Abstract
Objective
To determine the association of smoking, alcohol and nonsteroidal anti-inflammatory drugs (NSAIDs) use with presence and virulence of Helicobacter pylori (H. pylori) infection in a representative sample of a random adult population of asymptomatic subjects.
Methods
Non virulent 16S rRNA and virulent cag A and T genes from salivary samples of 854 asymptomatic subjects were determined using polymerase chain reaction. The presence and absence of virulent and non virulent infection was statistically compared with consumption of smoking, alcohol and NSAIDs.
Results
The prevalence of infection in male and female subjects was found to be 69.25% and 66.90%, respectively. The prevalence of infection in the population of asymptomatic subjects with respect to consumption of alcohol was as follows: current (31.22%), former (52.20%) and never (43.58%). The prevalence of infection in the population of asymptomatic subjects with respect to smoking of cigarettes was as follows: current (88.80%), former (57.14%) and never (33.33%). The prevalence of infection in the subject population consuming NSAIDs and not consuming NSAIDs frequently was found to be 82.75% and 21.16%, respectively. Virulence in male and female subjects was found to be 60.00% and 50.00%, respectively. The presence of virulent infection in the population of asymptomatic subjects with respect to consumption of alcohol was as follows: current (28.57%), former (40.15%) and never (50.00%). The prevalence of virulent infection in the population of asymptomatic subjects with respect to smoking of cigarettes was as follows: current (79.32%), former (75.00%) and never (50.00%). The prevalence of virulent infection in the subject population consuming NSAIDs and not consuming NSAIDs frequently was found to be 88.23% and 66.66%, respectively.
Conclusions
It can be concluded that smoking and NSAIDs consumption are aggravating factors for virulence of H. pylori and alcohol can inhibit H. pylori infection in asymptomatic subjects.
Keywords: Smoking, Alcohol, NSAIDs, Helicobacter pylori, Cag A, Cag T, 16S rRNA, PCR, Agarose gel electrophoresis, Virulence, Prevalence, Infection, Asymptomatic
1. Introduction
Helicobacter pylori (H. pylori), termed as class I carcinogen by World Health Organization, is a worldwide menace with the ability to transform gastric lesions into a carcinogenic lymphoma[1]–[3]. It has been studied in depth by many workers proving its disease causing potential to be immense[4],[5]. An array of factors like gender, age, smoking, chronic nonsteroidal anti-inflammatory drugs (NSAIDs) consumption and habitual alcohol have been studied by various researchers[6]. However, the difference between presence of virulent H. pylori and its presence in its dormant or viable but nonculturable (VBNC) form has been implicated[7].
The present investigation was designed to understand the association of NSAIDs use, smoking, alcohol and presence of virulent H. pylori. H. pylori strains possess a varied genetic diversity and many markers of virulence in H. pylori have been identified. Polymerase chain reaction (PCR) based methods have been used for the detection of H. pylori DNA in gastric mucosa and gastric juice, as well as in feces, saliva, dental plaque, and environmental samples. Determination of H. pylori in saliva serves as an excellent non invasive tool to detect H. pylori in human subjects. A number of target genes have been proposed as candidates for the PCR detection of H. pylori[8].
Cag A and cag T have been implicated to play an important role in the disease progression and can be used as biomarkers of virulent disease causing state of H. pylori[8]. In the present investigation, 16S rRNA, cag A and T have been targeted to determine the presence and virulence of the H. pylori in salivary samples of asymptomatic subjects, respectively.
2. Materials and methods
2.1. Chemicals
All the chemicals for DNA extraction were procured from S.D. Fine Chemicals, India. The reagents for PCR, gel preparation, and visualization were purchased from Vivantis India, Thane. The forward and reverse primers for 16S rRNA and cag A, E, T genes were synthesized at Ocimum Biosolutions, Hyderabad, India. Gel electrophoresis unit (Bangalore genie, Bangalore) was used to perform gel electrophoresis and gel documentation unit (Alpha Innotech Inc. USA) was used to visualize and capture the gel image.
