Abstract
Objective
To evaluate the berries of Phytolacca dodecandra (P. dodecandra) for its effect on Histoplasma capsulatum var. farciminosum (HCF) and for the treatment of cases of epizootic lymphangitis (EL).
Methods
Samples were collected from un-ruptured nodules of cases of EL at Debre Zeit and Akaki (central Ethiopia). Mycological culture and isolation of HCF were performed at the Aklilu Lemma Institute of Pathobiology. Phytochemical screening was done for n-butanol extract of P. dodecandra to detect alkaloids, saponins, phenolic compounds and flavonoids. The minimum inhibitory concentrations (MICs) and minimum fungicidal concentrations (MFCs) of aqueous and n-butanol extracts of P. dodecandra against HCF were determined by agar dilution assay. For the in vivo trial, 5% simple ointment was prepared from n-butanol extract and applied topically to 24 (twelve early and twelve moderate) cases of EL.
Results
Phytochemical screening showed that n-butanol extract of P. dodecandra was positive for alkaloids, saponins and phenolic compounds but negative for flavonoids. The MICs of n-butanol and aqueous extracts of P. dodecandra were (0.039%–0.078%) and (0.625%–1.250%), respectively. The MFCs of n-butanol and aqueous extracts of P. dodecandra were (0.078%–0.156%) and (1.250%–2.500%), respectively. The MIC and MFC of ketoconazole (positive control) was (1.200×10−5%–2.500×10−5%) and (5.000×10−5%–1.000×10−4%), respectively while growth was observed on free medium (negative control). From the total of 24 treated cases of EL, 14 (58.3%) responded to treatment; however, 10 (41.7%) did not respond to treatment. There was no significant difference in the degree of response to treatment between early and moderate cases (χ2=0.686; P=0.408).
Conclusions
It can be concluded that n-butanol extract of P. dodecandra demonstrates antifungal effects while the aqueous extract shows no antifungal activity.
Keywords: Epizootic lymphangitis, Phytolacca dodecandra, Histoplasma capsulatum var. farciminosum, Minimum inhibitory concentration, Minimum fungicidal, concentration, Phytochemical screening, Antifungal activity, Agar dilution assay, Mycological culture, In vivo trial, Contagious disease, Chronic disease, Lymphangitis
1. Introduction
Epizootic lymphangitis (EL) is a contagious, chronic disease which mainly affects horses, mules and donkeys. It is caused by Histoplasma capsulatum var. farciminosum (HCF). The disease is characterized clinically by a suppurative, ulcerating, and spreading pyogranulomatous, multifocal dermatitis and lymphangitis. It is seen most commonly in the extremities, chest wall and the neck, but it can also be manifested as an ulcerating conjunctivitis of the palpebral conjunctiva, or rarely as a multifocal pneumonia. The organism may also invade open lesions including ruptured strangles abscesses and castration wounds[1]. EL is more common in tropical and subtropical regions than in temperate zones. Currently, HCF is endemic in some countries in the Mediterranean region, and in parts of Africa and Asia including India, Pakistan and Japan[1]. HCF infects animals through wounds. The source of the organisms can be the skin lesions, nasal and ocular exudates of infected animals, or the soil. This organism can also be spread by fomites and biting flies mechanically.
Many treatment types have been tried, largely without success. Parenteral iodides and amphotericin B have been reported as effective. However, although the disease is highly prevalent and economically important in Ethiopia[2], the treatment options mentioned have not been employed because of the cost of the drugs and their absence in Ethiopia. This warrants for the need for other approaches including the use of traditional remedies. In Ethiopia, traditional medicine has long been practiced. It is common for Ethiopians to treat some common ailments using plants available around them[3]. Natural products and their derivatives have historically been sources of therapeutic agents.
