Abstract
Objective
To evaluate the antidiabetic and hypolipidemic activities of ethanolic leaf extract and fraction of Melanthera scandens (M. scandens) in alloxan-induced diabetic rats.
Methods
M. scandens leaf extract/fractions (37–111 mg/kg) were administered to alloxan-induced diabetic rats for 14 days and blood glucose levels (BGL) of the diabetic rats were monitored at intervals of 7 hours for acute study and 14 days for prolonged study. Lipid profiles of the treated diabetic rats were determined after the period of treatment.
Results
Treatment of alloxan-induced diabetic rats with the extract/fractions caused a significant (P<0.001) reduction in fasting bloodglucose levels (BGL) of the diabetic rats both in acute study and prolonged treatment (2 weeks). The activities of the extract and fractions were more than that of the reference drug, glibenclamide. The extract/fractions exerted a significant reduction in the levels of serum total cholesterol, triglycerides, LDL and VLDL of extract with increases in HDL levels of the diabetic rats.
Conclusions
These results suggest that the leaf extract/fractions of M. scandens possesses antidiabetic effect on alloxan induced diabetic rats and this justifies its use in ethno medicine and can be exploited in the management of diabetes.
Keywords: Antidiabetic, Hypolididemic, Melanthera scandens
1. Introduction
Melanthera scandens (Schumach&Thonn.) Roberty (Asteraceae) (M. scandens) is a perennial herb up to 1–4 m high, branches quadrangular and scabrid. It distributed geographically in East, West and South Africa[1]. It is known as ‘ayara edemerong’ by the Ibibios of Akwa Ibom State of Nigeria. The leaves are used traditionally to treat various ailments such as stomach ulcer and sores in Gosomtwi- Atwimakwanwoma district of Ghana [2]. In Nigeria, the leaves are used to treat dysmenorrhoea[3], diabetes and malaria (Philip Ikpe, personal communication). It is also used by the Bete people of Issia district of Cote d'Ivoire to treat malaria[4],[5]. The antioxidant [6], in vitro antiplasmodial[4],[5] and anti-inflammatory and analgesic[7], in vivo antiplasmodial and antiulcer[8] activities have been reported. Triterpenoid saponins have been reported in the leaves [9]. The leaf extract has been reported by Okokon et al[7] to have LD50 of (370.00± 23.33) mg/kg and phytochemical constituents made up of cardiac glycosides, tannins, saponins, terpenes and flavonoids. We reported in this study the in vivo antidiabetic and hypolipidemic activities of M. scadens alloxan-induced diabetic rats as the plant is use traditionally as remedy for diabetes.
2. Materials and methods
2.1. Plant materials
The plant (leaves) was identified and authenticated by Dr. Margaret Bassey, a taxonomist in the Department of Botany and Ecological Studies, University of Uyo, Uyo. The leaves were collected from a bush in Ukap in Ikono Local Government Area of Akwa Ibom State and were authenticated. A voucher specimen of the plant was deposited in the herbarium of Department of Botany and Ecological Studies, University of Uyo, Uyo.
2.2. Extraction
The plant parts (leaves) were washed and shade-dried for two weeks. The dried leaves were further chopped into small pieces and reduced to powder. The powdered leaf was divided into two parts, one part (1.5 kg) was macerated in 97% ethanol for 72 hours to give the crude ethanolic extract while the other part (1.5 kg) was successively and gradiently macerated for 72 hours in each of these solvents; chloroform, ethyl acetate and methanol to give the corresponding fractions of these solvents. The liquid filtrates were concentrated and evaporated to dryness in vacuoat 40 °C using rotary evaporator[10]. The yield of each extract was determined; crude ethanolic extract (3.98%), chloroform fraction (0.50%), ethyl acetate fraction (0.30%) and methanolic fraction (0.78%). The dried crude extract/ fractions were stored in a refrigerator at 4°C until use for the proposed experiment.
