Abstract
Objective
To investigate the scolicidal effect of the Satureja khuzistanica (S. khuzistanica)essential oil from aerial parts of this herbal plant.
Methods
The essential oil was obtained by hydrodistillation method. Gas chromatography (GC) and gas chromatography mass spectrometry (GC-MS) were employed to determine the chemical composition of the essential oil. Protoscolices were collected aseptically from sheep livers containing hydatid cyst. Protoscolices were exposed to various concentrations of the oil (3, 5 and 10 mg/mL) for 10, 20, 30, and 60 min. Viability of protoscolices was confirmed by 0.1% eosin staining.
Results
: A total of 19 compounds representing 97.6% of the total oil, were identified. Carvacrol (94.9%) was found to be the major essential oil constituent. Scolicidal activity of S. khuzistanica essential oil at concentration of 3 mg/mL was 28.58, 32.71, 37.20 and 42.02%, respectively. This essential oil at concentration of 5 mg/mL killed 51.33, 66.68, 81.12, and 100% of protoscolices after 10, 20, 30 and 60 min, respectively. One hundred scolicidal effect was observed with S. khuzistanica essential oil at the concentration of 10 mg/mL after 10 min (comparing with 7.19% for control group).
Conclusions
The essential oil of S. khuzistanica is rich in carvacrol and may be used as a natural scolicidal agent.
Keywords: Hydatid cyst, Scolicidal, Essential oil, Carvacrol, Satureja khuzistanica
1. Introduction
Human cystic echinococcosis (hydatid disease) continues to be a substantial cause of morbidity and mortality in many parts of the world[1]. Although scientists and clinicians have accumulated much experience in the diagnosis and treatment of human echinococcosis, there are still many questions and problems. Among the most pressing of these are early and accurate diagnosis by imaging, immunological techniques or needle biopsy, development of reliable follow-up methods and a need for rapidly acting, effective and safe protoscolicides, development of PAIR techniques under ultrasound guidance, improvement in surgical procedures and chemotherapeutic approaches for the increase of clinical cure, prevention of secondary infection and reduction of other complications[2].
In general, radical resection of the parasitic mass, if possible, represents the preferred treatment strategy. Surgery is usually complemented by pre-, and/or post-surgical chemotherapy, and in inoperable cases, chemotherapy is the only option. For this, benzimidazole carbamate derivatives such as albendazole and mebendazole are currently the drugs of choice[3].
With regard to benzimidazole chemotherapy for human hydatid disease, all studies show similar rates of success (10%-30%), improvement (40%-60%) and no change (10%-30%)[2]. In human patients, benzimidazoles have to be applied in high doses for extended periods of time, and adverse side effects are frequently observed[4].
Scolicidal solutions remain indispensable in the treatment of hydatid cyst disease and surgeons need less harmful but more effective drugs in hydatid disease[5]. Many efforts have been made to discover new antimicrobial compounds from various kinds of sources such as plants, animals and microorganisms. Recently, herbal medicines have increasingly been used to treat many diseases including several infections[6]. Recently, antibacterial[7], antiviral[8], and antifungal[9] effects of Satureja species have been reported from different parts of the world.
Satureja khuzistanica (S. khuzistanica) Jamzad belonging to the Lamiaceae family is an endemic plant that widely distributed in the southern parts of Iran. It is a subshrub, branched stem ± 30 cm high, densely leafy, broadly ovate-orbicular and covered with white hairs. Base of the leaves is attenuate and petioliform[10]. It is famous for its medical uses as an analgesic and antiseptic in folk medicine[11]. S. khuzistanica has been reported to be antispasmodic, antidiarrhea[12], vasodilator[13], anti-inflammatory[14], antihyperlipidemic[11] and antioxidant[11],[14]. S. khuzistanica has been also known to possess antifungal[15], antiviral[8] and antimicrobial[16], properties. Furthermore, it is effective in improvement in rat fertility[17] and is useful in the treatment of recurrent aphthous stomatitis (RAS)[18]. It also protects rats from hemorrhagic cystitis induced by cyclophosphamide (a widely used antineoplastic drug) by reduction of free radical-induced toxic stress[19].