As drugs such as aspirin, digitalis, morphine, and quinine were all originally isolated or synthesized from compounds derived from plants, knowledge gained from the use of medicinal plants and their active ingredients serves as the foundation for much of modern drugs[4]. Antifungal effect is one of the effects of secondary metabolites produced by plants. Phytolacca dodecandra (P. dodecandra) is one of the many plants claimed to have antifungal secondary metabolites. Many studies indicated that, saponins are responsible for its antifungal effect. The antifungal effect of the crude aqueous extract of P. dodecandra was demonstrated in vitro against different genera of dermatophytes of human pathogen and four clinical isolates of Candida albicans[5]. The crude aqueous extract was also found to have effect against HCF both in vitro and in vivo[6],[7]. Further evaluation of different extracts of P. dodecandra on HCF isolates and cases of EL would of paramount importance towards the effort made to control EL in endemic countries. The objective of the present study was, therefore, to investigate the in vitro and in vivo effects of P. dodecandra on HCF isolates and cases of EL.
2. Materials and methods
2.1. Test fungus
Pus samples were collected directly aseptically from un-ruptured nodules of EL cases of horses visiting Society for the Protection of Animals Abroad (SPANA) Veterinary Clinic with sterile disposable syringe after washing, shaving and disinfection of the area, and placed in an icebox. Then, the samples were transported to Aklilu Lemma Institute of Pathobiology (ALIPB) Laboratory by keeping the chain cold and cultured immediately within a day[8]. The pus samples were inoculated onto Sabourauds dextrose agar supplemented with 2.5% glycerol and 0.5 g/L of chloramphenicol. Thereafter, the inoculated medium was incubated at 27 °C for 6–8 weeks, and the growth of the fungal colony was checked continuously once a week. Then the primary colonies were sub-cultured to get pure colony.
2.2. Collection and preparation of P. dodecandra
The berries of P. dodecandra (type 44) were harvested in fully developed green stage collected from ALIPB[9]. The dried berries were garbled and grinded to powder for extraction. The powder was subjected to sieve with 250 µm size mesh to get a fine material and then stored at room temperature in dry place until use.
A known weight of the powdered P. dodecandra was defatted with petroleum ether (RFCL Limited, New Delhi), then the extract was removed and the marc was allowed to dry. The dried marc was extracted with n-butanol (Blulux Laboraory Pvt. Ltd., India) using maceration method of extraction. Maceration continued for 48 hours with frequent agitation and the resulting supernatant was filtered using filter paper (Whatman No. 1). The process of extraction was repeated three times and the filtrates of all portions were collected in one vessel. The organic solvent was removed by evaporation using Rota Vapor (BÜCHI Rota-vapor R-205). The residue was then placed on a water bath at 40 °C. In a similar way, the aqueous extract was prepared by soaking the defatted powder in the water after which, it was filtered with sterile muslin cloth and then freeze dried using lyophilizer. The resulting dried mass was weighed as percentage yield and packed into a glass vial and stored in a desiccator over silica gel until use. Fresh stock solution was prepared for the experiment whenever required[10].
2.3. Phytochemical screening
The n-butanol extract of P. dodecandra was subjected to phytochemical screening using a standard screening procedure since it showed better antifungal activity[10].
2.3.1. Test for alkaloids
Two grams of the n-butanol extract was stirred with 10 mL of 1% HCl and heated for 30 minutes in a steam bath. The mixture was then filtered with filter paper and 5 drops of Dragendorff's reagent were added. Turbidity with yellow-orange precipitate was concluded to indicate the presence of alkaloids.
2.3.2. Test for saponins
Two grams of n-butanol extract was stirred with 20 mL of distilled water. The mixture was heated in a steam bath for 5 minutes and filtered with filter paper. Ten millilitre of the filtered solutions were taken in 25 mL measuring cylinder and shaked vigorously. Formation of honey comb froth which persists upon warming was taken as a preliminary evidence for the presence of saponin.
2.3.3. Test for phenolic compounds
Two millilitre of 1% n-butanol extract solutions and 3 drops of a mixture of 1 mL of 1% FeCl3 and 1 mL of 1% K3Fe(CN)6 were mixed. Formation of green blue colour was taken to indicate the presence of phenolic compounds.