2.3. Animals
The animals (Swiss albino rats) of both sexes used for these experiments were obtained from University of Uyo animal house. The animals were housed in standard cages and were maintained on a standard pelleted feed (Guinea Feed) and water ad libitum. All animal treatments were strictly in accordance with international ethical guidelines concerning the care and use of laboratory animals and all the experiments were carried out under the approval of the ethical committee of the University.
2.4. Evaluation of antidiabetic and hypolipidemic activities of the extract and fractions
2.4.1. Induction of diabetes and animal treatment
The animals (male rats) were fasted for 24 hours and diabetes was induced by a single intraperitoneal injection of a freshly prepared solution of alloxan monohydrate (130 mg/kg) in ice cold 0.9% saline (NaCl) solution. The animals were given 2 mL of 5% dextrose solution using orogastric tube immediately after induction to overcome the drug induced hypoglycemia. 72 hours later, rats with blood glucose level (BGL) above 200 mg/dL were considered diabetic and selected for the experiment.
2.4.2. The animals were randomly divided into five groups of 6 rats each and treated as follows
Group I: Diabetic rats administered M. scandens extract (37 mg/kg/day) orally for 14 days.
Group II: Diabetic rats given M. scandens extract (74 mg/kg/day) orally for 14 days.
Group III: Diabetic rats administered orally with M. scandens extract (111 mg/kg/day) for 14 days.
Group IV: Diabetic rats administered orally with chloroform fraction of M. scandens (74 mg/kg/day) for 14 days.
Group V: Diabetic rats administered orally with ethyl acetate fraction of M. scandens(74 mg/kg/day) for 14 days.
Group VI: Diabetic rats administered with orally with methanol fraction of M. scandens (74mg/kg/day) for 14 days.
Group VII: Diabetic rats given Glibenclamide (10 mg/kg/day) for 14 days orally.
Group V: Diabetic control rats receiving normal saline (10 mL/kg) for 14 days.
The change in body weightand fasting BGL of all the rats were recorded at regular intervals during the experimental period. For acute study, the BGL was monitored after 1, 3, 5 and 7 h of administration of a single dose of the extract and at the end of 1, 3, 5, 7 and 14 days for prolonged treatments. The BGL was monitored in the blood of the diabetic rats by tail tipping method. The blood was dropped on the dextrostix reagent pad. This was inserted into microprocessor digital blood glucometer and the readings were recorded[12].
2.4.3. Evaluation of hypolipidaemic activity of the extract and fractions
After 14 days of treatments with the extract (24 h after the last dose), the rats were anaesthetized with ethyl ether vapour and the blood was collected through cardiac puncture into sample bottles devoid of anticoagulant. The samples were centrifuged at 1 000 rpm for 15 mins to obtain the sera. Serum cholesterol, triglyceride and High Density Lipoprotein (HDL) levels were measured by enzymatic colorimetric methods using Randox diagnostic kits. All samples were analysed with a wine light Unicam spectrophotometer. The concentrations of low density lipoprotein (LDL) and very low density lipoprotein (VLDL) were calculated from the formula of Friedwald et al.,[12]. These analyses were done at Department of Chemical Pathology, University of Uyo Teaching Hospital, (UUTH), Uyo.
2.4.4. Statistical analysis
Data are reported as mean ± SEM and were analyzed statistically using One way ANOVA followed by Tukey-kramer multiple comparison test and values of P< 0.01 were considered significant.
3. Results
3.1. Effect of leaf extract and fractions of M. scandens on alloxan-induced diabetic rats
There were observable changes in the body weight of treated and untreated alloxan-induced diabetic rats. Treatment of alloxan-induced diabetic rats with ethanolic leaf extract/fractions of M. scandens or glibenclamide did not improve the weights of treated diabetic rats compared to untreated diabetic rats except those treated with ethyl acetate and methanol fractions (Table 1). However, the weight losses were greatly reduced in the extract/fractions- treated groups compared to control.