As far as the authors are aware, there are no published reports regarding the protoscolicidal effect of essential oils. Therefore, the aim of the present work was to determine the in vitro protoscolicidal effect of the essential oil from S. khuzistanica at various concentrations and at different exposure times.
2. Materials and methods
2.1. Experimental design
Hydatid cyst protoscolices were exposed to the essential oil of S. khuzistanica for 10, 20, 30, and 60 min. Three concentrations (3, 5 and 10 mg/mL) of the essential oil were used in this study. The experiments were performed at 37 °C. To determine the scolicidal activity of S. khuzistanica essential oil, the treated protoscolices were stained with 0.1% eosin for 15 min and the mortality rate of protoscolices was monitored by a light microscope.
2.2. Preparation of plant material
Aerial parts including flowers and leaves of S. khuzistanica (Mazhin, Lorestan, Iran), were collected from the wild growing plants at the full flowering stage in September to October 2009 (Altitude 490 m, Coordinate N 33° 00′ E 47° 40′). Voucher specimen of the species (MPH-1582) was deposited at the Herbarium of Medicinal Plants and Drugs Research Institute (MPH), Shahid Beheshti University, Tehran, Iran.
2.3. Essential oil isolation
The leaves and flowers of S. khuzistanica were dried under shade, ground mechanically using a commercial electric blender. One hundred grams of the resulting powder was subjected to hydrodistillation for 3 h in an all glass Clevenger-type apparatus according to the method recommended by the European Pharmacopoeia[20]. The extracted oil samples were dried over anhydrous sodium sulphate and stored in sealed vials at 4 °C for gas chromatography (GC), GC/mass spectrometry (MS) analysis and scolicidal assessments.
2.4. GC analysis
GC analysis was performed using a Thermoquest gas chromatograph with a flame ionization detector (FID). The analysis was carried out on fused silica capillary DB-5 column (30 m × 0.25 mm i.d.; film thickness 0.25 µm). The injector and detector temperatures were kept at 250 °C and 300 °C, respectively. Nitrogen was used as the carrier gas at a flow rate of 1.1 mL/min, oven temperature program was 60-250 °C at the rate of 4 °C/min and finally held isothermally for 10 min. Split ratio was 1:50.
2.5. GC/MS analysis
GC-MS analysis was carried out by use of Thermoquest-Finnigan gas chromatograph equipped with fused silica capillary DB-5 column (60 m × 0.25 mm i.d.; film thickness 0.25 µm) coupled with a TRACE mass (Manchester, UK). Helium was used as carrier gas with ionization voltage of 70 eV. Ion source and interface temperatures were 200 °C and 250 °C, respectively. Mass range was from 35 to 456 amu. Oven temperature program was the same given above for the GC.
2.6. Identification and quantification of the oil components
The constituents of essential oils were identified by calculation of their retention indices under temperature-programmed conditions for n-alkanes (C6-C24) and the oil on a DB-5 column under the same chromatographic conditions. Identification of individual compounds was made by comparison of their mass spectra with those of the internal reference mass spectra library (Adams and Wiley 7.0) or with authentic compounds and confirmed by comparison of their retention indices with authentic compounds or with those of reported in the literature[21],[22]. For quantification purposes, relative area percentages obtained by FID were used without the use of correction factors.
2.7. Collection of protoscolices
Hydatid cysts from livers of naturally infected sheep were obtained from Shiraz abattoir in southern Iran. Protoscolices were removed from cysts under aseptic conditions and washed several times with normal saline. Viability was assessed by muscular movements and 0.1% eosin staining test. The live protoscolices were finally transferred into a dark container containing normal saline solution and stored at 4 °C for further use.