2.2. Sample collection
A total of 854 healthy subjects were included in the present study. The sampling for the study was undertaken during May to October 2010. An informed consent was obtained from each individual. The study protocol was approved by Institutional Human Ethics Committee of Bharati Medical College, Bharati Vidyapeeth Deemed University, Pune. The study population consisted of men and women of more than 18 years of age. A questionnaire in local language or English was filled up by each participant to determine that none of the participants had symptoms suggestive of acid peptic diseases. The medication history of each subject was recorded and it was ascertained that they did not consume proton pump inhibitors, H2 blockers and antibiotics before one month of saliva sampling. Saliva samples were collected by visiting homes, colleges and villages. Unstimulated saliva in the volume of 1.5 mL was collected in presterilized microcentrifuge tube and stored at -80 °C until processed. Approximately 1.5 mL of non-stimulated saliva was collected in a 2 mL microtube. After collection saliva was homogenized by vigorous shaking with the use of a vortex mixer and clarified by centrifugation (10 000 g, 4 °C, 4 min).
2.3. Collection of data
The questionnaire was available in local language and English for data collection that included gender, history of cigarette smoking, alcohol consumption, and NSAIDs use by the asymptomatic subjects. All the subjects who consumed NSAIDs more than 10 day per month were considered as NSAIDs users[9].
2.4. Preparation of genomic DNA for PCR
DNA isolation from salivary samples was performed according to phenol chloroform C-TAB method[8]. All the steps were performed in aseptic conditions to minimize contamination using cryocentrifuge (Eppendorf). The DNA was extracted and preserved at -20 °C until polymerase amplification by chain reaction was performed. Amplification of the DNA template was carried out using forward and reverse primers[8] as mentioned in Table 1. 16S rRNA (534 base pair fragment) was amplified in a programmable thermal cycler (Eppendorf). DNA sample from the same subject was used to amplify cag A using specific primers[8]. At each amplification, H. pylori DNA from strain ATCC 26695 was used as a positive control, while sterile water for injection instead of DNA served as a negative control. The products were analyzed by agarose gel electrophoresis unit (Bangalore Genei, India) and the gel image was captured using gel documentation unit (Apha Innotech Inc. USA).
Table 1. Primer sequences and respective product sizes of H. pylori specific genes.
| Primer | Sequence | Amplicon size (base pair) |
| 16S rRNA-F | 5′TAAGAGATCAGCCTATGTCC3′ | 534 |
| 16S rRNA-R | 5′TCCCACGCTTTAAGCGCAAT3′ | |
| Cag A-F | 5′CCATGAATTTTTGATCCGTTCGG3′ | 349 |
| Cag A-R | 5′GATAACAGGCAAGCTTTTGAGAGGGA3′ | |
| Cag T-F | 5′ATGAAAGTGAGAGCAAGTGT3′ | 301 |
| Cag T-R | 5′TCACTTACCACTGAGCAAAC3′ |
F: forward primer; R: reverse primer.
2.5. Statistical analysis
Statistical analysis was carried out to examine the association between the various study variables with saliva PCR positivity for H. pylori using Fischer exact test. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA), odds ratio, 95% confidence interval of odds ratio, and relative risk.
3. Results
The DNA isolated from all the samples was amplified to get a 534 base pair fragment amplicon corresponding to 16S rRNA gene in the subjects who had H. pylori infection. The same template was used to amplify cag A and T genes to find out expression of virulence factors in the detected H. pylori (Figure 1). The amplicons obtained were of 349 and 301 base pairs, respectively.
Figure 1. Successful amplification of H. pylori specific genes.
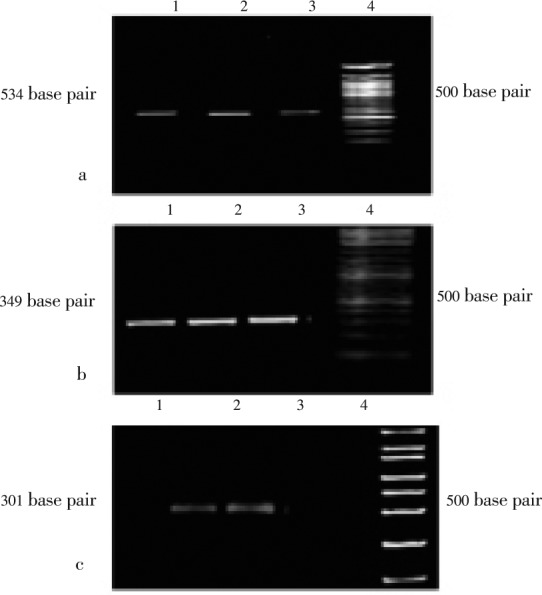
a: 16S rRNA (534 base pair); b: cag A gene (349 base pair); c: cag T gene (301 base pair).