Table 1. Effect of ethanolic leaf extract of M. scandens on body weight of alloxan - induced diabetic rats.
| Treatment | Dose (mg/kg) | Day 0 | Day 15 | % increase/ decrease in body weight |
| Control | 0.2 mL | 157.50±16.97 | 134.50±51.62 | -14.60 |
| Extract | 37 | 170.60±18.06 | 159.60±5.82 | -6.45 |
| 74 | 174.48±25.76 | 167.80±2.41 | -3.83 | |
| 111 | 161.90±17.98 | 142.60±15.70 | -11.93 | |
| Chloroform fraction | 74 | 143.80±25.98 | 140.10±2.60 | -2.57 |
| Ethyl acetate fraction | 74 | 143.60±23.15 | 154.80±22.98 | +7.79 |
| Methanol fraction | 74 | 165.20±14.73 | 167.70±24.0 | +1.51 |
| Glibenclamide | 10 | 163.20±27.04 | 142.60±15.78 | -12.63 |
Data are represented as mean ± SEM. a P< 0.001, significant difference when compared with control group (n=6).
The crude extract (37–111 mg/kg) produced dose-dependent reductions in blood glucose level (BGL) of alloxan-induced diabetic rats relative to control during acute studies. These effect were statistically significant (P<0.01-0.001) and progressed for 7 hours (Figure 1). Similar BGL reductive effect was observed in the groups treated with the fractions with the chloroform fraction having the highest effect (Figure 2). The effect of the highest dose of the extract (111 mg/kg) at the end of 7 hr as well as those of chloroform and methanol fractions were more than that of the standard drug, glibenclamide (Figures 1 and 2).
Figure 1. Antidiabetic effect of ethanoliccrude leaf extract of M. scandens on blood glucose level of alloxan- induced diabetic rats during acute study.
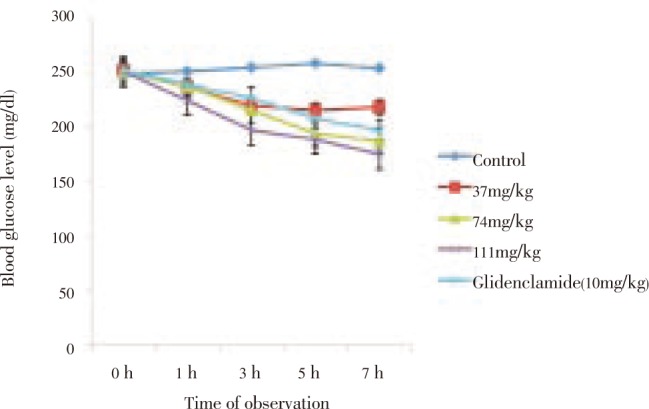
Figure 2. Antidiabetic effect of leaf fractions of Melanthera scandens on blood glucose level of alloxan-induced diabetic rats during acute study.