2.8. Scolicidal assay
In this study, four concentrations of S. khuzistanica essential oil (1, 3, 5, and 10 mg/mL) were used for 10, 20, 30 and 60 min. To prepare the above concentrations, 10, 30, 50 and 100 µL of essential oil was dissolved in 9.7 mL of normal saline, in a test tube respectively. To enhance the dispersion of the essential oil in normal saline, 0.3 mL of tween 80 (Sigma,Germany) was added to the test tube. The resulting solution was mixed properly using a magnetic stirrer. In each experiment, 2.5 mL of the solution was placed in a test tube, to which a drop of protoscolex-rich sediment was added. The contents of the tube was gently mixed. The tube was then incubated at 37 °C. At the end of each incubation time (10, 20, 30 and 60 min) the upper phase was carefully removed so as not to disturb the protoscolices. One milliliter of 0.1% eosin stain was then added to the remaining settled protoscolices and mixed gently. The upper portion of the solution was discarded after 15 min of incubation. The remaining pellet of protoscolices was then smeared on a manually scaled glass slide, covered with a cover glass (24 mm × 50 mm), and examined under a light microscope. The percentages of dead protoscolices were determined by counting a minimum of 700 (usually more than 1 000) protoscolices. Protoscolices in the control group were treated only with normal salin containing tween 80. The experiments were performed in triplicate[23].
2.9. Viability test
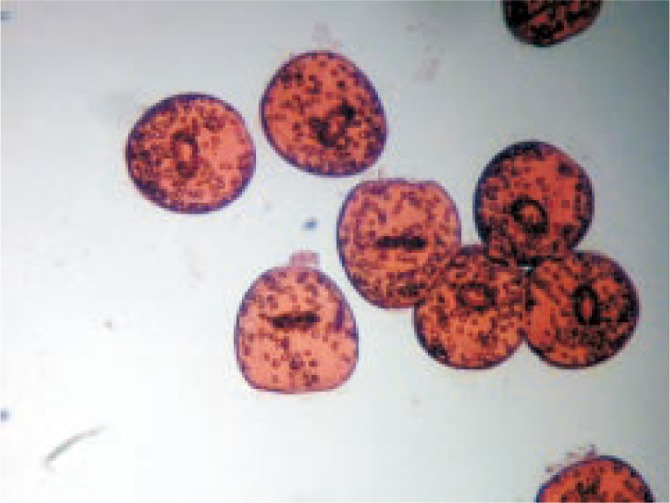
In the present study, eosin stain with the concentration of 0.1% (1 g of eosin powder in 1 000 mL distilled water) was used to check the viability of the protoscolices[23]. Fifteen minutes after exposure to the stain the protoscolices with no absorbed dye were considered potentially viable (Figure 1); otherwise, they were recorded as dead (Figure 2).
Figure 1. Live protoscolices after staining with 0.1% eosin.
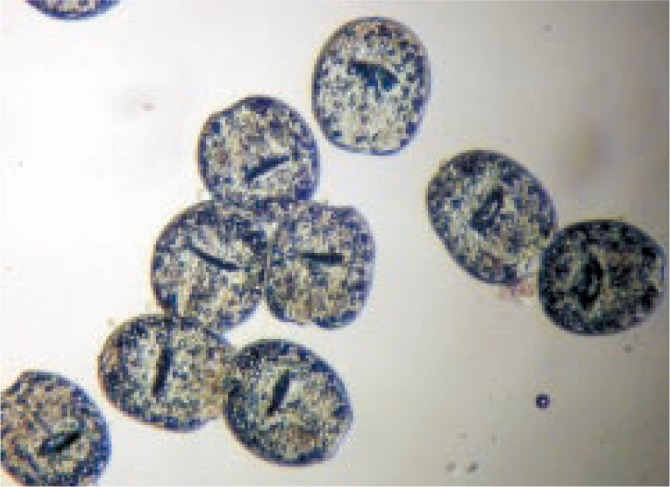
Figure 2. Dead protoscolices after exposure to S. khuzistanica essential oil and staining with 0.1% eosin.
2.10. Statistical analysis
Differences between the test and control groups were analyzed with Chi-square test. Statistical analysis was performed with GraphPad InStat software. P values less than 0.05 were considered to be significant.
