Abstract
Objective
To determine the protective effect of morin, a flavonoid against deoxycorticosterone acetate (DOCA)-salt induced hypertension in male Wistar rats.
Methods
Hypertension was induced in uninephrectomized rats by weekly twice subcutaneous injection of DOCA (25 mg/kg bw) and 1% NaCl in the drinking water for six consecutive weeks. Effect of morin against DOCA-salt induced hypertension was evaluated by measuring blood pressure and performing biochemical estimations and histopathological examination of renal tissues.
Results
DOCA-salt hypertensive rats showed considerably increased systolic and diastolic blood pressure, serum hepatic marker enzyme activities such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT) and renal function markers (urea, uric acid and creatinine) in plasma. Oral administration of morin (25, 50 and 75 mg/kg bw) brought back all the above parameters to near normal level. Histopathology of kidney also confirmed the biochemical findings of this study. The effect at a dose of 50 mg/kg bw of morin was more pronounced than that of the other two doses (25 and 75 mg/kg bw).
Conclusions
These findings indicate that morin exhibits strong antihypertensive effect against DOCA-salt induced hypertension.
Keywords: Morin, Uninephrectomy, DOCA-salt, Oxidative stress, Hypertension, Antihypertensive effect, Blood pressure
1. Introduction
Cardiovascular disease (CVD) is a major health problem across the world, accounting for 30% of all deaths[1]. CVD has reached epidemic proportions in India[2] and is estimated to result in more than 3 million deaths each year[3]. Hypertension is a major risk factor for cardiovascular diseases and a large body of evidence suggests oxidative stress, an increase in the production of reactive oxygen species (ROS), as a strong underlying factor in hypertension[4],[5]. Excessive ROS generation has been suggested to be involved in a variety of cardiovascular diseases including hypertension, inflammation in the cardiovascular system, cardiac hypertrophy, cardiomyopathies and ischemic heart disease[6]–[8]. Various genetic and environmental factors are known to be involved in the pathogenesis of primary hypertension, among which excess sodium intake has long been regarded as the pivotal environmental factor for this disorder[9]. The long-term administration of deoxycorticosterone acetate (DOCA)-salt to rats induces sodium retention and high salt intake, producing volume-dependent hypertension[10]. Considerable evidence demonstrated that high blood pressure is accompanied by oxidative stress and impaired renal function in salt-sensitive hypertension[11].
During the last few years, efforts have been made to increase the number of synthetic antihypertensive drugs, but their use is often limited because of their adverse effects. In recent years, dietary agents such as increased consumption of fruits, vegetables, whole grains, and fish have been shown to be important in the control of CVD including hypertension[12]. Flavonoids are polyphenolic compounds that can be found in dietary components such as food products, beverages and herbal medicines with different health benefits shown in a large number of studies[13]. Most flavonoids have an antioxidant activity[14],[15].
Morin (3,5,7,2′,4′-pentahydroxyflavone; a light yellowish pigment) is a kind of flavonoid belonging to the group of flavonols (Figure 1), found in guava leaves[16], onion, apple[17] and other Moraceae which are used as dietary agents and also as herbal medicines[18]. Morin exhibits many biological activities, including antioxidant[19],[20], anti-inflammatory[21], antitumour[22] activities and possibly even protective effects against chronic diseases[23]. Extensive literature survey has shown that no sufficient work has been done to study its antihypertensive effect. Therefore, the present study was designed to determine the dose-dependent effect of chronic administration of morin on blood pressure, activities of hepatic marker enzymes, renal function markers and histopathological findings against DOCA-salt induced hypertension in male albino Wistar rats.
Figure 1. Chemical structure of morin (3,5,7,2′,4′-pentahydroxyflavone).
2. Materials and methods
2.1. Experimental animals
Male albino Wistar rats (Rattus norvegicus; 11–13 week old) were obtained from the Central Animal House, Department of Experimental Medicine, Rajah Muthiah Medical College and Hospital, Annamalai University, Tamilnadu, India. They were housed (3 rats/cage) in polypropylene cages (47 cm × 34 cm × 20 cm) lined with husk, renewed every 24 h under a 12:12 h light/dark cycle at around 22 °C and had free access to tap water and food. The rats were fed on a standard pellet diet (Kamadhenu Agencies, Bangalore, India). The whole experiment was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, New Delhi, India and approved by the Animal Ethical Committee of Annamalai University (Reg no: 160/1999/CPCSEA, Approval no: 622).