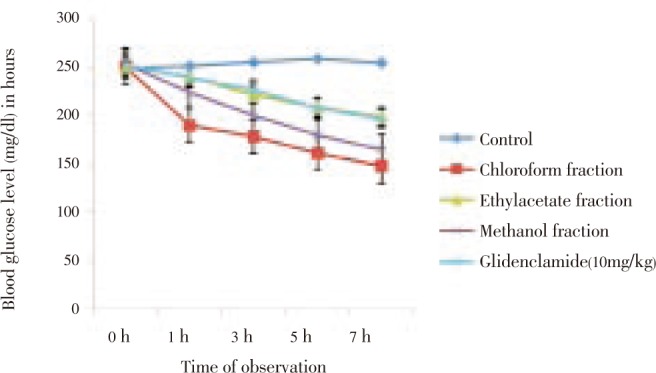
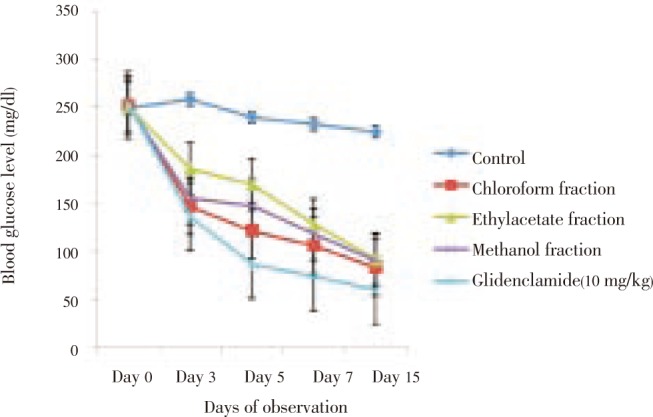
On prolonged treatment (14 days),the crude extract produced sustained reductions of BGL in diabetic rats in a dose-dependent fashion. These reductions were significant (P<0.001) when compared to control. The effect of the highest dose of the extract was comparable to that of standard drug, glibenclamide, 10 mg/kg, on day 15 (Figure 3). The fractions exerted sustained significant (P<0.001) reduction in blood glucose level of the animals on day 15. Chloroform and methanol fractions had comparable antidiabetic effect (Figure 4).
Figure 3. Antidiabetic effect of ethanolic crude leaf extract of M. scandens on blood glucose level of alloxan- induced diabetic rats during prolonged treatment.
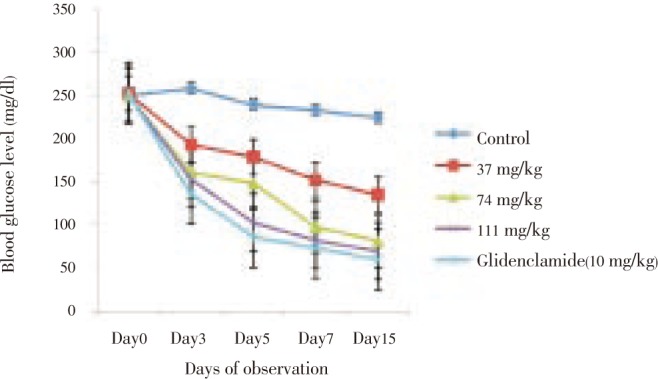
Figure 4. Antidiabetic effect of leaf fractions of M. scadens on blood glucose level of alloxan-induced diabetic rats during prolonged treatment.
3.2. Effect of leaf extract and fractions of M. scandens on lipid profile of alloxan- induced diabetic rats
Ethanolic leaf crude extract/fractions of M. scandens as well as glibenclamide caused decreases in the levels of serum total cholesterol, triglycerides, LDL and VLDL of alloxan-induced diabetic rats treated with them. However, HDL levels of the crude extract/fractions and glibenclamide-treated diabetic rats were elevated. These observed changes were statistically significant (P<0.01 -0.001) (Table 2).
Table 2. Effect of ethanolic leaf extract and fractions of M. scandens on serum total cholesterol, triglycerides, HDL - cholesterol, LDL - cholesterol and VLDL – chol of alloxan- induced diabetic rats.
| Drug | Dose (mg/kg) | Average Serum lipids profile (MMOL/L) |
||||
| Total cholesterol | Triglycerides | HDL cholesterol | LDL cholesterol | VLDL cholesterol | ||
| Control( Normal saline) | 0.2 mL | 5.84±0.19 | 4.26±0.55 | 0.07±0.01 | 2.64±0.09 | 1.77±0.09 |
| Crude extract | 37 | 3.67±0.41c | 3.25±0.70 | 0.14±0.01 | 2.57±0.58 | 0.66±0.26c |
| 74 | 4.17±0.86c | 2.94±0.55a | 0.16±0.01c | 2.45±0.04 | 0.60±0.06c | |
| 111 | 4.80±0.13a | 2.57±0.74c | 0.18±0.01c | 1.72±0.07c | 0.58±0.04c | |
| Chloroform fraction | 74 | 4.03±0.35c | 3.25±0.53 | 0.15±0.01c | 2.45±0.17 | 0.61±0.04c |
| Ethyl acetate fraction | 74 | 3.63±0.47c | 2.40±0.20c | 0.13±0.01b | 1.10±0.13c | 0.48±0.01c |
| Methanol fraction | 74 | 5.32±0.14 | 2.90±0.56a | 0.15±0.01c | 1.81±0.06c | 0.58±0.04c |
| Glibenclamide | 10 | 4.38±0.83c | 3.30±0.79 | 0.16±0.01c | 0.95±0.02c | 0.70±0.03c |
Data are expressed as mean ± SEM. Significant at a P< 0.05, b P< 0.01, c P<0.001 when compared with the control group (n=6).