3. Results
The chemical composition of S. khuzistanica essential oil is shown in Table 1. A total of 19 compounds, representing of 97.6 % of total oil, were identified. The major constituent of S. khuzistanica essential oil was carvacrol (94.9%). p-Cymene and γ-terpinene, as precursors of carvacrol, were present in low concentrations (Table 1). The mortality rates of E. granulosus protoscolices after exposure to different concentrations of S. khuzistanica essential oil at various exposure times are shown in Tables 2–4. The scolicidal activity of S. khuzistanica essential oil at the concentration of 3 mg/mL was 28.58, 30.12, 37.20, and 42.02% after 10, 20, 30 and 60 min of application, respectively (comparing with 7.19% for control group). The difference between the scolicidal effect of S. khuzistanica essential oil at this concentration was statistically highly significant comparing to the control group (P<0.001).The scolicidal power of S. khuzistanica essential oil at the concentration of 5 mg/mL was 51.33, 66.68, 81.12, and 100%, respectively. One hundred scolicidal effect was observed with S. khuzistanica essential oil at the concentration of 10 mg/mL after 10 min of application. All experiments of our work, exhibited dose-dependent and also time-dependent scolicidal effect of S. khuzistanica essential oil on the protoscolices of hydatid cyst. The results of the present study indicated that the essential oil of S. khuzistanica has high scolicidal activity and might be used as a scolicidal agent.
Table 1. Essential oil composition (%) of S. khuzistanica.
| No | RI | Percentage (%) | Compound | Identification method |
| 1 | 925 | t | α-Thujene | RI, MS |
| 2 | 933 | t | α-Pinene | RI, MS, CoI |
| 3 | 981 | t | Myrcene | RI, MS |
| 4 | 1013 | 0.26±0.02 | α-Terpinene | RI, MS, CoI |
| 5 | 1017 | 0.55±0.28 | p-Cymene | RI, MS, CoI |
| 6 | 1026 | 0.32±0.54 | Limonene | RI, MS, CoI |
| 7 | 1036 | 0.36±0.17 | Z-β-Ocimene | RI, MS |
| 8 | 1053 | 0.49±0.07 | γ-Terpinene | RI, MS, CoI |
| 9 | 1081 | 0.11±0.04 | trans-Sabinene hydrate | RI, MS |
| 10 | 1163 | t | Terpin-4-ol | RI, MS |
| 11 | 1175 | t | α-Terpinole | RI, MS |
| 12 | 1266 | t | Thymol | RI, MS, CoI |
| 13 | 1282 | 94.97±0.68 | Carvacrol | RI, MS, CoI |
| 14 | 1329 | t | Thymyl acetate | RI, MS |
| 16 | 1425 | 0.13±0.01 | β-Caryophyllene | RI, MS, CoI |
| 17 | 1427 | t | α-Humulene | RI, MS |
| 18 | 1501 | 0.36±0.17 | β-Bisabolene | RI, MS |
| 19 | 1522 | t | trans-β-Bisabolene | RI, MS |
RI: Retention indices relative to C6-C24 n-alkanes on the DB-5 column; MS: Mass spectroscopy; CoI: Co-injection; t: Trace <0.05%.
Table 2. Scolicidal effect of S. khuzistanica essential oil at concentration of 3 mg/mL following various exposure times (mean±SD).
| Exposure time (min) | Protoscoleces | Dead protoscoleces | Mortality rate |
| 10 | 808.00±33.15 | 231.00±15.39 | 28.58 |
| 20 | 901.66±46.73 | 295.00±25.23 | 32.71 |
| 30 | 898.66±55.82 | 334.33±36.85 | 37.20 |
| 60 | 1 140.66±95.13 | 479.33±35.92 | 42.02 |
| Control | 1014.00 | 73.00 | 7.19 |
Table 4. Scolicidal effect of S. khuzistanica essential oil at concentration of 10 mg/mL following various exposure times (mean ± SD).
| Exposure time (min) | Protoscoleces | Dead protoscoleces | Mortality rate |
| 10 | 875.66±160.00 | 875.66±160.00 | 100.00 |
| Control | 1 014.00 | 73.00 | 7.19 |
Table 3. Scolicidal effect of S. khuzistanica essential oil at concentration of 5 mg/mL following various exposure times (mean±SD).
