Abstract
Objective
To investigate the anticonvulsant activity of the lobeline isolated from the Lobelia nicotianaefolia in chemoconvulsant-induced seizures and its biochemical mechanism by investigating relationship between seizure activities and altered gamma amino butyric acid (GABA) in brain of mice in Pentylenetetrazol (PTZ) seizure models.
Methods
The anticonvulsant activity of the isolated lobeline (5, 10, 20 and 30 mg/kg, i.p.) was investigated in PTZ and strychnine induced seizures in mice and the effect of isolated lobeline on brain GABA level in seizures induced by PTZ. Diazepam was used as reference anticonvulsant drugs for comparison.
Results
Isolated lobeline (10, 20 and 30 mg/kg, i.p.) significantly delayed and antagonized (P < 0.050–0.001) the onset of PTZ-induced seizures. It also antagonized strychnine induced seizures. The mortality was also prevented in the test group of animals. In biochemical evaluation, isolated lobeline (5, 10 and 20 mg/kg, i.p.) significantly increased the brain GABA level. And at dose of 30 mg/kg GABA level showed slight decrease in PTZ model.
Conclusions
In our findings, isolated lobeline (20mg/kg) exhibited potent anticonvulsant activity against PTZ induced seizures. Also a biochemical evaluation suggested significant increase in barain GABA level at 20 mg/kg i.p. of isolated lobeline. Hence, we may propose that lobeline reduces epileptic seizures by enhancing the GABA release supporting the GABAergic mechanism.
Keywords: Lobelia nicotianaefolia, Lobeline, Brain GABA level, Antiepileptic activity, Pentylenetetrazol (PTZ), Strychnine
1. Introduction
Epilepsy is the second most common neurological disorder in India. Epilepsy affects an estimated 7 million people in India, and 50 million worldwide. Approximately 40% of them are women. The prevalence of epilepsy is 0.7% in India, which is comparable to the USA and other developing nations. The estimated incidence rate range from 40 to 60 per 1000000 population per year. The WHO estimated that approximately 80% people with epilepsy live in developing countries and most of them do not get adequate medical treatment[1]. The introduction of each new antiepileptic drug into the market raises valid expectations in patients and physicians for more effective treatment of epilepsy. Although safety, tolerability, and interactions are important, better efficacy is a crucial feature for a new antiepileptic drug[2].
In the Indian traditional system of medicine, various herbs have been used to treat epileptic seizures. Lobelia has been recognized and used in traditional systems of medicine for their antiepileptic activity in crude and their respective dosage forms. Lobelia nicotianaefolia (L. nicotianaefolia) is a rich source of alkaloids of the lobeline group and used as a substitute for Lobelia inflata[3],[4]. The plant has been recorded to contain several alkaloids with the main alkaloid lobeline apparently observed, revealed higher concentrations. The total alkaloidal content ranges from 1.04–1.18%[5]. According to ethnobotanical survey, leaves and inflorescence are antispasmodic and used for the treatment of asthma, bronchitis and fever[6]. In Ayurveda, this plant is used for sciatia and back pain[7]. Roots are useful in the treatment of eye diseases. Decoction of flowers was given orally in asthma[8]. Leaves has been used for speedy healing of wounds[9]. The use of lobeline as a smoking deterrent was reported in 1936, but several later studies led to a dispute between positive and negative reports[10]. In a recent patent, studies on a new formulation of drugs to deliver an effective amount of lobeline to sublingual mucosa were done. Sublingual formulation of lobeline sulfate does not appear to be an effective smoking cessation aid[11].
Lobeline was shown to attenuate self-administration of heroin and amphetamine[12],[13], also used in the treatment of alcohol abuse[14]–[16]. Lobeline and its analogues could represent a novel class of therapeutic agents with a great potential for the treatment of psychostimulant abuse[17],[18]. Lobelane, a molecule formed from deoxygenation of the lobeline molecule, has a shorter duration of action compared to lobeline[19]. The potassium large conductance calcium-activated channel KCNMA1 a protein might be targeted by Lobeline which was thought to be one of the reason in treating drug abuse[20]. Zheng et al. suggested that the noncompetitive inhibition of VMAT2 function by lobeline might be the mechanism in the treatment of drug abuse[21].