4. Discussion
Evaluation of antidiabetic and hypolipidemic activities of M. scandens leaf extract/fractions were carried out in alloxan- induced diabetic rats. The extract was observed to demonstrate significant antidiabetic and hypolipidemic activities in alloxan-induced diabetic rats. There are a lot of reports implicating some phytochemical compounds in plants as being responsible for their antidiabetic activities[14]–[16]. Some of these phytochemical compounds revealed to be present in this extract include terpenes,saponins,tannins and alkaloids[7]. These constituents may in part be responsible for the observed significant activity of this extract either singly or in synergy with one another[11].These constituents may in part be responsible for the observed significant activity of this extract either singly or in synergy with one another. Alloxan is reported to selectively destroy insulin secretory β-cells to impair insulin secretion and function[17].Sulphonylureas cause hypoglycemia by stimulating insulin secretion from the pancreas and these compounds are potent in mild alloxan induced diabetes and inactive in intense alloxan induced diabetes whereby nearly all β-cells have been destroyed [18]–[20]. The observed reduction in BGL of the diabetic rats by glibenclamide in this study portrays an insevere state of diabetes. In this study, continous treatment with the leaf extract and fractions of M. scandens for a period of 2 weeks caused significant decrease in BGL of treated rats compared to untreated diabetic rats.This was followed by a significant increase in body weight of the treated rats. This is a reverse to diabetic state characterised by a severe loss in body weight due to loss or degradation of structural proteins[21]. This condition was alleviated by the treatment of the diabetic rats with root extract/fractions of M. scandens. Some plants' extracts are reported to exert hypoglycemic action by potentiating the insulin effect, either by stimulating the pancreatic secretion of insulin from the cells of islets of langerhans[22] or its release from bound insulin[23]. While others act through extra pancreatic mechanisms by inhibition of hepatic glucose production [24] or corrections of insulin resistance[25]. These leaf extract and fractions may have acted through one of the above mechanisms. Serum lipids are known to be elevated during severe diabetes and have been implicated in the development of artherosclerosis[26]. The serum lipid levels of the extract treated diabetic rats were significantly reduced after 2 weeks of treatment as against that in the untreated diabetic rats in this study. Diabetes-induced hyperlipidemia is attributable to excess mobilization of fat from the adipose due to the under utilization of glucose[27]. The regression of the diabetic state due to the administration of the root extract may have increased the utilization of glucose, thereby depressing the mobilization of fat. Phytochemical compounds like phenols, tannins, alkaloids, steroids, cardiac glycosides and terpenes present in this extract have been reported to exert antilipidemic activity[28]. Adesegun et al[6] had reported high phenolic compounds content in M. scandens with antioxidant and free radical scavenging potentials. These may in part be responsible for both the antidiabetic and hypolipidemic activities of this leaf extract and fractions.
In conclusion,the results of this study show that ethanolic leaf extract/fractions of M. scandens possessed antidiabetic and hypolipidemic properties. This confirmation justifies its use in ethnomedical medicine for the treatment of diabetes[29]–[30].