3.1. Prevalence
The results in Table 2, indicated that the prevalence of infection in male and female subjects was found to be 68.25% and 66.90%, respectively. The P value was found to be equal to 0.698 1 and hence the prevalence was not dependent upon gender. The prevalence of infection in the population of asymptomatic subjects with respect to consumption of alcohol was as follows: current (31.22%), former (52.20%) and never (43.58%). The infection status in the people who had never consumed alcohol was not significant (P=0.144 0) whereas it was found to be significant in the people who were former alcohol consumers (P<0.000 1). The prevalence of infection in the population of asymptomatic subjects with respect to smoking of cigarettes was as follows: current (88.80%), former (57.14%) and never (33.33%). The differences of infection status in the people who were former smokers and current smokers were statistically significant (P=0.043 4 and P<0.000 1, respectively). The prevalence of infection in the subject population consuming NSAIDs and not consuming NSAIDs frequently was found to be 82.75% and 21.16%, respectively. Frequent consumption of NSAIDs was associated with H. pylori infection (P<0.000 1).
Table 2. Various risk factors with H. pylori infection status (16S rRNA) [n (%)].
| Variables | No. of subjects | H. pylori positive | H. pylori negative | Odds ratio | 95% CI of odds ratio | |
| Gender | Male | 570 (66.74) | 389 (68.25) | 181 (31.75) | 0.94 | 0.69-1.27 |
| Female | 284 (33.25) | 190 (66.90) | 94 (33.10) | Referent | ||
| Consumption of alcohol | Current | 506 (59.30) | 158 (31.22) | 348 (68.78) | Referent | |
| Never | 78 (9.00) | 34 (43.58) | 44 (56.41) | 1.70 | 0.86-3.38 | |
| Former | 272 (31.77) | 142 (52.20) | 130 (47.80)**** | 2.41 | 1.57-3.70 | |
| Smoking | Current | 768 (79.50) | 682 (88.80) | 86 (11.20)**** | 15.86 | 7.19-35.01 |
| Never | 30 (3.50) | 10 (33.33) | 20 (66.66) | Referent | ||
| Former | 56 (17.00) | 32 (57.14) | 24 (42.86)* | 2.67 | 1.06-6.73 | |
| NSAIDs use | Yes | 580 (67.91) | 480 (82.75) | 100 (17.25)**** | 17.88 | 10.72-29.79 |
| No | 274 (32.09) | 58 (21.16) | 10 (78.84) | Referent | ||
Statistical analysis between groups was carried out using Fischer exact test. The values in paranthesis indicate the percentage of the number of subjects. *: P<0.05, ****: P<0.000 1 comparing with H. pylori positive individuals.
The odds ratio and 95% CI of odds ratio in the population of asymptomatic subjects with respect to gender were (0.94, 0.69–1.27) in males when females subjects were taken as referent. The odds ratio and 95% CI of odds ratio in the population of asymptomatic subjects with respect to consumption of alcohol were as follows: former (1.70, 0.86–3.38) and never (2.41, 1.57–3.70) when current consumers of alcohol were taken as referent. The odds ratio and 95% CI of odds ratio in the population of asymptomatic subjects with respect to smoking of cigarettes were as follows: current (15.86, 7.19–35.01) and former (2.67, 1.06–6.73) when people who were never smokers were taken as referent. The odds ratio and 95% CI of odds ratio in the population of asymptomatic subjects who frequently consumed NSAIDs were (17.88, 10.72–29.79) when subjects who do not consume NSAIDs frequently were taken as referent.