Lobeline is used as a pharmacotherapy for substance use disorder and attention deficit hyperactivity disorder[22]. The effect of lobeline in improving learning may be useful in the development of novel treatments for learning deficits[23].
However, no significant data supporting the efficacy of lobeline as an antiepileptic is available in official literature. Hence, the present study was designed to verify anti-epileptic effect of isolated lobeline and its effect on gamma amino butyric acid (GABA) level in mice brain.
2. Materials and methods
2.1. Collection and authentification of plant material
The plant L. nicotianaefolia Roth E. &S. was collected from the dense forest of Varandha Ghat, Bhor, cleaned and dried at room temperature in shade and kept away from direct sunlight and it was authenticated by Botanical Survey of India, Koregaon Park, Pune (Voucher specimen number ALLALON1BSI/WRC/Tech/2010/).
2.2. Extraction and isolation of lobeline
The powdered herb (800 g) was moistened with water (3.5 L), acidified with acetic acid (700 mL) and left macerated for three hours. The mass was then pressed and the process of moistening and pressing was repeated and the collected pressed solutions were filtered. The filtrate was alkalinized with 10% sodium bicarbonate solution and then extracted with petroleum ether successively (100 mL×3) for purification. The ether extract was then shaken with water (100 mL×3) acidulated with sulphuric acid (pH 3). The acidic solution was rendered alkaline with 10% sodium bicarbonate solution and shaken with ether (50 mL×3). The ether fraction was evaporated to dryness, and the resulting yellow oily residue containing the total alkaloids was again dissolved in water (100 mL) acidified with dilute hydrochloric acid (50 mL), then it was filtered and shaken successively with chloroform (50 mL×3); the chloroform extracted only lobeline hydrochloride leaving the salt of the other alkaloids in the aqueous layer. The chloroform was then evaporated under reduced pressure. The brownish oily residue was then stirred with twice its volume of hot water at about 60 °C. The aqueous solution was then left sometimes under vacuum over sulphuric acid when lobeline hydrochloride crystallizes out. Isolated lobeline was recrystallized from alcohol to obtain off-white needle shaped lobeline[24],[25].
2.3. Animals
Swiss albino male mice weighing around 22–25 g were obtained from the Yash Farm, Pune. The animals were housed in groups of 6–8 per cage and maintained at (24 ± 2) °C, with relative humidity of 45–55% and 12:12 h dark/light cycle. The animals had free access to standard chew pellets and water. All the animals were fasted for 16 h, but still allowed free access to tap water before the commencement of our experiments. The Institutional Animal Ethics Committee of Allana College of Pharmacy, Pune approved the pharmacological and acute toxicity protocol (IAEC No: Ref/ACP/IAEC/10-11/18-09).
2.4. Chemicals
The PTZ was procured from Sigma chemicals, USA. Strychnine was procured from Indus Biotech Pvt Ltd., Pune. Diazepam was procured from Ranbaxy Lab, Mumbai.
2.5. Acute toxicity study
Acute toxicity study was performed in mice according to OECD guidelines. The isolated lobeline was administered intraperitoneally (i.p) at doses of 1.75, 5.5, 17.5, 55 mg/kg. They were then observed for signs of toxicity, continuously for 2 h, and for mortality up to 24 h, after injection[26].
2.6. Assessment of anti-convulsant activity
2.6.1. PTZ induced convulsions
The mice were divided into six groups of six animals each. The isolated lobeline was administered at doses 5, 10, 20, 30 mg/kg i.p. Group I was treated with vehicle (saline) and Group II with diazepam at a dose of 1 mg/kg i.p. Group III, IV, V and VI were administered with isolated lobeline in a dose of 5, 10, 20, 30 mg/kg respectively. All the drug treatment was given thirty minutes before the injection of PTZ (90 mg/kg i.p). Each animal was placed in individual plastic cage for observation lasting forty five minutes. The onset of general clonus was recorded and tonic clonic convulsions were studied. Protection of the animals from mortality was recorded[27].
2.6.2. Strychnine induced convulsions
For the evaluation of anti-convulsant activity against strychnine induced convulsions, the same procedure was adopted as mentioned in PTZ induced convulsions. Instead, strychnine (3 mg/kg i.p) was used for inducing convulsions[12].