Acknowledgments
The authors are grateful to Mr. Nsikan Malachy and Sifon Akpan of Pharmacology and Toxicology Department for their technical assistance.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Burkill HM. The useful plants of West tropical Africa. 2nd ed. vol.2. Keaw, London: Royal Botanic Garden; 1985. p. 127. [Google Scholar]
- 2.Agyare C, Asase A, Lechtenberg M, Niehues M, Deters A, Hensel A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. J Ethnopharmacol. 2009;125:393–403. doi: 10.1016/j.jep.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Ajibesin KK, Ekpo BA, Bala DN, Essien EE, Adesanya SA. Ethnobotanical survey of Akwa Ibom State of Nigeria. J Ethnopharmacol. 2008;115:387–408. doi: 10.1016/j.jep.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Guede NZ, Mambu L, Guede Guina F, Bodo B, Grellier P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J Ethnopharmacol. 2005;98:281–285. doi: 10.1016/j.jep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Guede NZ, N'guessan K, Dibie TE, Grellier P. Ethnopharmacological study of plants used to treat malaria in traditional medicine by Bete populations of Issia (Cote d'voire) J Pharmaceut Sci Res. 2010;2(4):216–227. [Google Scholar]
- 6.Adesegun SA, Alabia SA, Olabanja PA, Alexander H, Coker B. Evaluation of antioxidant potential of Melanthera scandens. J Acupunct Meridian Stud. 2010;4:267–271. doi: 10.1016/S2005-2901(10)60047-7. [DOI] [PubMed] [Google Scholar]
- 7.Okokon JE, Udoh AE, Amazu L, Frank SG. Antiinflammatory and analgesic activities of Melanthera scandens. Asian Pac J Trop Biomed. 2012;2:144–148. doi: 10.1016/S2221-1691(11)60209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okokon JE, Etebong EO, Udobang JA, Obot JS. Antiplasmodial and antiulcer activities of Melanthera scandens. Asian Pac J Trop Biomed. 2012;1:16–20. doi: 10.1016/S2221-1691(11)60182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pender A, Delande C. Triterpenoid saponins from Melanthera scandens. Phytochemistry. 1994;37(3):821–825. doi: 10.1016/s0031-9422(00)90364-9. [DOI] [PubMed] [Google Scholar]
- 10.Okokon JE, Nwafor PA. Antiplasmodial activity of ethanolic root extract and fractions of Croton zambesicus. J Ethnopharmacol. 2009;121:74–78. doi: 10.1016/j.jep.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 11.Patil RN, Patil RY, Ahirwar B. Dheeraj AhirwarEvaluation of antidiabetic and related actions of some Indian medicinal plants in diabetic rats. Asian Pac J Trop Med. 2011;4(1):20–23. doi: 10.1016/S1995-7645(11)60025-4. [DOI] [PubMed] [Google Scholar]
- 12.Antia BS, Okokon JE, Umoh EE, Udobang JA. Antidiabetic activity of ethanolic leaf extract of Panicum maximum. Int J Drug Dev & Res. 2010;2(3):488–492. [Google Scholar]
- 13.Friedewald WT, Hevy RT, Frederickson D. Estimation of the concentration of low protein cholesterol in plasma without use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Bnouham M, Ziyyat A, Mekhfi H, Tahri A, Legssyer A. Medicinal plants with potential antidiabetic activity - A review of ten years of herbal medicine research (1990–2000) Int J Diabetes & Metabolism. 2006;14:1–25. [Google Scholar]
- 15.Kumar D, Kumar S, Kohli S, Arya R, Gupta J. Antidiabetic activity of methanolic bark extract of Albizia odoratissima Benth. in alloxan induced diabetic albino mice. Asian Pac J Trop Med. 2011;4(11):900–903. doi: 10.1016/S1995-7645(11)60215-0. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Kumar PD, Prasad SK, Sairam K, Hemalatha S. Antidiabetic activity of alcoholic leaves extract of Alangium lamarckii Thwaites on streptozotocin-nicotinamide induced type2 diabetic rats. Asian Pac J Trop Med. 2011;4(11):904–909. doi: 10.1016/S1995-7645(11)60216-2. [DOI] [PubMed] [Google Scholar]