| Exposure time (min) | Protoscoleces | Dead protoscoleces | Mortality rate |
| 10 | 951.33±88.55 | 488.30±30.03 | 51.33 |
| 20 | 1 194.66±28.50 | 796.66±15.56 | 66.68 |
| 30 | 1 054.33±29.00 | 855.33±52.99 | 81.12 |
| 60 | 938.33±53.59 | 938.33±53.59 | 100.00 |
| Control | 1 014.00 | 73.00 | 7.19 |
4. Discussion
The control of helminthosis and, generally of all parasitic diseases is usually made with synthetic anthelmintics. The appearance of resistance to synthetic anthelmintics stimulated the research of alternatives, such as medicinal plants active substances[24]. According to circumstances and depending on their efficacy, naturally produced plant anthelmintics offer an alternative that can overcome some of these problems and is both sustainable and environmentally acceptable[25]. essential oils which are accumulated in aromatic plants, are chiefly used as flavors or fragrances, but currently a renewal interest in natural substances has focused attention on plants rich in bioactive compounds. Among these components, essential oils well known for their antimicrobial properties[26]. In the present study, the essential oil of S. khuzistanica represented high scolicidal activity. The scolicidal power of this oil at the concentration of 5 mg/mL was 51.33, 66.68, 81.12, and 100% after 10, 20, 30 and 60 min, respectively. One hundred scolicidal effect was observed with S. khuzistanica essential oil at the concentration of 10 mg/mL after 10 min of application(comparing with 7.19% for control group).
Up to date, many scolicidal agents have been used for inactivation of the hydatid cyst protoscolices. Many of these scolicidal agents may cause undesirable complications that limit their use. For example adverse side effects has been reported for 20% hypertonic saline, 20% silver nitrate, 0.5% -1% cetrimide, ethyl alcohol, and 20 mg/mL albendazole sulfoxide[23].
According to the results of this work, the scolicidal activity of S. khuzistanica essential oil at the concentration of 5 mg/mL (60 min) or 10 mg/mL (10 min) was comparable with scolicidal power of 20% hypertonic saline (15 min), 20% silver (20 min), 0.5%-1% cetrimide (10 min), and 95% ethyl alcohol (15 min).
In the present study, the results of essential oil analysis showed that carvacrol was the major oil component of S. Khuzistanica (94.9%). Carvacrol is a monoterpenoid phenol, biosynthesized via aromatization of γ-terpinene to p-cymene and the subsequent hydroxylation of p-cymene. This phenol along with its precursors (γ-terpinene and p-cymene) appears as the major components in numerous phenolic essential oils (thyme, oregano and savory) of Lamiaceae family. It is also one of the most important components of many species including those belong to Satureja genus. This component has been reported as one of the strongest antimicrobial agents[27]. This phenolic compound has shown antiseptic, antibacterial, antifungal as well as anti-noceceptive and anti-inflammatory properties[28]. It has been shown that the antimicrobial activity of several essential oils has been attributed to the presence of phenolic compounds such as carvcrol[26].
Ultee et al[29] hypothesized that the hydroxyl group and the presence of a system of delocalized electrons are important for the antimicrobial activity of phenolic compounds, such as carvacrol and thymol[29]. Such a particular structure would allow compounds to act as proton exchanger, thereby reducing the gradient across the cytoplasmic membrane. The resulting collapse of the proton motrice force and depletion of the ATP pool lead eventually to cell death[26].
As far as we know, this is the first report on the scolicidal activity of S. khuzistanica essential oil. The results of this study allowed us to suggest that S. khuzistanica is a rich source of carvacrol that could be used as an effective scolicidal agent. S. khuzistanica is an edible plant therefore it is safe for human. Oral administration of S. khuzistanica essential oil to rats induced a marked antioxidant, antidiabetic, antihyperlipidemic, and reproduction stimulatory effects without occurrence of any toxic or adverse effects[30].The results of present study open the possibility of more investigations of in vivo scolicidal effect of this traditional medicine.