2.7. Biochemical evaluation by estimation of brain GABA
Animals were divided into seven groups consisting six mice in each group. Treatment schedule was as shown in Table 1. Forty-five min after vehicle or lobeline and 30 min after diazepam, mice were sacrificed. Group three was sacrificed as soon as onset of convulsions occurs or 65 sec after PTZ treatment. Brain was isolated immediately and transferred to homogenization tube containing 5 mL of 0.01 M hydrochloric acid and homogenized. Brain homogenate was transferred to bottle containing 8 mL of ice cold absolute alcohol and kept for 1 h at 0 °C. The content was centrifuged for 10 min at 16 000 rpm, supernatant was collected in petridish. Precipitate was washed with 5 mL of 75% alcohol for three times and washes were combined with supernatant. Contents in petridish were evaporated to dryness at 70 °C on water bath under stream of air. To the dry mass 1 mL water and 2 mL chloroform were added and centrifuged at 2 000 rpm. Upper phase containing GABA (2.0 mL) was separated and 10 µL of it was applied as spot on Whatman paper (No.41).
Table 1. Table showing treatment schedule of biochemical evaluation by estimation of brain GABA.
| Groups | Treatment | Dose |
| 1 | Vehicle | 1 mL/kg i.p. |
| 2 | Lobeline | 5 mg/kg i.p. |
| 3 | Lobeline | 10 mg/kg i.p. |
| 4 | Lobeline | 20 mg/kg i.p. |
| 5 | Lobeline | 30 mg/kg i.p. |
| 6 | Pentylenetetrazole (PTZ) | 80 mg/kg i.p. |
| 7 | Diazepam | 5 (mg/kg i.p. |
The mobile phase consisted of n-butanol (50 mL) acetic acid (12 mL) and water (60 mL). The chamber was saturated for half an hour with mobile phase. The paper chromatogram was developed with ascending technique. The paper was dried in hot air and then spread with 0.5% ninhydrin solution in 95% ethanol. The paper was dried for 1 h at 90 °C. Blue color spot developed on paper was cut and heated with 2 mL ninhydrin solution on water bath for 5 min. Water (5.0 mL) was added to solution and kept for 1h. Supernatant (2.0 mL) was decanted and absorbance was measured at 570 nm[28].
2.8. Statistical analysis
The statistical analysis of all the observations was carried out using one-way ANOVA followed by Dunnett's test. Values were presented as Mean ± SEM using Graph Pad Prism – 5 Statistical Software Package. The level of significance was set as P< 0.05.
3. Results
3.1. Acute toxicity study
Intraperitoneal administrations of stepwise, escalated doses of isolated lobeline in mice gave an LD50 value as 40 mg/kg.
3.2. PTZ induced convulsions
The onset of seizure was significantly delayed in the animals treated with isolated lobeline at 10, 20 and 30 mg/kg dose (Figure 1). The onset was most significantly delayed in 20 mg/kg dose, when compared with the vehicle (saline) treated group. Also, lobeline in a dose of 20 mg/kg was found to be the most significant in duration of clonic and tonic seizures (Figure 2, 3). The mortality was also prevented in the test group of animals but not in a dose dependent manner (Table 2).
Figure 1. The onset of seizures using PTZ. * P<0.05, ** P<0.01, *** P<0.001. All data compared with the control using one way ANOVA followed by Dunnet's post hoc test.
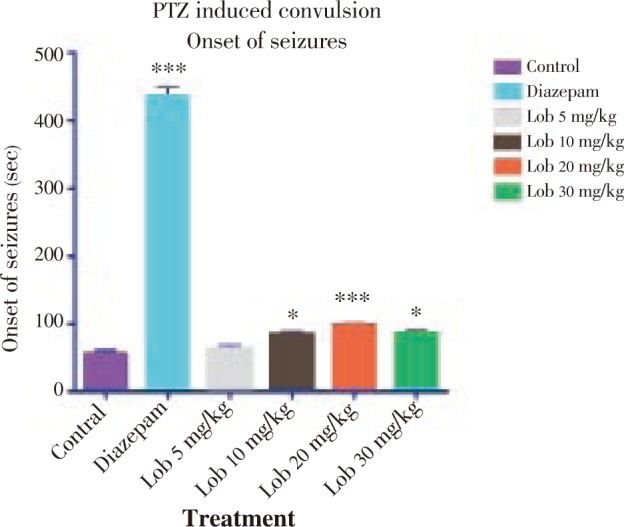
Figure 2. Duration of clonic seizures using PTZ. * P<0.05, ** P<0.01, *** P<0.001. All data compared with the control using one way ANOVA followed by Dunnet's post hoc test.