3.2. Virulence
The results in Table 3 indicated that, the virulence in male and female subjects was found to be 60% and 50%, respectively. The P value was found to be equal to 0.548 1 and hence the virulence was not dependent upon gender. The presence of virulent infection in the population of asymptomatic subjects with respect to consumption of alcohol was as follows: current (28.57%), former (40.15%) and never (50.00%). The virulent infection status in the people who had never consumed alcohol was significant (P=0.026 1) and non significant in the people who were former consumers of alcohol (P=0.051 6) when it was compared with people who were current consumers of alcohol. The prevalence of virulent infection in the population of asymptomatic subjects with respect to smoking of cigarettes was as follows: current (79.32%), former (75.00%) and never (50.00%). The virulent infection status in the people who were former smokers was not significant (P=0.238 3) whereas it was found to be significant in the people who were current smokers (P=0.039 4) when compared with people who had never smoked. The prevalence of virulent infection in the subject population consuming NSAIDs and not consuming NSAIDs frequently was found to be 88.23% and 66.66%, respectively. Frequent consumption of NSAIDs was associated with virulent H. pylori infection (P<0.000 1).
Table 3. Various risk factors with virulent H. pylori infection status (cag A and cag T positive) [n (%)].
| Variables | No. of HP positive subjects | Virulent HP | Non virulent HP | Odds ratio | 95% CI of odds ratio | |
| Gender | Male | 389 | 233 (60.00) | 156 (40.00) | 1.50 | 0.44-5.10 |
| Female | 190 | 95 (50.00) | 95 (50.00) | Referent | ||
| Consumption of alcohol | Current | 158 | 46 (28.57) | 112 (71.43) | Referent | |
| Never | 34 | 17 (50.00) | 17 (50.00)* | 2.44 | 1.14-5.18 | |
| Former | 142 | 57 (40.15) | 85 (59.85) | 1.63 | 1.01-2.64 | |
| Smoking | Current | 682 | 541 (79.32) | 141 (20.68)* | 3.84 | 1.10-13.44 |
| Never | 10 | 5 (50.00) | 5 (50.00) | Referent | ||
| Former | 32 | 24 (75.00) | 8 (25.00) | 3.00 | 0.69-13.12 | |
| NSAIDs use | Yes | 480 | 423 (88.23) | 57 (11.76)**** | 3.91 | 2.13-7.18 |
| No | 58 | 38 (66.66) | 20 (33.33) | Referent | ||
Statistical analysis between groups was carried out using Fischer exact test. The values in paranthesis indicate the percentage of the number of subjects. HP: H. pylori; *: P<0.05, ****: P<0.000 1 comparing with virulent H. pylori infection individuals.
The odds ratio and 95% CI of odds ratio in the population of asymptomatic subjects with respect to gender were (1.50, 0.44–5.10) in males when females subjects were taken as referent. The odds ratio and 95% CI of odds ratio in the population of asymptomatic subjects with respect to consumption of alcohol was as follows: never (2.44, 1.14–5.18) and former (1.63, 1.01–2.64) when current consumers of alcohol were taken as referent. The odds ratio and 95% CI of odds ratio in the population of asymptomatic subjects with respect to smoking of cigarettes were as follows: current (3.84, 1.10–13.44) and former (3.00, 0.69–13.12) when people who were never smokers were taken as referent. The odds ratio and 95% CI of odds ratio in the population of asymptomatic subjects who frequently consumed NSAIDs were (3.91, 2.13–7.18) when subjects who do not consume NSAIDs frequently were taken as referent.
4. Discussion
H. pylori is the causative agent of chronic gastritis, peptic ulcers, gastric adenocarcinoma, and lymphoma of the stomach[10]. Saliva has been a source to detect H. pylori from asymptomatic subjects as well as patients[13]. H. pylori can migrate from an infected individual to an uninfected individual via oral oral or fecal oral route. Oral cavity provides a harbor for the residence of H. pylori. Thereafter, it migrates to the gastric mucosa and resides in the acidic environment[14]. This concept has been used by various research workers to evaluate the infection status of H. pylori using the saliva from the oral cavity of H. pylori[13],[14]. H. pylori may exist in the oral cavity of such asymptomatic individuals without showing its pathological manifestations. It may exist in a dormant VBNC form. It is worth considering that other diagnostic techniques than PCR are unable to detect this form of H. pylori.