2.3.4. Test for flavonoids
Two millilitres of 1% n-butanol extract solutions and 5 drops of 2% lead acetate solutions were mixed. The development of yellow orange colour was taken as an indication for the presence of flavonoids.
2.4. Evaluation of in vitro antifungal activity of P. dodecandra
2.4.1. Agar dilution assay
Agar dilution assay is one of the methods used to test the antifungal effect of natural products[11]. In this study, a stock solution of the extracts (aquoeus and n-butanol), was prepared in a saline as 20% concentration with 10 mL volume, by mixing 8 mL saline with 2 g of the extract. The stock solutions were then filtered with a filter paper (Whatman No. 1). For positive control, ketoconazole was used since amphotorcin B was not available in the market. Ketoconazole (with 99.1% potency) was prepared in the range of 1.2 µg/10 mL to 640 µg/10 mL which was the range for moulds[12]. For the negative control, 10 mL saline (0.85%) was used as a stock solution.
Two fold serial dilutions of the extracts and positive control were prepared in Sabourauds dextrose agar in proportions of 10%, 5%, 2.5%, 1.25%, 0.625%, 0.313%, 0.156%, 0.078%, 0.039%, and 0.019 5%[13] in triplicates, and allowed to solidify in slant positions at room temperature overnight.
2.4.2. Preparation of the test fungus and inoculation
The colonies were scrapped by a sterile inoculating wire loop and transferred to a sterile saline (0.85%). Tween 80 (Sigma) was added in order to wet the moulds and then mixed by shaking vigorously using Vortex mixer. The turbidity of the inoculum was compared with 0.5 MacFarland standard (which is equivalent to 0.4×104 to 5×104 cfu/mL for moulds). The density of the test suspension was compared with that of the standard and it was adjusted by adding either more fungal colony or sterile saline. The prepared agar dilutions of the extract and controls were inoculated by dipping sterile swab into the test suspensions. Excess inoculum was removed by pressing and rotating the swab firmly against the side of the tube above the level of the liquid suspension. Then, the swab was streaked all over the surface of the agar medium in radial pattern. Finally, the lids of the inoculated agar plates were closed and incubated at 27 °C for 6-8 weeks with followup[14].
2.4.3. Determination of minimum inhibitory concentration (MIC)
In order to determine the MIC, two fold serial dilutions of n-butanol and aqueous extracts in the range of 0.019 5% to 10%, ketoconazole in the range of 1.2 µg/10 mL to 640 µg/10 mL (standard) and saline were inoculated with the test fungus. The agar dilution assay and inoculation of the test fungus were repeated three times to see the reputability of the experiment. The lowest concentrations that inhibit visible growth of the fungus after 6–8 weeks incubation were considered to be the MIC. The results of MIC were given in mg/10 mL or percentage[13].
2.4.4. Determination of minimum fungicidal concentration (MFC)
The MFC was determined by re-inoculation of each of the inhibited media on the extract (standard) free Sabourauds dextrose agar media separately. The inoculated media was incubated at 27 °C for 6–8 weeks with followup. Absence of mycelia growth after 6–8 weeks indicated fungicidal activity. The lowest concentrations of the test extract or standard that prevented growth of the fungus were considered to be MFC and the results were given in mg/10 mL or percentages[13].
2.5. Evaluation of in vivo antifungal activity of P. dodecandra
2.5.1. Topical ointment preparation
The ointments were prepared according to the formulated preparations stated by British Pharmacopoeia. Simple ointment was prepared from 50 g of wool fat, 50 g of hard paraffin, 50 g of cetostearyl alcohol and 850 g of white soft paraffin[15].
The four ingredients were mixed by heating gently with stirring to make homogenous. Some drops of dimethlsulfoxide (DMSO) were used to mix the n-butanol extract with the ointments uniformly[15]. The ointment was formulated at 5% concentration, and the formulated ointments were stored at 4 °C until used.