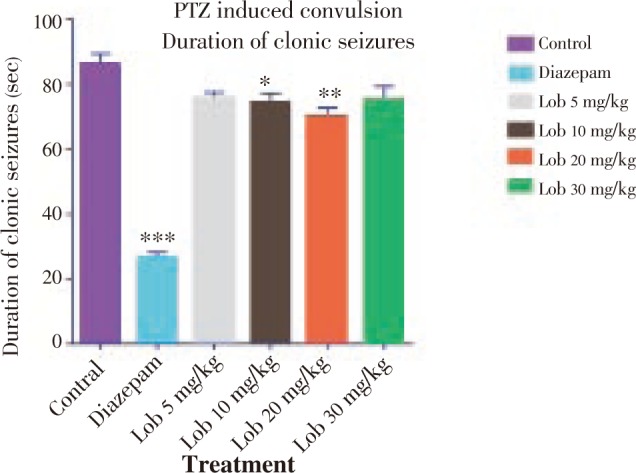
Figure 3. Duration of tonic seizures using PTZ. * P<0.05, ** P<0.01, *** P<0.001. All data compared with the control using one way ANOVA followed by Dunnet's post hoc test.
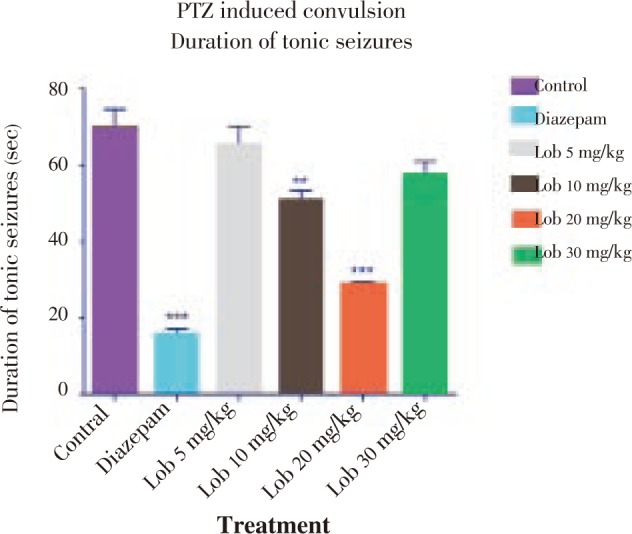
Table 2. Mortality of PTZ induced seizures.
| Groups | Mortality | % Mortality |
| Control | 6/6 | 100.00 |
| Diazepam | 0/6 | 0.00 |
| Lobeline (5mg/kg) | 4/6 | 66.66 |
| Lobeline (10mg/kg) | 2/6 | 33.33 |
| Lobeline (20mg/kg) | 1/6 | 16.66 |
| Lobeline (30mg/kg) | 2/6 | 33.33 |
Mortality = No. of animals dead after seizures / total No. of animals used;
% Mortality = (No. of animals dead after seizures / total No. of animals used) *100.
3.3. Strychnine induced convulsions
The onset of seizure was comparatively less significantly delayed in the animals treated with isolated lobeline at 10, 20 and 30 mg/kg dose with 20 mg/kg dose showing better results (Figure 4). Also with reference to Figure 5 and Figure 6, it was observed that lobeline in a dose of 20 mg/kg significantly reduced duration of clonic and tonic seizures however the results were not as impressive as that of PTZ induced seizure including percentage mortality (Table 3).
Figure 4. Onset of seizures using strychnine. * P<0.05, ** P<0.01, *** P<0.001. All data compared with control using one way ANOVA followed by Dunnet's post hoc test.