2.2. Drug and chemicals
Morin, DOCA and dimethyl formamide (DMF) were purchased from Sigma-Aldrich Chemical Company, St. Louis, Missouri, USA. All other chemicals used in this study were of the highest analytical grade and obtained from Sisco Research Laboratories (SRL) or Himedia, Mumbai, India.
2.3. Method of uninephrectomy
Left uninephrectomy was performed on all rats. Rats were anaesthetized with intraperitonial injection of ketamine (75 mg/kg bw), kidney was visualized by a left lateral abdominal incision (1 cm long), and the left renal artery and ureter were ligated by silk thread, and then the left kidney was removed and weighed. The muscle and skin layer (incision site) were sutured with highly sterile suture needles.
2.4. DOCA-salt hypertensive rats
After uninephrectomy, UNX-rats were allowed to drink tap water ad libitum, with no further treatment, and DOCA-salt hypertensive rats were given 1% NaCl in the drinking water with weekly twice subcutaneous injection of DOCA [25 mg/kg bw in 0.4 mL of DMF (vehicle) with mild heating] for six consecutive weeks[24],[25].
2.5. Suspension of the test compound and mode of administration
Morin was freshly suspended in water[26],[27] and was administered to rats orally using an intragastric tube daily for a period of six consecutive weeks. Rats were monitored daily for general health.
2.6. Experimental timeline
The rats were randomly divided into six groups each consisting of six rats. Group 1: UNX-rats; group 2: UNX-rats were treated with morin alone (75 mg/kg bw); group 3: DOCA-salt hypertensive rats; groups 4, 5 and 6 were hypertensive rats, which received different doses of morin 25, 50 and 75 mg/kg bw.
At the end of the sixth week, all the rats were anesthetized with intramuscular injection of ketamine and sacrificed by cervical dislocation. Blood was collected in two different tubes, i.e., one with anticoagulant for the separation of plasma and another without anticoagulant for the serum. Plasma and serum were separated by centrifugation for the estimation of various biochemical parameters.
2.7. Measurement of blood pressure by non-invasive method
Systolic and diastolic blood pressures were recorded every week during the entire period of the study by tail-cuff method (IITC, model 31, Woodland Hills, CA, USA). The animals were placed in heated chamber at an ambient temperature of (30–34 °C) for 15 min and from each animal, 1–9 blood pressure values were recorded. The lowest three readings were averaged to obtain a mean blood pressure. All the recordings and data analyses were done using a computerized data acquisition system and software.
2.8. Biochemical estimations
The activities of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT) were assayed by the method of Reitman and Frankel[28], Kind and King[29], Rosalki and Rau[30], respectively. The levels of urea, uric acid and creatinine in plasma were estimated by diagnostics kit (Qualigens Diagnostics, Mumbai, India) based on the method of Fawcett and Scott[31], Caraway[32] and Tietz[33].
2.9. Histopathological examination of renal tissues
The kidney tissues obtained from all experimental groups were washed immediately with 0.9% saline and then fixed in 10% buffered neutral formalin. After fixation, the kidney tissues were processed by embedding in paraffin wax. Then, the tissues were sectioned (5–6 µm) and stained with haematoxylin and eosin (H&E) dye and examined under a high power microscope (Nikon ELWD 0.3/OD75; Japan) and photomicrographs were taken.
2.10. Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT) using Statistical Package for the Social Science (SPSS) software version 12.00. Results were expressed as mean±SD for six rats in each group. A value of P≤0.05 was considered to be statistically significant.
3. Results
3.1. Effect of morin on blood pressure
Figures 2 and 3 showed the dose dependent effect of morin in UNX and DOCA-salt hypertensive rats. The systolic and diastolic blood pressure was considerably (P<0.05) increased in DOCA-salt hypertensive rats (group 3) compared to UNX-rats (group 1). Oral administration of morin (25, 50 and 75 mg/kg bw) for a period of six consecutive weeks considerably (P<0.05) decreased systolic and diastolic blood pressure in DOCA-salt treated rats (groups 4, 5 and 6). The effect exerted by 50 mg/kg bw of morin was better than the other two doses (25 and 75 mg/kg bw). Oral administration of morin alone (75 mg/kg bw) to UNX-rats (group 2) did not show any effect.