In the present study, 16S rRNA, cag A and T genes were amplified to detect the presence and virulence of H. pylori in the oral cavity. 16S rRNA gene is present in a highly conserved region of H. pylori genome and serves as a gold standard in the detection of H. pylori. The cag pathogenecity island is the hallmark of virulence of H. pylori[5]. The cag PAI is an important virulence component for infection and it gives shape to the pathological manifestations of H. pylori infection. A considerable proportion of the population in Asia is infected with H. pylori[13]. In brief, cag pathogenecity island comprises the various components of type IV bacterial secretion system which acts as a molecular needle and syringe to inject pathogenic proteins into the gastric epithelial cells[13],[14]. Injected cag A modulates signal transduction leading to detrimental host cell responses like up regulated proliferation of cells, apoptosis, and necrosis. Cag A positive strains of H. pylori significantly increase the risk for severe gastritis, peptic ulceration, and distal gastric cancer compared to strains that lack the cag island[15]. Hence, this gene was targeted in the present investigation to unravel the infection status and virulence in the infected asymptomatic subjects. Cag T has been implicated to be an important facet of the genetic make up of H. pylori and can be implicated to serve as an identification mark for the pathogenic state of H. pylori[8].
Smoking has been implicated as a decisive factor promoting the infection of H. pylori[17]. Our results are in concert with the previous workers who have examined the effect of smoking in the promotion of H. pylori infection[16]–[21].
Chronic NSAIDs consumption leads to decrease in mucin synthesis and promotes aggravation of ulcers and H. pylori colonization. It is evident that chronic NSAIDs consumption is a risk factor in H. pylori infection as the salivary samples of subjects on chronic NSAIDs therapy were found to be infected with H. pylori. Cag A gene was also successfully amplified in some of these subjects. This reflects that NSAIDs consumption is a risk factor for virulent H. pylori infection.
The present investigation shows that a small population of asymptomatic subjects possesses a virulent strain of H. pylori and needs to take precautionary measures. These subjects were asymptomatic during sampling but it may be hypothesised that they may have a higher chance of acquiring an active gastric malady in the near future.
Recently, alcohol consumption has been studied in detail with the disease status and it has been elucidated in other parts of world that alcohol consumption has a negative relation with H. pylori infection[22].
The detection of non virulent and virulent H. pylori from saliva of the asymptomatic subjects indicates that H. pylori exists in a non pathogenic form in a group of subjects. This form is called VBNC bacterial form. This refers that bacteria are in a state of very low metabolic activity and do not divide, but are alive and have the ability and become culturable once resuscitated[23].
Hence, a large population in western Indian population is a carrier of non virulent strain of H. pylori out of which a small population carries virulent (cag A positive) H. pylori. Hence, the individuals have a risk of acquiring active infection if the VBNC form transforms back to its helical form. In this form H. pylori can exist in a plethora of substrates like food, water or biofilm[24]–[26]. Thereafter, it can easily be transmitted to an uninfected person.
PCR is the technique that detects low levels of H. pylori as well as determines the virulent and non virulent strains. This form of H. pylori can't be detected by culture and PCR provides a perfect method for the detection of VBNC form. In this state, H. pylori is detectable but non virulent. Hence, cag A gene could not be amplified in most of the salivary samples of asymptomatic subjects.
The present study demonstrates that people need to abstain from smoking and habitual consumption of NSAIDs which pose a serious threat to the asymptomatic subjects of acquiring active infection and are aggravating factors for H. pylori infection. NSAIDs consumption has been previously demonstrated to be a significant risk factor for H. pylori infection[16]–[20].
The study shows that H. pylori exists in the oral cavity of a considerable portion of the asymptomatic subjects. However, the cag pathogenecity island genes cag A and T were not detected in a substantial portion of population in which 16S rRNA gene was amplified. Hence, it could be deduced that H. pylori is present in the asymptomatic subjects but the virulent factors are expressed in a fraction of them. However, virulent strains may give rise to a plethora of gastric diseases when the suitable pathobiological conditions culminate in the gastric environment of the patients[27]–[37].