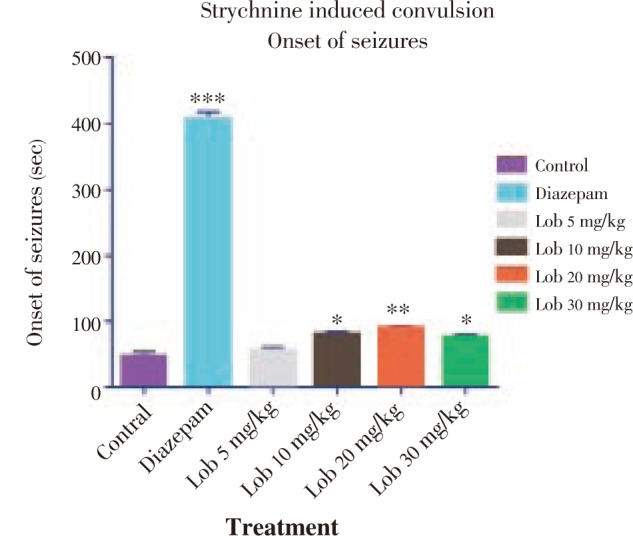
Figure 5. Duration of clonic seizures using strychnine. * P<0.05, ** P<0.01, *** P<0.001. All data compared with control using one way ANOVA followed by Dunnet's post hoc test.
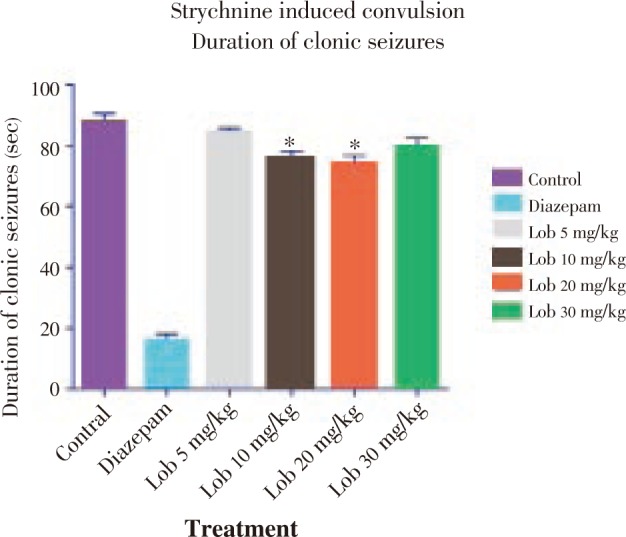
Figure 6. Duration of tonic seizures using strychnine. * P<0.05, ** P<0.01, *** P<0.001. All data compared with control using one way ANOVA followed by Dunnet's post hoc test.
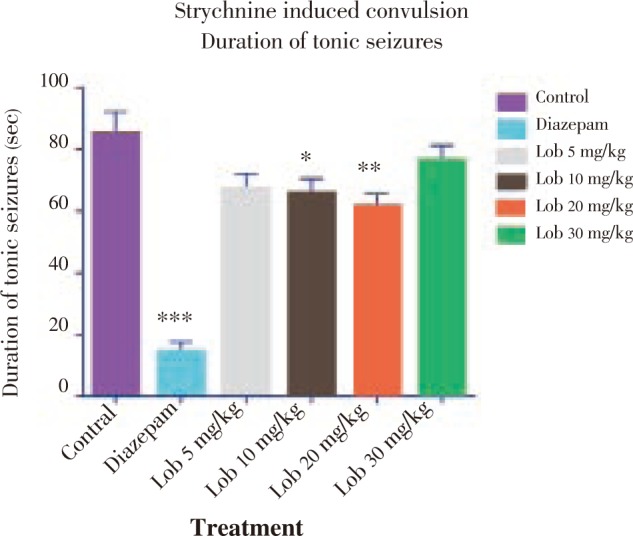
Table 3. Mortality of strychnine induced seizures.
| Groups | Mortality | %Mortality |
| Control | 6/6 | 100.00 |
| Diazepam | 0/6 | 0.00 |
| Lobeline (5mg/kg) | 4/6 | 66.66 |
| Lobeline (10mg/kg) | 3/6 | 50.00 |
| Lobeline (20mg/kg.) | 1/6 | 16.66 |
| Lobeline (30mg/kg) | 4/6 | 66.66 |
Mortality = No. of animals dead after seizures / Total No. of animals used; % Mortality = No. (of animals dead after seizures / Total No. of animals used) *100.