Figure 2. Effect of morin on systolic blood pressure of UNX and DOCA-salt hypertensive rats.

Values are expressed as mean±SD for six rats in each group. Values not sharing a common superscript differ significantly at P<0.05 (DMRT).
Figure 3. Effect of morin on diastolic blood pressure of UNX and DOCA-salt hypertensive rats.
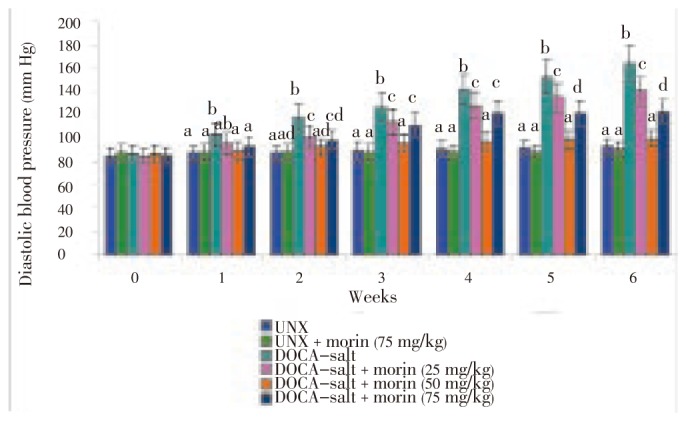
Values are expressed as mean±SD for six rats in each group. Values not sharing a common superscript differ significantly at P<0.05 (DMRT).
3.2. Effect of morin on the activities of hepatic marker enzymes
The activities of AST, ALT, ALP and GGT were depicted in Table 1. DOCA-salt hypertensive rats (group 3), exhibited a considerable (P<0.05) increase in the activities of these marker enzymes in serum when compared to UNX-rats (group 1). Oral administration of morin (25, 50 and 75 mg/kg bw) considerably (P<0.05) decreased the activities of these enzymes in serum of DOCA-salt treated rats (groups 4, 5 and 6). The morin at a dose of 50 mg/kg bw was more effective for reducing these enzyme activities compared with other two doses (25 and 75 mg/kg bw).
Table 1. Effect of morin on the activities of serum AST, ALT, ALP and GGT of UNX and DOCA-salt hypertensive rats (mean±SD) (n=6).
| Groups | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | GGT (IU/L) |
| UNX | 68.31±4.57a | 28.75±1.78a | 76.27±5.03a | 14.69±0.92a |
| UNX + morin (75 mg/kg bw) | 67.06±3.95a | 26.16±1.33a | 75.16±4.43a | 13.43±0.79a |
| DOCA-salt | 122.11±11.84b | 65.23±5.60b | 137.13±12.75b | 32.96±3.06b |
| DOCA-salt + morin (25 mg/kg bw) | 103.06±9.37c | 57.81±4.68c | 109.06±9.92c | 25.09±2.28c |
| DOCA-salt + morin (50 mg/kg bw) | 73.08±5.18a | 32.91±2.23d | 81.06±5.75a | 17.66±1.43d |
| DOCA-salt + morin (75 mg/kg bw) | 92.47±7.95d | 49.65±3.77e | 96.76±7.83d | 22.96±1.97c |
Values not sharing a common superscript differ significantly at P<0.05 (DMRT).
3.3. Effect of morin on renal function markers
In DOCA-salt hypertensive rats (group 3), the levels of urea, uric acid and creatinine in plasma were considerably (P<0.05) increased when compared to UNX-rats (group 1). Oral treatment with morin (25, 50 and 75 mg/kg bw) throughout the experimental period to DOCA-salt treated rats (groups 4, 5 and 6) considerably (P<0.05) decreased the levels of these markers in plasma. Among the three different doses tested, 50 mg/kg bw of morin elicited the highest significant effect (Table 2).