Acknowledgments
This work was supported by the financial support from Shiraz University (grant No. 87-GR-VT-24).
Footnotes
Foundation Project: Supported by the financial support from Shiraz University (Grant No. 87-GR-VT-24).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Craig S, McManus DP, Lightowlers MW, Chabalgoity JA, Hector H, Garcia HH, et al. et al. Prevention and control of cystic echinococcosis. Lancet Infect Dis. 2007;7:385–394. doi: 10.1016/S1473-3099(07)70134-2. [DOI] [PubMed] [Google Scholar]
- 2.Wen H, New RRC, Craig PS. Diagnosis and treatment of human hydatidosis. Br J Clin Pharmac. 1993;35:565–574. doi: 10.1111/j.1365-2125.1993.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spicher M, Naguleswaran A, Luis M, Ortega-Mora LMO, Müller J, Gottstein B, et al. In vitro and in vivo effects of 2-methoxyestradiol, either alone or combined with albendazole, against Echinococcus metacestodes. Exp Parasitol. 2008;119:467–474. doi: 10.1016/j.exppara.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Walker M, Rossignol JF, Torgerson P, Hemphill A. In vitro effects of nitazoxanide on Echinococcus granulosus protoscoleces and metacestodes. J Antimicrob Chemother. 2004;54:609–616. doi: 10.1093/jac/dkh386. [DOI] [PubMed] [Google Scholar]
- 5.Adas G, Arikan S, Kemik O, Oner A, Sahip N, Karatepe O. Use of albendazole sulfoxide, albendazole sulfone, and combined solutions as scolicidal agents on hydatid cysts (in vitro study) W J Gastroenterol. 2009;15:112–116. doi: 10.3748/wjg.15.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan R, Zakir M, Afaq SH, Latif A, Khan AU. Activity of solvent extracts of Prosopis spicigera, Zingiber officinale and Trachyspermum ammi against multidrug resistant bacterial and fungal strains. J Infect Dev Ctries. 2010;4:292–300. doi: 10.3855/jidc.621. [DOI] [PubMed] [Google Scholar]
- 7.Sahin F, Karaman I, Gulluce M. Evaluation of antimicrobial activities of Saturega hortensis L. J Ethnopharmacol. 2003;87:61–65. doi: 10.1016/s0378-8741(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 8.Abad MJ, Bermejo P, Gonzales E, Iglesias I, Irurzun A, Carrasco L. Antiviral activity of Bolivian plant extracts. Gen Pharmacol. 1999;32:499–503. doi: 10.1016/s0306-3623(98)00214-6. [DOI] [PubMed] [Google Scholar]
- 9.Boyraz N, Ozcan M. Inhibition of phytopathogenic fungi by essential oil. Hydrosol, ground material and extract of summer savory (Satureja hortensis L.) growing wild in Turkey. Int J Food Microbiol. 2005;107:238–242. doi: 10.1016/j.ijfoodmicro.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Jamzad Z. A new species of the genus Satureja (Labiatae) from Iran. Iran J Bot. 1994;6:215–218. [Google Scholar]
- 11.Vosough-Ghanbari S, Rahimi RS, Kharabaf S, Zeinali S, Mohammadirad A, Amini S, et al. et al. Effects of Satureja khuzistanica on serum glucose, lipids and markers of oxidative stress in patients with type 2 diabetes mellitus: A double-blind randomized controlled trial. eCAM. 2008;27:1–6. doi: 10.1093/ecam/nen018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajhashemi V, Sadraei H, Ghannadi AR, Mohseni M. Antispasmodic and antidiarrhoeal effect of Satureja hortensis L. JJM. 2010;3:36–40. doi: 10.1016/s0378-8741(99)00209-3. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez de Rojas V, Somoza B, Ortega T, Villar AM, Tejerina T. Vasodilatory effect in rat aorta of eriodictyol obtained from Satureja obovata. Planta Med. 1999;65:234–238. doi: 10.1055/s-1999-13986. [DOI] [PubMed] [Google Scholar]