3.4. Biochemical evaluation by estimation of brain GABA
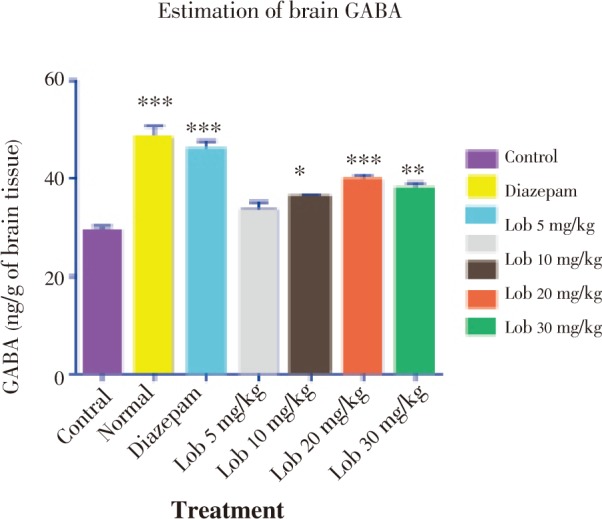
In biochemical evaluation, lobeline exerted a biphasic effect on GABA level i.e. GABA level was found to be increased up to dose of 20 mg/kg and it decreased at dose of 30 mg/kg (Figure 7). Hence, we may propose that lobeline reduces epileptic seizures by enhancing the GABA release thereby supporting GABAergic mechanism.
Figure 7. Estimation of brain GABA (ng/g of brain tissue). * P<0.05, ** P<0.01, *** P<0.001. All data compared with control using one way ANOVA followed by Dunnet's post hoc test.
4. Discussion
In the present investigation, it is evident that lobeline was able to ameliorate the epileptic seizures induced by PTZ and strychnine in laboratory animals. The various epileptogenic agents used in the investigation were PTZ and strychnine. It is understood from the literature that GABAergic neurotransmission is closely associated with the induction of epilepsy in the animals[29],[30]. GABA is the major inhibitory neurotransmitter in the central nervous system and even slight deficiencies in GABAergic transmission may lead to hyperexcitability and pathological neuronal discharges leading to epilepsy. GABA is an endogenous agonist at GABAA receptor (ionotropic receptor) thereby opening the channels to Cl− ions in the neuronal membrane. An enhancement of GABAergic inhibitory transmission is responsible for the antiepileptic effects of drugs that directly bind and activate GABAA receptors or influence GABA release, transport and metabolism. GABA mediated Cl− channel GABA-benzodiazepine receptor complex are closely associated with induction and onset of seizures[31]. The benzodiazepines do not substitute for GABA but appear to enhance GABA's effects allosterically without directly activating GABAA receptors or opening the associated chloride channels. Since PTZ is a noncompetitive antagonist it blocks GABA-mediated Cl− influx. Hence, this accentuates the excitatory neurons to release neurotransmitters such as The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) and N-Methyl-D-aspartic acid (NMDA). This leads to neuronal excitotoxicity leading to seizures and convulsions characterized by clonic and tonic phases. PTZ-induced convulsion decreases the GABA content in brain when compared to the vehicle group[32].
Lobeline was able to inhibit the epileptic seizures at doses of 5, 10, 20, 30 mg/kg. However, an interesting phenomenon of ceiling effect of efficacy was seen. At doses of 5, 10 and 20 mg/kg lobeline exhibited dose dependant attenuation epileptic seizures. However at higher doses of 30mg/kg effect waned off. This shows that at higher doses lobeline was ineffective. There are numerous molecular mechanisms through which drugs can block seizure spread and/or elevate seizure threshold.
The paradoxical nature of lobeline is exhibited in the pharmacological activity at a dose of 20 mg/kg. Lobeline protects the animals from epilepsy at 20 mg/kg, whereas at 30 mg/kg it might be an epileptogenic agent.
Strychnine is an alkaloid which is an inhibitor of glycine (inhibitory neurotransmitter) synthesis. It is an antagonist to excitatory monotransmittors like AMPA and NMDA and it also opposes Ca2+ induced excitotoxicity in the neurons. These factors are pro epileptogenic and precipitate seizures. Hence, inhibition of glycine leads to clonic and tonic seizures in laboratory animals. Thus, it is used as a research tool to induce seizures in the animals.
