DOI] [PubMed] [Google Scholar]
- 20.Kariba RM. Antimicrobial activity of Hymenodictyon parvifolium. Fitoterapia. 2002;73(6):523–525. doi: 10.1016/s0367-326x(02)00176-4. [DOI] [PubMed] [Google Scholar]
- 21.Begum S, Hassan SI, Ali SN, Siddiqui BS. Chemical constituents from the leaves of Psidium guajava. Nat Prod Res. 2004;18(2):135–140. doi: 10.1080/14786410310001608019. [DOI] [PubMed] [Google Scholar]
- 22.Sanches NR, Cortez DAG, Schiavini MS, Nakamura CV, Dias Filho BP. An evaluation of antibacterial activities of Psidium guajava (L.) Braz Arch Biol Tech An Int J. 2005;48(3):429–436. [Google Scholar]
- 23.Shariff N, Sudarshana MS, Umesha S, Hariprasad P. Antimicrobial activity of rauvolfia tetraphylla and Physalis minima leaf and callus extracts. Afr J Biotechnol. 2006;5(10):946–950. [Google Scholar]
- 24.Dwivedi S. Terminalia A, A useful drug for cardiovascular disorders. J Ethnopharmacol. 2007;114(2):114–129. doi: 10.1016/j.jep.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Kamath JV, Rahul N, Ashok Kumar CK, Mohana Laksmi S. Psidium guajava L: A review. Int J Green Pharm. 2008;2(1):9–12. [Google Scholar]
- 26.Singh DV, Gupta MM, Santha Kumar TR, Saikia D, Khanuja SPS. Antibacterial principles from the bark of Terminalia arjuna. Curr Sci. 2008;94(1):27–29. [Google Scholar]
- 27.John De Britto A, Herin Sheeba Gracelin D, Azadiracta Indica A. A potential antimicrobial agent against Xanthomonas campestris. IJABPT. 2011;3(2):374–378. [Google Scholar]
- 28.Banso A, Adeyemo SO. Evaluation of antibacterial properties of tannins isolated from Dichrostachys cinerea. Afr J Biotechnol. 2007;6(15):1785–1787. [Google Scholar]
- 29.SK Dey A, Debdulal BB, Sourav CA, Krishnendu BK. Antimicrobial activities of some medicinal plants of West Bengal. Int J Pharm BioSci. 2010;1(3):1–10. [Google Scholar]
- 30.Sukumaran S, Kiruba S, Mahesh M, Nisha SR, Miller PZ, Ben CP, et al. Phytochemical constituents and antibacterial efficacy of the flowers of Peltophorum pterocarpum (DC.) Baker ex Heyne. Asian Pac J Trop Med. 2011;4(9):735–738. doi: 10.1016/S1995-7645(11)60183-1. [DOI] [PubMed] [Google Scholar]
- 31.Kannathasan Krishnan, Senthilkumar Annadurai, Venkatesalu Venugopalan. In vitro antibacterial potential of some Vitex species against human pathogenic bacteria. Asian Pac J Trop Med. 2011;4(8):645–648. doi: 10.1016/S1995-7645(11)60164-8. [DOI] [PubMed] [Google Scholar]
- 32.Raja RDA, Jeeva S, Prakash JW, Antonisamy JM, Irudayaraj V. Antibacterial activity of selected ethnomedicinal plants from South India. Asian Pac J Trop Med. 2011;4(11):375–378. doi: 10.1016/S1995-7645(11)60107-7. [DOI] [PubMed] [Google Scholar]
- 33.Madhumitha G, Saral AM. Preliminary phytochemical analysis, antibacterial, antifungal and anticandidal activities of successive extracts of Crossandra infundibuliformis. Asian Pac J Trop Med. 2011;4(3):192–195. doi: 10.1016/S1995-7645(11)60067-9. [DOI] [PubMed] [Google Scholar]
- 34.Johnson M, Wesely EG, Kavitha MS, Uma V. Antibacterial activity of leaves and inter-nodal callus extracts of Mentha arvensis L. Asian Pac J Trop Med. 2011;4(3):196–200. doi: 10.1016/S1995-7645(11)60068-0. [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee SK, Bhattacharjee I, Chandra G. Isolation and identification of bioactive antibacterial components in leaf extracts of Vangueria spinosa (Rubiaceae) Asian Pac J Trop Med. 2011;4(1):35–40. doi: 10.1016/S1995-7645(11)60028-X. [DOI] [PubMed] [Google Scholar]
- 36.Mirnejad R, Jeddi F, Kiani J, Khoobdel M. Etiology of spontaneous bacterial peritonitis and determination of their antibiotic susceptibility patterns in Iran. Asian Pac J Trop Dis. 2011;1(3):116–118. [Google Scholar]
- 37.Ani AE, Diarra B, Dahle UR, Lekuk C, Yetunde F, Somboro AM, et al. Identification of mycobacteria and other acid fast organisms associated with pulmonary disease. Asian Pac J Trop Dis. 2011;1(4):259–262. [Google Scholar]
- 38.Ezeani MC, Agba MI, Onyenekwe CC, Anahalu I, Azikiwe CC, Unaezeb BE, et al. Aerobacteriology of laboratories and offices: Evidence of high risk exposure to immune complex formation in Nigeria. Asian Pac J Trop Dis. 2011;1(3):131–136. [Google Scholar]
- 39.Dahanukar SA, Kulkarni RA, Rege NN. Pharmacology of medicinal plants and natural products. Indian J Pharm. 2000;32:S81–S118. [Google Scholar]
- 40.Tomoko N, Takashi A, Hiromu T, Yuka I, Hiroko M, Munekazu I, et al. Antibacterial activity of extracts preparated from tropical and subtropical plants on methicillin-resistant Staphylococcus aureus. J health Sci. 2000;48:273–276. [Google Scholar]
- 41.Saravanakumar A, Venkateshwaran K, Vanitha J, Ganesh M, Vasudevan M, Sivakumar T. Evaluation of antibacterial activity phenol and flavonoid contents of Thespesia populnea flower extracts. Pak J Pharm Sci. 2009;22(3):282–286. [PubMed] [Google Scholar]