Hence, there is a need to ascertain that smoking and NSAIDs use are limited in the population to halt the progression of the H. pylori mediated gastritis and lymphomas. Our investigation also confirms that the H. pylori inhabiting the oral cavity is non virulent and if the virulent genes, like cag A and T corresponding to cag PAI are targeted in the oral cavity, it may serve as a tool to monitor disease progression in asymptomatic subjects.
Our investigation will help epidemiologists and physicians to understand the pattern of disease in this geographical domain and its implications on public health. The outcome of this study is the finding that a considerable portion of population is under constant threat of being affected with fulminant manifestations of H. pylori infection if rapid measures are not initiated to eradicate this menace from food, water and other contamination sources.
Acknowledgments
The authors would like acknowledge Dr. SS Kadam, Vice-Chancellor and Dr. KR Mahadik, Principal, Poona College of Pharmacy, Bharati Vidyapeeth University, Pune, India, for providing necessary facilities to carry out the study. The authors are thankful to Dr. Aleem A Khan, Dr. Santosh K Tiwari and Dr. Manoj Gopi of Centre for Liver Research and Diagnostics, Owaisi Hospital, Hyderabad for valuable guidance in molecular biology techniques in the investigation. The authors acknowledge all India Council of Technical Eductaion for financial support as National Doctoral Fellowship to Pinaki Ghosh (RID/NDF-37/2009/10).
Footnotes
Foundation Project: This work was financially supported by India Council of Technical Education as National Doctoral Fellowship (grant No. RID/NDF-37/2009/10).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Ahmed N, Tenguria S, Nandanwar N. Helicobacter pylori-a seasoned pathogen by any other name. Gut Pathog. 2009;1:24. doi: 10.1186/1757-4749-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamangar F, Qiao YL, Blaser MJ, Sun XD, Katki H, Fan JH, et al. et al. Helicobacter pylori and oesophageal and gastric cancers in a prospective study in China. Br J Cancer. 2007;96(1):172–176. doi: 10.1038/sj.bjc.6603517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akhter Y, Ahmed I, Devi SM, Ahmed N. The co-evolved Helicobacter pylori and gastric cancer: trinity of bacterial virulence, host susceptibility and lifestyle. Infect Agent Cancer. 2007;2:2. doi: 10.1186/1750-9378-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, et al. et al. Helicobacter pylori Cag A phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5(1):23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Romo-González C, Salama NR, Burgeño-Ferreira J, Ponce-Castañeda V, Lazcano-Ponce E, Camorlinga-Ponce M, et al. et al. Differences in genome content among Helicobacter pylori isolates from patients with gastritis, duodenal ulcer, or gastric cancer revealnovel disease-associated genes. Infect Immun. 2009;77(5):2201–2211. doi: 10.1128/IAI.01284-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogihara A, Kikuchi S, Hasegawa A, Kurosawa M, Miki K, Kaneko E, et al. et al. Relationship between Helicobacter pylori infection and smoking and drinking habits. J Gastroenterol Hepatol. 2000;15(3):271–276. doi: 10.1046/j.1440-1746.2000.02077.x. [DOI] [PubMed] [Google Scholar]