In the present investigation, lobeline was able to halt the progression and precipitation of seizures. This leads to suppression of clonic-tonic seizures and delays the onset of seizures. In the estimation of GABA levels in the PTZ model, lobeline exerted a biphasic effect on GABA level i.e. GABA level was found to be increased up to dose of 20 mg/kg and it decreased at dose of 30 mg/kg. However, the induction of seizures via strychnine does not involve GABA mediated Cl− channel or benzodiazepine modulation. Hence, GABA levels were not determined in the study. Hence, we may propose that lobeline reduces epileptic seizures by enhancing the GABA release thereby GABAergic mechanism.
However, since lobeline exhibited antiepileptic profile, it could be postulated that lobeline exerts its pharmacological effects via multiple mechanisms through GABAergic transmission. Concrete proof to this hypothesis could be derived by estimating the levels of glycine, AMPA, NMDA and monoamines such as noradrenalin, 5-hydroxytryptamine in the further study. And further studies are required to check any binding of lobeline with GABA receptor which can be achieved by radioligand binding assays.
Acknowledgments
The authors would like acknowledge Dr. Kiran Bhise, Principal, Allana College of Pharmacy, Pune, India for providing necessary facilities to carry out the study. The authors are thankful to Dr. KR Mahadik, Principal, Poona College of Pharmacy, Bharati Vidyapeeth University, Pune, India for making available the infrastructure of the college.
Footnotes
Foundation Project: Supported by University of Pune (Grant No. BCUD/OSD/390).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Reddy DS. Pharmacotherapy of catamenial epilepsy. Indian J Pharmacol. 2005;37:288–293. [Google Scholar]
- 2.Dieter S. Efficacy of new antiepileptic drugs. Epilepsy Curr. 2011:9–11. doi: 10.5698/1535-7511-11.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karuppasamy S. Medicinal plants used by Paliyan tribes of Sirumalai hills of Southern India. Nat Prod Radiance. 2007;6(5):436–442. [Google Scholar]
- 4.Jarald E. Textbook of pharmacognosy and phytochemistry. New Delhi: CBS Publication; 2009. p. 255. [Google Scholar]
- 5.Pullaiah T. Encyclopedia of world medicinal plants. Delhi, India: Regency Publications; 2006. pp. 1264–1265. [Google Scholar]
- 6.Jegan G, Kamalraj P, Muthuchelian K. Medicinal plants in tropical evergreen forest of Pachakumachi Hill, Cumbum Valley, Western Ghats, India. Ethnobot Leafl. 2008;12:254–260. [Google Scholar]
- 7.Kunwar RM, Keshab PS, Rainer WB. Traditional herbal medicine in Far-west Nepal: A pharmacological appraisal. J Ethnobiol Ethnomed. 2010;6:35. doi: 10.1186/1746-4269-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wabale AS, Kharde MN, Salunke KJ. Costus speciosus Lobelia nicotianaefolia Urginea indica (Koeing.) J. E. Sm., Roth. And Kunth- the important ethnomedicinal plants from the Western Ghats. Asian J Exp Biol Sci. 2011:169–170. [Google Scholar]
- 9.Udayan PS, Harinarayanan MK, Tushar KV, Balachandran I. Some common plants used by Kurichiar tribes of Tirunelli forest, Wayanad district, Kerala in medicine and other traditional uses. Indian J Tradit Knowl. 2008;7(2):250–255. [Google Scholar]
- 10.Stead LF, Hughes JR. Lobeline for smoking cessation. Cochrane Database Syst Rev. 2000;(2):CD000124. doi: 10.1002/14651858.CD000124. [DOI] [PubMed] [Google Scholar]
- 11.Glover ED, Rath JM, Sharma E, Glover PN, Laflin M, Tonnesen P, et al. et al. A multicenter phase 3 trial of lobeline sulfate for smoking cessation. Am J Health Behav. 2010;34(1):101–109. doi: 10.5993/ajhb.34.1.12. [DOI] [PubMed] [Google Scholar]