- 17.Lenzen S. The mechanisms of alloxan- and streptozotocin induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 18.Yallow RS, Black H, Villazan M, Berson SA. Comparison of plasma insulin levels following administration of tolbutamide and glucose. Diabetes. 1960;9:356–362. doi: 10.2337/diab.9.5.356. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran S, Rajasekaran A, Kumar KTM. Antidiabetic, antihyperlipidemic and antioxidant potential of methanol extract of Tectona grandis flowers in streptozotocin induced diabetic rats. Asian Pac J Trop Med. 2011;4(8):624–631. doi: 10.1016/S1995-7645(11)60160-0. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Kumar V, Prakash O. Antidiabetic, hypolipidemic and histopathological analysis of Dillenia indica (L.) leaves extract on alloxan induced diabetic rats. Asian Pac J Trop Med. 2011;4(5):374–352. doi: 10.1016/S1995-7645(11)60101-6. [DOI] [PubMed] [Google Scholar]
- 21.Rajkumar L, Govindarajulu P. Increased degradation of dermal collagen in diabetic rats. Indian J Exp Biol. 1991;29:1081–1083. [PubMed] [Google Scholar]
- 22.Xu Z, Wang X, Zhou M, Ma L, Deng Y, Zhang H, et al. et al. The antidiabtic activity of total lignan from Fructusarctii against alloxan-induced diabetes in mice and rats. Phytother Res. 2008;22:97–101. doi: 10.1002/ptr.2273. [DOI] [PubMed] [Google Scholar]
- 23.Pari L, Amarnath S. Antidiabetic activity of Boerhavia diffusa L.: effect on hepatic key enzymes in experimental diabetes. J Ethnopharmacol. 2004;91:109–113. doi: 10.1016/j.jep.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Edduoks M, Jouad H, Maghrani M, Lemhadri A, Burcelin R. Inhibition of endogenous glucose production accounts for hypoglycemic effect of Spergularaia purpurea in streptozotocin mice. Phytomedicine. 2003;10:594–599. doi: 10.1078/094471103322331890. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Sato J, Oshida Y, Xu M, Bajotto G, Sato Y. Effect of Goshajinki-Gan(Chinese herbal medicine:Niu-Che-Sen-Qi-Wan) on insulin resistance in streptozotocin induced diabetic rats. Diabetes Res & Clin Pract. 2003;59:103–111. doi: 10.1016/s0168-8227(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 26.Mironova MA, Klein RL, Virella GT, Lopes-Virella MF. Antimodified LDL antibodies, LDL - containing immune complexes and susceptibility of LDL to in vitro oxidation in patients with type-2 diabetes. Diabetes. 2000;49:1033–1049. doi: 10.2337/diabetes.49.6.1033. [DOI] [PubMed] [Google Scholar]
- 27.Krishnakumar K, Augustti KT, Vijayammal PL. Hypolipidaemic effect of Salacia oblonga Wall. root bark in streptozotocin diabetic rats. Med Sci. 2000;28:65–67. [Google Scholar]
- 28.Tandon S. Phytochemicals and cardiovascular health. High Current R and D. 2005;28:18–22. [Google Scholar]
- 29.Osadebe PO, Omeje EO, Nworu SC, Esimone CO, Uzor PF, David EK, Uzoma JU. Antidiabetic principles of Loranthus micranthus Linn. parasitic on Persea americana. Asian Pac J Trop Med. 2010;3(8):619–623. [Google Scholar]
- 30.Osadebe PO, Omeje EO, Uzor PF, David EK, Obiorah DC. Seasonal variation for the antidiabetic activity of Loranthus micranthus methanol extract. Asian Pac J Trop Med. 2010;3(3):196–199. [Google Scholar]