2.5.2. Selection of animals for the trial
Cases of EL visiting SPANA Veterinary Clinic were used for the trial. The cases were clinically categorized as early and moderate according to the criteria used by SPANA. Distance from the clinic and willingness of owners to bring their horse every week for treatment and followup were used as additional criteria for selection of cases. A total of 24 EL cases of horses were used.
2.5.3. Application of the ointment
When cases came to the clinic, they were examined clinically and only early and moderate stages of the disease were selected to take the treatment. To apply the ointment, the area of the lesions (nodules) was washed, shaved, and the pus was drained by using surgical blade. The owner of the animals were taught and advised about the application, dosage regimen of the ointment (the ointment applied twice a day after cleaning the area) and to bring their horses every week for 2 months. The progress of the disease was recorded every week by taking picture. Horses which did not cure at the end of the trial were treated free by SPANA Veterinary Clinic.
2.5.4. Ethical considerations
Owners were informed about their rights and responsibilities (absence of compensation fee, the right to withdraw their horse from the trial, aim and outcome of the study). Willingness of owners was asked verbally before the horse started the treatment. Horses that did not responded for the new drug entity were managed and treated by SPANA Veterinary Clinic free of charge. There were no controlled groups, negative controls (because not ethically accepted) and positive controls (because of the absence of standard drugs) but only the test groups were given the new drug entity.
2.6. Data analysis
Variation in the degree of response, which was measured through the observation of the healing of the lesions, to treatment between early and moderate stages of the disease was analyzed by the Chi-square (χ2) test using SPSS 17.0 software.
3. Results
3.1. Yields of the crude extracts
The average yields for n-butanol and aqueous extracts of P. dodecandra were 32.82% and 20.8%, respectively. The results of phytochemical screening showed that n-butanol extract of P. dodecandra berries was positive for alkaloids, saponins and phenolic compounds but negative for flavonoids.
3.2. In vitro antifungal activity
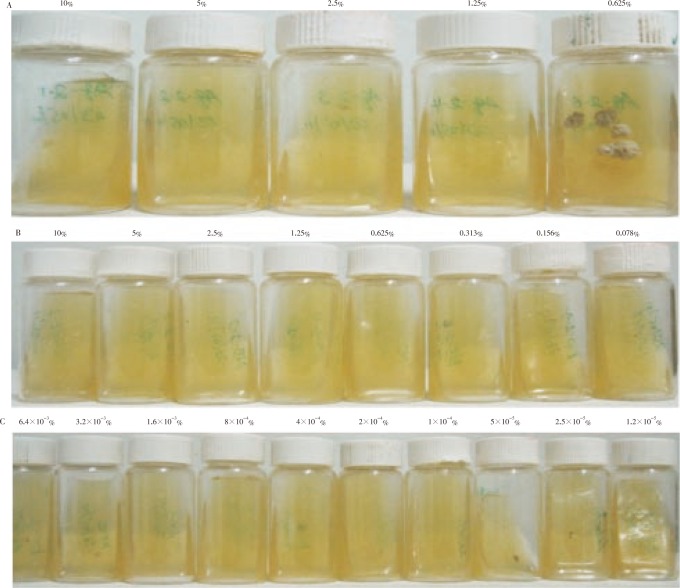
The MICs of aqueous and n-butanol extracts of P. dodecandra against HCF were (0.625%–1.250%) and (0.039%–0.078%), respectively, while that of the positive control (ketoconazole) was (1.200×10−5%–2.500×10−5%) (Figure 1).
Figure 1. The MICs of aqueous and n-butanol extracts of P. dodecandra against HCF.
A: MIC of aqueous extract: growth was observed starting at 0.625%; B: MIC of n-butanol extract: growth was observed starting from 0.039%; C: MIC of ketoconazole (standard): growth was observed at a concentration of 1.2×10−5%; D: Saline diluted Sabourauds dextrose agar (negative control): growth was observed in all agar plates.