Table 2. Effect of morin on the levels of urea, uric acid and creatinine in plasma of UNX and DOCA-salt hypertensive rats (mean±SD) (n=6).
| Groups | Urea (mg/dL) | Uric acid (mg/dL) | Creatinine (mg/dL) |
| UNX | 19.36±1.33a | 1.36±0.08a | 0.88±0.06a |
| UNX + morin (75 mg/kg bw) | 18.03±1.26a | 1.24±0.06a | 0.83±0.04a |
| DOCA-salt | 44.06±3.92b | 3.73±0.32b | 2.87±0.27b |
| DOCA-salt + morin (25 mg/kg bw) | 35.06±2.98c | 3.02±0.24c | 2.19±0.20c |
| DOCA-salt + morin (50 mg/kg bw) | 22.67±1.63d | 1.89±0.13d | 1.06±0.07d |
| DOCA-salt + morin (75 mg/kg bw) | 30.16±2.44e | 2.59±0.19e | 1.76±0.15e |
Values not sharing a common superscript differ significantly at P<0.05 (DMRT).
3.4. Effect of morin on the histopathology of kidney
Histopathological changes in the kidney tissue of all groups were evaluated microscopically (Figure 4A–F). Figure 4A showed the histological section of UNX-rats (group 1), which exhibited normal glomeruli and tubules. UNX-rats treated with morin alone (75 mg/kg bw) showed normal kidney architecture without pathological changes (group 2; Figure 4B). However, DOCA-salt hypertensive rats kidney showed segmented glomerulo nephritics in glomeruli, tubules contain protein precipitate and cloudy swelling, small blood vessels show thickening of the wall, parenchymal hemorrhage and inflammatory cell infiltrate in the parenchyma (group 3; Figures 4C and C1). The above changes were reduced in kidney of rats treated with morin (25, 50 and 75 mg/kg bw) (groups 4, 5 and 6; Figures 4D–F). From the histopathological findings, oral administration of morin at a dose of 50 mg/kg bw remarkably minimized the structural changes in kidney than other two doses (25 and 75 mg/kg bw).
Figure 4. Histopathological changes in the kidney (H&E 40×).
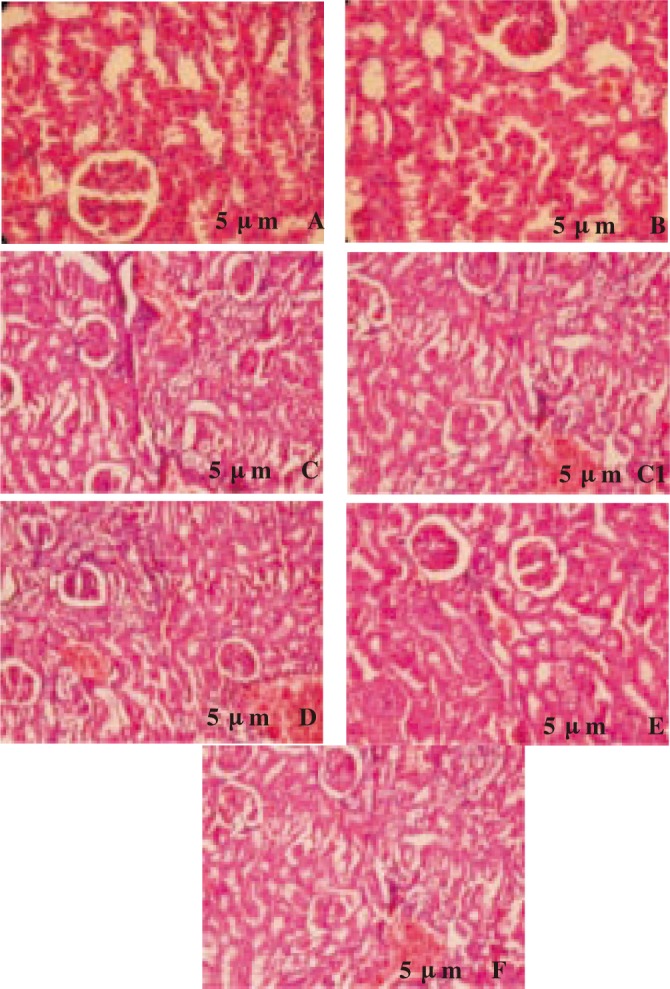
A: UNX-rat kidney (group 1) showing normal glomeruli and tubules; B: UNX + morin alone (75 mg/kg bw) treated kidney (group 2) showing normal architecture without pathological changes; C & C1: DOCA-salt hypertensive kidney (group 3) showing segmented glomerulo nephritics in glomeruli, tubules contain protein precipitate and cloudy swelling, small blood vessels showing thickening of the wall, parenchymal hemorrhage and inflammatory cell infiltrate in the parenchyma; D: DOCA-salt + morin (25 mg/kg bw) treated kidney (group 4) showing mild swelling in tubules and few inflammatory cells; E: DOCA-salt + morin (50 mg/kg bw) treated kidney (group 5) showing fewer inflammatory cells and near normal architecture; F: DOCA-salt + morin (75 mg/kg bw) treated kidney (group 6) showing mild glomerular hypertrophy and less congestion in interstitial vessels.