- 12.Nigel H, Angelica R, Dennis KM, Jack RN. Dose dependent attenuation of heroin self-administration with lobeline. J Psychopharmacol. 2010;24(1):51–55. doi: 10.1177/0269881108092119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller DK, Crooks PA, Teng L, Witkin JM, Munzar P, Goldberg SR, et al. et al. Lobeline inhibits the neurochemical and behavioral effects of amphetamine. J Pharmacol Exp Ther. 2001;296:1023–1034. [PubMed] [Google Scholar]
- 14.Richard LB, Bill JAE, Jason BC, Shafiqur R. Nicotinic receptor ligands reduce ethanol intake by high alcoholdrinking HAD-2 rats. Alcohol. 2009;43(8):581. doi: 10.1016/j.alcohol.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farook JM, Lewis B, Gaddis JG, Littleton JM, Barron S. Lobeline, a nicotinic partial agonist attenuates alcohol consumption and preference in male C57BL/6J mice. Physiol Behav. 2009;97;(3–4):503–506. doi: 10.1016/j.physbeh.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Sajja RK, Rahman S. Lobeline and cytisine reduce voluntary ethanol drinking behavior in male C57BL/6J mice. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):257–64. doi: 10.1016/j.pnpbp.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Francois XF, Lebreton J. History, chemistry and biology of alkaloids from Lobelia inflata. Tetrahedron. 2004;60:10127–10153. [Google Scholar]
- 18.Polston JE, Cunningham CS, Rodvelt KR, Miller DK. Lobeline augments and inhibits cocaine-induced hyperactivity in rats. Life Sci. 2006;79(10):981–990. doi: 10.1016/j.lfs.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Neugebauer NM, Harrod SB, Stairs DJ, Crooks PA, Dwoskin LP, Bardo MT. Lobelane decreases methamphetamine self-administration in rats. Eur J Pharmacol. 2007;571(1):33–38. doi: 10.1016/j.ejphar.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu G, Agarwal P. Human disease-drug network based on genomic expression profiles. PLoS ONE. 4(8):E6536. doi: 10.1371/journal.pone.0006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng G, Dwoskin LP, Crooks PA. Vesicular monoamine transporter 2: Role as a novel target for drug development. AAPS J. 2006;78:E682–E692. doi: 10.1208/aapsj080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrod SB, Horn MLV. Sex differences in tolerance to the locomotor depressant effects of lobeline in periadolescent rats. Pharmacol Biochem Behav. 2009;94(2):296–304. doi: 10.1016/j.pbb.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin ED, Christopher CN. Lobeline-induced learning improvement of rats in the radial-arm maze. Pharmacol Biochem Behav. 2003;76(1):133–139. doi: 10.1016/s0091-3057(03)00216-8. [DOI] [PubMed] [Google Scholar]
- 24.Kar A. Pharmacognosy and pharmacobiotechnology. New Delli: New Age International Publishers; 2007. pp. 443–444. [Google Scholar]
- 25.Florey K. Analytical profiles of drug substances. New York: Academic Press; 2005. p. 262. [Google Scholar]
- 26.OECD . Guidelines for the testing of chemicals. Paris: OECD; 2009. pp. 1–24. [Google Scholar]
- 27.Ojewole AOJ. Anticonvulsant effect of Rhus chirindensis (BakerF.) (Anacardiaaceae) stem bark aqueous extract in mice. J Ethnopharmacol. 2008;117(1):130–135. doi: 10.1016/j.jep.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Maynert EW, Klingman GI, Kaji HK. Tolerance to morphine. II. Lack of effects on brain 5-hydroxytryptamine and γ-aminobutyric acid. J Pharmacol Exp Ther. 1962;135:296–299. [PubMed] [Google Scholar]
- 29.Rang HP, Dale MM, Ritter JM, Moore PK. Textbook of pharmacology. London: Churchill Livingstone; 2007. pp. 575–584. [Google Scholar]
- 30.Katzung Bertram G. Basic and clinical pharmcology. New York, USA: McGraw-Hill Inc; 2009. pp. 357–369. [Google Scholar]
- 31.Tripathi KD. Essentials of medical pharmacology. New Delhi, India: Jaypee Publishers; 2008. pp. 394–395. [Google Scholar]
- 32.Yoo YM. Inhibitory effects of the Korean red ginseng extract on the content of neurotransmitter-related components of the mouse brain in convulsion-induced model. Natr Prod Sci. 2007;13(4):384–389. [Google Scholar]