The MFCs of aqueous and n-butanol extracts of P. dodecandra against HCF were (1.250%–2.500%) and (0.078%–0.156%), respectively (Figure 2), while that of the positive control (ketoconazole) was (5.000×10−5%–1.000×10−4%).
Figure 2. MFCs of aqueous and n-butanol extracts of P. dodecandra against HCF.
A: MFC of aqueous extract; B: MFC of n-butanol; C: MFC of the positive control (ketoconazole).
3.3. In vivo antifungal activity
The results of the in vivo trial showed that 14 (58.3%) were completely cured from the fungal infections with 8 in early stage and 6 in moderate stage (Figure 3). On the other hand, no response was observed in 10 (41.7%) of the cases with 4 in early stage and 6 in moderate stage. No significant difference in the degree of response to treatment was observed between early and moderate cases (χ2= 0.686, OR=2, 95%CI=0.384-10.409, P=0.408).
Figure 3. Lesions of EL before treatment (left column) and after completing the treatment (right column).

The ulcers completely healed, forming scar after application of ointment for two weeks.
4. Discussion
In the present study, n-butanol and aqueous extracts of P. dodecandra were evaluated for their effects on the isolates of HCF and for the treatment of cases of EL. The phytochemical analysis of P. dodecandra showed the presence of saponins, alkaloids, and phenolic compounds in the berries of P. dodecandra. Thus, the secondary metabolites identified in the berries are all active antifungal compounds[16], which could imply that these secondary metabolites could be responsible for the antifungal activity of the berries observed in the n-butanol extract of the berries.
The antifungal effect of n-butanol extract was observed to be much greater than that of the aqueous extract. The MIC of n-butanol extract ranged from (0.039%–0.078%); whereas that of the aqueous extract was in the range of (0.625%–1.250%). Similar finding for the aqueous extract was reported in which the MIC of P. dodecandra against the yeast forms of different Candida species were higher than 0.5%[5]. Another study showed that the MIC of the aqueous extract of P. dodecandra was 1%[6]. The MIC for novel pharmacological compounds should be <0.1%[17]. As the MIC of the aqueous extract of P. dodecandra against HCF was greater than the recommended level (0.1%), this extract could be considered inactive. On the other hand, the MIC of n-butanol extract of P. dodecandra was found to be below 0.1% and hence this extract could be considered active.
The MFCs of aqueous and n-butanol extract of P. dodecandra ranged from (1.250%–2.500%) and (0.078%–0.156%), respectively. Similar results were reported against different dermatophyte strains of human pathogen[5]. The MIC and MFC of n-butanol extract were found to be higher than the standard indicating that it is less potent as compared to the standard.
For the in vivo trial, the prepared ointment was topically applied and the result showed that, 58.3% were completely healed, while 41.7% did not cure. Comparable results were reported with the aqueous extracts[6],[7]. The present study showed that, n-butanol extract of P. dodecandra was effective against HCF. Previous toxicity studies on P. dodecandra indicated that human and guinea pigs can tolerate skin irritation of P. dodecandra. Moreover, oral LD50 were found to be 2.6 and 2.2 g/kg in mice and rats, respectively[18]. This could also indicate the wide safety margin of the berries. The observations that there was no skin reaction when the topical ointments were applied on horses could further strengthen the safe nature of the extracts with high therapeutic index.
Different limitations were encountered during the study periods. Amphotericin B would have been used as a standard drug for the in vivo and in vitro trial but due to its absence in the market no positive control was included in the in vivo trial but ketoconazole used as a positive control for the in vitro trial. Owners were advised to apply the ointment twice a day but it is unknown whether each owner was strictly followed the advice of the researchers. Thus, to avoid such discrepancies, controlled experiment is recommended where the experimental horses will be under the full control of the researcher.
Nevertheless, the finding of the preliminary study seems promising as the n-butanol extract of P. dodecandra demonstrated MIC and MFC values that are considered to have antifungal properties. Therefore, since antifungals are not available for veterinary use in Ethiopia and also as they are expensive, searching for available and affordable antifungals such as n-butanol extract of P. dodecandra is recommended for the treatment of EL.