4. Discussion
Increased concentrations of aldosterone lead to increased reabsorption of sodium ions and water from epithelial cells in the distal nephron of the kidney, thereby influencing blood pressure levels[34]. In addition, increased aldosterone concentrations may activate oxidative stress through an unregulated NADPH oxidase in the DOCA-salt model[35]. In agreement with previous report[25] we also observed that systolic and diastolic blood pressure was considerably increased in DOCA-salt hypertensive rats (group 3), which might be due to increased oxidative stress and decreased bioavailability of nitric oxide. Daily oral administration of morin, resulted in a remarkable reduction in systolic and diastolic blood pressure which might be due to the antioxidant ability of this compound. Flavonoids and triterpine were recently shown to reduce hypertension in experimental animal models[36]. Several cohort studies have suggested that high intake of flavonoids may decrease the risk of coronary heart diseases[37].
Oxidative stress and alteration of cellular redox state are linked to many types of acute and chronic liver and kidney injury. Disruption of liver tissue architecture and vacuolation under hypertension and nitric oxide-deficiency are an indication of hepatic fatty infiltration and hepatocellular injury[38]. AST, ALT, ALP and GGT are the relatively liver specific enzymes. Their estimation in the serum is useful as a quantitative marker of the extent and type of liver damage. In our results, AST, ALT, ALP and GGT activities were increased considerably in the serum of DOCA-salt hypertensive rats (group 3), which is a clear evidence for liver damage. The reason behind this elevation may be due to the necrotic and oxidative action of liver tissues which causes leakage of these enzymes from hepatocytes as a result of membrane damage[39]. Treatment with morin considerably reduced the activities of these enzymes. It is suggested that morin aids in parenchymal cell regeneration in liver, thus protecting membrane integrity, thereby decreasing enzyme leakage.
The kidney plays a central role in the regulation of the balance of the body salt and water, and then disordered regulation of renal functions is responsible for the altered balance of salt and water in pathophysiological states including some experimental models of hypertension[40]. Our results revealed that a considerable increase in plasma urea, uric acid and creatinine levels might indicate a hypertension in DOCA-salt rats (group 3) and may be due to kidney damage caused by the oxidative stress by increasing the formation of superoxide. These findings are correlated well with the kidney histology examination. Oral administration of morin considerably reduced the raised plasma urea, uric acid and creatinine levels, bringing about remarkable recovery in kidneys as evidenced microscopically. Considerable reduction in these levels might be due to the antioxidant property of morin. These findings suggest that renal damage is remarkably prevented by treatment of morin in DOCA-salt hypertensive rats, which also might be beneficial in the control of blood pressure. Interestingly, it has been suggested that kidney and cardiac injury can be avoided or minimized by reducing oxidative stress through increased intake of antioxidants[41].
UNX-rats treated with morin alone (75 mg/kg bw) showed normal appearance of the kidney without any pathological changes. This indicates that morin does not possess any adverse effect under normal conditions. Thus, histopathological findings of the present study confirmed the biochemical observations of this study.
In conclusion, our results demonstrate that morin, possesses strong antihypertensive effect against DOCA-salt hypertensive rats, which is evidenced by a considerable decrease in blood pressures, hepatic and renal function markers. Our results also revealed that the renal damage was ameliorated by morin supplementation. Thus, it is a safe antioxidant. Among the three dosages tested, 50 mg/kg bw exhibited the highest considerable antihypertensive effect. However, further studies are needed to find out the exact mechanism of action of morin.
Acknowledgments
This study was supported by the grants from the Department of Science and Technology (DST; SR/FT/LS-150/2008), New Delhi, India. The authors are grateful to Dr. Viswanathan P, Professor, Department of Pathology, Rajah Muthiah Medical College and Hospital, Annamalai University, Tamilnadu, India for his assistance in histopathological studies.