- 7.Andersen LP, Rasmussen L. Helicobacter pylori-coccoid forms and biofilmformation. FEMS Immunol Med Microbiol. 2009;56(2):112–115. doi: 10.1111/j.1574-695X.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 8.Tiwari SK, Khan AA, Ahmed KS, Ali M, Habeeb A, Kauser F, et al. et al. PCR based analysis of the cag-PAI of Helicobacter pylori from saliva: an approach for rapid molecular genotyping in correlation with disease status. J Gastroenterol Hepatol. 2005;20(10):1560–1568. doi: 10.1111/j.1440-1746.2005.03955.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka E, Singh G, Saito A, Syouji A, Yamada T, Nakajima WUA, et al. et al. Prevalence of Helicobacter pylori infection and risk of upper gastrointestinal ulcer in patients with rheumatoid arthritis in Japan. Mod Rheumatol. 2005;15:340–345. doi: 10.1007/s10165-005-0419-5. [DOI] [PubMed] [Google Scholar]
- 10.Ghoshal UC, Chaturvedi R, Correa P. The enigma of Helicobacter pylori and gastric cancer. Indian J Gastroenterol. 2010;29(3):95–100. doi: 10.1007/s12664-010-0024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh V, Mishra S, Rao GR, Jain AK, Dixit VK, Gulati AK, et al. et al. Evaluation of nested PCR in detection of Helicobacter pylori targeting a highly conserved gene: HSP60. Helicobacter. 2008;13(1):30–34. doi: 10.1111/j.1523-5378.2008.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra S, Singh V, Rao GR, Dixit VK, Gulati AK, Nath G. Prevalence of Helicobacter pylori in asymptomatic subjects-a nested PCR based study. Infect Genet Evol. 2008;8(6):815–819. doi: 10.1016/j.meegid.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Kumar A, Dixit VK. Diversity in the cag pathogenicity island of Helicobacter pylori isolates in populations from North and South India. J Med Microbiol. 2010;59(1):32–40. doi: 10.1099/jmm.0.013763-0. [DOI] [PubMed] [Google Scholar]
- 14.Safak B, Ciftci IH, Dilek FH, Uslam I, Cetinkaya Z, Asik G, et al. et al. Prevalance of cag A and vac A genotypes of Helicobacter pylori isolated from Turkish patients with active or non active chronic gastritis. Scand J Infect Dis. 2010;42(6–7):435–438. doi: 10.3109/00365540903563418. [DOI] [PubMed] [Google Scholar]
- 15.Said Essa A, Alaa Eldeen Nouh M, Mohammed Ghaniam N, Graham DY, Said Sabry H. Prevalence of cagA in relation to clinical presentation of Helicobacter pylori infection in Egypt. Scand J Infect Dis. 2008;40(9):730–733. doi: 10.1080/00365540802023725. [DOI] [PubMed] [Google Scholar]
- 16.Yeh JM, Goldie SJ, Kuntz KM, Ezzati M. Effects of Helicobacter pylori infection and smoking on gastric cancer incidence in China: a population-level analysis of trends and projections. Cancer Causes Control. 2009;20(10):2021–2029. doi: 10.1007/s10552-009-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SW, Chang CS, Lee TY, Yeh HZ, Tung CF, Peng YC. Risk factors and therapeutic response in Chinese patients with peptic ulcer disease. World J Gastroenterol. 2010;16(16):2017–2022. doi: 10.3748/wjg.v16.i16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma B, Sharma N, Chauhan V, Thakur S, Kaushal SS. Relationship of smoking with H. pylori incidence in non-ulcer dyspepsia patients. J Indian Acad Clin Med. 2006;7(1):22–24. [Google Scholar]
- 19.Shi R, Xu S, Zhang H, Ding Y, Sun G, Huang X, et al. et al. Prevalence and risk factors for Helicobacter pylori infection in Chinese population. Helicobacter. 2008;13:157–165. doi: 10.1111/j.1523-5378.2008.00586.x. [DOI] [PubMed] [Google Scholar]
- 20.Peleteiro B, Lunet N, Figueiredo C, Carneiro F, David L, Barros H. Smoking, Helicobacter pylori virulence, and type of intestinal metaplasia in Portuguese males. Cancer Epidemiol Biomarkers Prev. 2007;16(2):322–326. doi: 10.1158/1055-9965.EPI-06-0885. [DOI] [PubMed] [Google Scholar]
- 21.Stec-Michalska K, Peczek L, Michalski B, Adamczyk M, Chojnacki J, Nawrot B. Influence of cigarette smoking on the level of mRNA of somatostatin receptor 3 (SSTR3) in the gastric mucosa of patients with functional dyspepsia. Adv Med Sci. 2010;55(1):53–58. doi: 10.2478/v10039-010-0026-3. [DOI] [PubMed] [Google Scholar]