Footnotes
Foundation Project: This work was financially supported by the Graduate Program the Addis Ababa University and the Aklilu Lemma Institute of Pathobiology Addis Ababa University (grant No. CAOR/PY-059/2011).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.OIE . Update for epizootic lymphangitis. USA: Center for Food Security and Public Health (CFSPH), and Institute for International Cooperation in Animal Biologics, an OIE Collaborating Centre; 2009. pp. 1–4. [Google Scholar]
- 2.Ameni G. Epidemiology of equine histoplasmosis (epizootic lymphangitis) in carthorses in Ethiopia. Vet J. 2006;172:160–165. doi: 10.1016/j.tvjl.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Yirga G. Ethnobotanical study of medicinal plants in and around Alamata, Southern Tigray, Northern Ethiopia. Curr Res J Biol Sci. 2010;2(5):338–344. [Google Scholar]
- 4.Ansari JA, Inamdar NN. The promise of traditional medicines. Int J Pharmacol. 2010;6(6):808–812. [Google Scholar]
- 5.Woldeamanuel Y, Abate G, Chryssanthou E. In vitro activity of Phytolacca dodecandra (Endod) against dermatophytes. Ethiop Med J. 2005;43(1):31–34. [PubMed] [Google Scholar]
- 6.Ameni G, Tilahun G. Preliminary laboratory and field evaluation of Endod for treatment of epizootic lymphangitis. Bull Anim Health Prod Afr. 2003;51:153–160. [Google Scholar]
- 7.Hadush B, Ameni G, Medhin G. Equine histoplasmosis: treatment trial in cart horses in Central Ethiopia. Trop Anim Health Prod. 2008;40:407–411. doi: 10.1007/s11250-007-9099-9. [DOI] [PubMed] [Google Scholar]
- 8.OIE . Terrestrial manual for epizootic lymphangitis. Paris: Office International des Epizootics (OIE); 2008. pp. 852–857. [Google Scholar]
- 9.Lemma A, Brody G, Newell GW, Parkhurst RM, Skinner WA. Studies on the molluscicidal properties of Endod (Phytolacca dodecandra) increased potency with butanol extraction. J Parasitol. 1972;58:104–107. [PubMed] [Google Scholar]
- 10.Evans WC, Trease GE. Trease and Evans' pharmacognosy. 16th ed. Edinburgh: W.B. Saunders; 2009. pp. 214–393. [Google Scholar]
- 11.Das K, Tiwari RKS, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: current methods and future trends. J Med Plant Res. 2010;4(2):104–111. [Google Scholar]
- 12.EUCAST . EUCAST E.DEF 9.1: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. Europe: European Committee on Antimicrobial Susceptibility Testing (EUCAST); 2008. pp. 1–13. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis. 2009;49(11):1749–1755. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- 14.CLSI . Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. 2nd ed. Pennsylvania, USA: CLSI; 2008. pp. 1–35. [Google Scholar]
- 15.Gaur R, Azizi M, Gan J, Hansal P, Harper K, Manna R, et al. et al. Formulated preparations, specific monographs simple ointment. London: Medicines and Healthcare Products Regulatory Agency (MHRA); 2009. p. 9983. [Google Scholar]
- 16.Arif T, Bhosale JD, Kumar N, Mandal JK, Bendre RS, Lavekar GS, et al. et al. Natural products-antifungal agents derived from plants. J Asian Nat Prod Res. 2009;11(7):621–638. doi: 10.1080/10286020902942350. [DOI] [PubMed] [Google Scholar]
- 17.Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med. 2010a;76:1479–1491. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- 18.Lemma A. Laboratory and field evaluation of the molluscicidal properties of Phytolacca dodecandra. Bull World Health Organ. 1970;42:597–617. [PMC free article] [PubMed] [Google Scholar]