Footnotes
Foundation Project: This work was financially supported by Department of Science and Technology, New Delhi, India (grant No. SR/FT/LS-150/2008).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Gaziano TA. Economic burden and the cost-effectiveness of treatment of cardiovascular disease in Africa. Heart. 2008;94:140–144. doi: 10.1136/hrt.2007.128785. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R, Joshi PP, Mohan V, Reddy KS, Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Mortality and burden of disease estimates for WHO member states in 2004. Geneva: WHO; 2004. Available from: http://www.whoint/entity/healthinfo/global_burden/disease/gbddeathdalycountryestimates2004.xls. [Accessed on 20 July, 2009] [Google Scholar]
- 4.Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care. 2008;31:181–184. doi: 10.2337/dc08-s245. [DOI] [PubMed] [Google Scholar]
- 5.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 6.Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signalling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47:15–22. doi: 10.1016/j.yjmcc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Fukagawa NK. Age-related changes in redox signalling and VSMC function. Antioxid Redox Signal. 2010;1:641–655. doi: 10.1089/ars.2009.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pechanova O, Simko F. Chronic antioxidant therapy fails to ameliorate hypertension: potential mechanisms behind. J Hypertens Suppl. 2009;27:32–36. doi: 10.1097/01.hjh.0000358835.25934.5e. [DOI] [PubMed] [Google Scholar]
- 9.Adrogue HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356:1966–1978. doi: 10.1056/NEJMra064486. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, Luo P, Chang HH. Clofibrate attenuates blood pressure and sodium retention in DOCA-salt hypertension. Kidney Int. 2008;74:1040–1048. doi: 10.1038/ki.2008.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seifi B, Kadkhodaee M, Karimian SM, Zahmatkesh M, Xu J, Soleimani M. Evaluation of renal oxidative stress in the development of DOCA-salt induced hypertension and its renal damage. Clin Exp Hypertens. 2010;32:90–97. doi: 10.3109/10641960902993111. [DOI] [PubMed] [Google Scholar]
- 12.Retelny VS, Neuendorf A, Roth JL. Nutrition protocols for the prevention of cardiovascular disease. Nutr Clin Pract. 2008;23:468–476. doi: 10.1177/0884533608323425. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Liu JH, Zhang J, Yu BY. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J Agric Food Chem. 2008;56:3876–3883. doi: 10.1021/jf073520n. [DOI] [PubMed] [Google Scholar]
- 14.Kim MK, Jung HS, Yoon CS, Ko JH, Chun HJ, Kim TK, et al. et al. EGCG and quercetin protected INS-1 cells in oxidative stress via different mechanisms. Front Biosci. 2010;2:810–817. doi: 10.2741/e142. [DOI] [PubMed] [Google Scholar]
- 15.Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rattanachaikunsopon P, Phumkhachorn P. Contents and antibacterial activity of flavonoids extracted from leaves of Psidium guajava. J Med Plant Res. 2010;4:393–396. [Google Scholar]
- 17.Lotito SB, Frei B. Consumption of flavonoid rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon. Free Radic Biol Med. 2006;41:1727–1746. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Xie MX, Long M, Liu Y, Qin C, Wang YD. Characterization of the interaction between human serum albumin and morin. Biochim Biophys Acta. 2006;1760:1184–1191. doi: 10.1016/j.bbagen.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Subash S, Subramanian P. Effect of morin on the levels of circulatory liver markers and redox status in experimental chronic hyperammonaemic rats. Singapore Med J. 2008;49:650–655. [PubMed] [Google Scholar]
- 20.Subash S, Subramanian P. Morin a flavonoid exerts antioxidant potential in chronic hyperammonemic rats: a biochemical and histopathological study. Mol Cell Biochem. 2009;32:153–161. doi: 10.1007/s11010-009-0053-1. [DOI] [PubMed] [Google Scholar]