- 22.Gao L, Weck MN, Stegmaier C, Rothenbacher D, Brenner H. Alcohol consumption, serum gamma-glutamyltransferase, and Helicobacter pylori infection in a population-based study among 9 733 older adults. Ann Epidemiol. 2010;20(2):122–128. doi: 10.1016/j.annepidem.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Giao MS, Azevedo NF, Wilks SA, Vieira MJ, Keevil CW. Persistence of Helicobacter pylori in heterotrophic drinking-water biofilms. Appl Environ Microbiol. 2008;74(19):5898. doi: 10.1128/AEM.00827-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gião MS, Azevedo NF, Wilks SA, Vieira MJ, Keevil CW. Effect of chlorine on incorporation of Helicobacter pylori into drinking water biofilms. Appl Environ Microbiol. 2010;76(5):1669–1673. doi: 10.1128/AEM.01378-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno Y, Piqueres P, Alonso JL, Jiménez A, González A, Ferrús MA. Survival and viability of Helicobacter pylori after inoculation into chlorinated drinking water. Water Res. 2007;41(15):3490–3496. doi: 10.1016/j.watres.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Buck A, Oliver JD. Survival of spinach-associated Helicobacter pylori in the viable but nonculturable state. Food Control. 2010;21:1150–1154. [Google Scholar]
- 27.Venkateshwari A, Krishnaveni D, Venugopal S, Shashikumar P, Vidyasagar A, Jyothy A. Helicobacter pylori infection in relation to gastric cancer progression. Indian J Cancer. 2011;48:94–98. doi: 10.4103/0019-509X.75840. [DOI] [PubMed] [Google Scholar]
- 28.Ani AE, Diarra B, Dahle UR, Lekuk C, Yetunde F, Somboro AM, et al. et al. Identification of mycobacteria and other acid fast organisms associated with pulmonary disease. Asian Pac J Trop Dis. 2011;1(4):259–262. [Google Scholar]
- 29.Mahapatra SK, Chakraborty SP, Das S, Kumar HA, Roy S. Prevalence of severe chloroquine resistance associates the point mutation in pfcrt and pfmdrI gene in eastern India. Asian Pac J Trop Dis. 2011;1(4):263–269. [Google Scholar]
- 30.Kouassi-M'Bengue A, Boni CC, Ouattara D, Berthé K, Doumbia M, Sévédé D, et al. et al. Co-infection of HIV and HBV in voluntary counseling and testing center in Abidjan. Asian Pac J Trop Dis. 2011;1(4):275–278. [Google Scholar]
- 31.Magana-Arachchi DN, Medagedara D, Thevanesam V. Molecular characterization of Mycobacterium tuberculosis isolates from Kandy, Sri Lanka. Asian Pac J Trop Dis. 2011;1(3):181–186. [Google Scholar]
- 32.Ophori EA, Isibor C, Onemu SO, Johnny EJ. Immunological response to Helicobacter pylori among healthy volunteers in Agbor, Nigeria. Asian Pac J Trop Dis. 2011;1(1):38–40. [Google Scholar]
- 33.Kumar TD, Pankaja SS, Rao HK, Kate V. Evaluation of Helicobacter pylori infection and other risk factors in patients with benign peptic ulcer disease. Asian Pac J Trop Dis. 2011;1(1):50–51. [Google Scholar]
- 34.Ravikumar H, Ramachandraswamy N, Puttaraju HP. Molecular strain typing of Wolbachia infection from Indian mosquitoes using wsp gene. Asian Pac J Trop Dis. 2011;1(2):106–109. [Google Scholar]
- 35.Daniel O, Osman E, Adebiyi P, Mourad G, Declarcq E, Bakare R. Non tuberculosis mycobacteria isolates among new and previously treated pulmonary tuberculosis patients in Nigeria. Asian Pac J Trop Dis. 2011;1(2):113–115. [Google Scholar]
- 36.Kurashima Y, Murata-Kamiya N, Kikuchi K, Higashi H, Azuma T, Kondo S, et al. et al. Deregulation of β-catenin signal by Helicobacter pylori Cag A requires the Cag A-multimerization sequence. Int J Cancer. 2008;122:823–831. doi: 10.1002/ijc.23190. [DOI] [PubMed] [Google Scholar]
- 37.Kenneth EL. McColl Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]