- 21.Sivaramakrishnan V, Shilpa P, Kumar V, Devaraj S. Attenuation of N-nitrosodiethylamine-induced hepatocellular carcinogenesis by a novel favonol-morin. Chem Biol Int. 2008;171:79–88. doi: 10.1016/j.cbi.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Sivaramakrishnan V, Devaraj SN. Morin regulates the expression of NF-kB-p65, COX-2 and matrix metalloproteinases in diethylnitrosamine induced rat hepatocellular carcinoma. Chem Biol Int. 2009;180:353–359. doi: 10.1016/j.cbi.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Parihar VK, Prabhakar KR, Veerapur VP, Priyadarsini KI, Unnikrishnan MK, Rao CM. Anticlastogenic activity of morin against whole body gamma irradiation in Swiss albino mice. Eur J Pharmacol. 2007;557:58–65. doi: 10.1016/j.ejphar.2006.09.073. [DOI] [PubMed] [Google Scholar]
- 24.Iyer A, Fenning A, Lim J, Le GT, Reid R, Halili A, et al. et al. Antifibrotic activity of an inhibitor of histone deacetylases in DOCA-salt hypertensive rats. Br J Pharmacol. 2010;159:1408–1417. doi: 10.1111/j.1476-5381.2010.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veeramani C, Aristatile B, Pushpavalli G, Pugalendi KV. Effects of Melothriamaderaspatana leaf extract on antioxidant status in sham-operated and uninephrectomized DOCA-salt hypertensive rats. Saudi J Biol Sci. 2011;18:99–105. doi: 10.1016/j.sjbs.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sreedharan V, Venkatachalam KK, Namasivayam N. Effect of morin on tissue lipid peroxidation and antioxidant status in 1, 2-dimethylhydrazine induced experimental colon carcinogenesis. Invest New Drugs. 2009;27:21–30. doi: 10.1007/s10637-008-9136-1. [DOI] [PubMed] [Google Scholar]
- 27.Karthik Kumar V, Vennila S, Nalini N. Inhibitory effect of morin on DMH-induced biochemical changes and aberrant crypt foci formation in experimental colon carcinogenesis. Environ Toxicol Pharmacol. 2010;29:50–57. doi: 10.1016/j.etap.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamate oxaloacetic and glutamate pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 29.Kind PN, King EJ. Estimation of plasma phosphate by determination of hydrolyzed phenol with aminopyrines. J Clin Pathol. 1954;7:322–323. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosalki SB, Rau D. Serum gamma-glutamyl transpeptidase activity in alcoholism. Clin Chim Acta. 1972;39:41–47. doi: 10.1016/0009-8981(72)90297-5. [DOI] [PubMed] [Google Scholar]
- 31.Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caraway WT. Determination of uric acid in serum by carbonate method. Am J Clin Pathol. 1955;25:840–845. doi: 10.1093/ajcp/25.7_ts.0840. [DOI] [PubMed] [Google Scholar]
- 33.Tietz NW. Fundamentals of clinical chemistry. Philadelphia: WB Saunders Company; 1987. p. 638. [Google Scholar]
- 34.Tomaschitz A, Pilz S, Ritz E, Pietsch B, Pieber TR. Aldosterone and arterial hypertension. Nat Rev Endocrinol. 2010;6:83–93. doi: 10.1038/nrendo.2009.263. [DOI] [PubMed] [Google Scholar]
- 35.Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology. 2008;149:1009–1014. doi: 10.1210/en.2007-0864. [DOI] [PubMed] [Google Scholar]
- 36.Jalili T, Carlstrom J, Kim S. Quercetin-supplemented diets lower blood pressure and attenuate cardiac hypertrophy in rats with aortic constriction. J Cardiovasc Pharmacol. 2006;47:531–541. doi: 10.1097/01.fjc.0000211746.78454.50. [DOI] [PubMed] [Google Scholar]
- 37.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, et al. et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 38.Hoetzel A, Welle A, Schmidt R, Loop T, Humar M, Ryter SW, et al. et al. Nitric oxide-deficiency regulates hepatic heme oxygenase-1. Nitric Oxide. 2008;18:61–69. doi: 10.1016/j.niox.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharjee N, Pathak S, Khuda-Bukhsh AR. Amelioration of carcinogen-induced toxicity in mice by administration of a potentized homeopathic drug, natrum sulphuricum 200. 2009;6:65–75. doi: 10.1093/ecam/nem067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohring J, Mohring B, Naumann HJ, Philippi A, Homsy E, Orth H, et al. et al. Salt and water balance and renin activity in renal hypertension of rats. Am J Physiol. 1975;228:1847–1855. doi: 10.1152/ajplegacy.1975.228.6.1847. [DOI] [PubMed] [Google Scholar]
- 41.Schnackenberg CG, Welch W, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane permeable superoxide dismutase mimetic role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]