- 14.Ghazanfari G, Minaie B, Yasa N, Ashtaral Nakhai L, Mohammadirad A, Nikfar S, et al. et al. Biochemical and histopathological evidences for beneficial effects of Satureja khuzistanica Jamzad essential oil on the mouse model of inflammatory bowel diseases. Toxicol Mech Meth. 2006;16:365–372. doi: 10.1080/15376520600620125. [DOI] [PubMed] [Google Scholar]
- 15.Sadeghi-Nejad B, Shiravi F, Ghanbari S, Alinejadi M, Zarrin M. Antifungal activity of Satureja khuzistanica (Jamzad) leaves extracts. JJM. 2010;3:36–40. [Google Scholar]
- 16.Amanlou M, Fazeli MR, Arvin A. Antimicrobial activity of crude methanolic extract of Satureja khuzistanica. Fitoterapia. 2004;75:768–770. doi: 10.1016/j.fitote.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Haeri S, Minaie B, Amin G, Nikfar S, Khorasani R, Esmaily H, et al. et al. Effect of Satureja khuzistanica essential oil on male rat fertility. Fitoterapia. 2006;77:495–499. doi: 10.1016/j.fitote.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Amanlou M, Babaee N, Saheb-Jamee M, Salehnia A, Farsam H, Tohidast Akrad Z. Efficacy of Satureja khuzistanica extract and its essential oil preparations in the management of recurrent aphthous stomatitis. DARU. 2007;15:231–235. [Google Scholar]
- 19.Rezvanfara MA, Farshid AA, Sadrkhanlou RA, Ahmadi A, Rezvanfar MA, Salehni A, et al. et al. Benefit of Satureja khuzistanica in subchronically rat model of cyclophosphamide-induced hemorrhagic cystitis. Exp Toxicol Pathol. 2010;62:323–230. doi: 10.1016/j.etp.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Anonymous . European Pharmacopoeia. 3rd Ed. Strasbourg: Council of Europe; 1997. pp. 121–122. [Google Scholar]
- 21.Shibamoto T. Retention indices in essential oil analysis. In: Sandra P, Bicchi C, editors. Capillary gas chromatography in essential oil analysis. New York: Alfred Heuthingverlag; 1987. pp. 259–275. [Google Scholar]
- 22.Adams RP. Identification of essential oils components by gas chromatography/quadra pole mass spectroscopy. 4th edn. Carol Stream: Allured; 2007. pp. 361–367. [Google Scholar]
- 23.Moazeni M, Nazer A. In vitro effectiveness of garlic (Allium sativum) extract on scolices of hydatid cyst. W J Surg. 2010;34:2677–2681. doi: 10.1007/s00268-010-0718-7. [DOI] [PubMed] [Google Scholar]
- 24.Pessoa LM, Morais SM, Bevilaqua CML, Luciano JHS. Anthelmintic activity of essential oil of Ocimum gratissimun Linn. and eugenol against Haemonchus contortus. Vet Parasitol. 2002;109:59–63. doi: 10.1016/s0304-4017(02)00253-4. [DOI] [PubMed] [Google Scholar]
- 25.Elissondo MC, Albani CM, Gende L, Eguaras M, Denegri G. Efficacy of thymol against Echinococcus granulosus protoscoleces. Parasitol Int. 2008;57:185–190. doi: 10.1016/j.parint.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Arfa AB, Combes S, Preziosi-Belloy L, Gontard N, Chalier P. Antimicrobial activity of carvacrol related to its chemical structure. Letters Appl Microbiol. 2006;43:149–154. doi: 10.1111/j.1472-765X.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim JM, Marshall MR, Cornell JA, Preston JF, Wei CI. Antibacterial activity of carvacrol, citral, and geraniol against Salmonella typhimurium in culture medium and on fish cubes. J Food Sci. 1995;60:1364–1368. [Google Scholar]
- 28.Hajhashemi V, Ghannadi A, Pezeshkian SK. Antinocicieptive and anti-inflammatory effects of Satureja hortensis L. extracts and essential oil. J Ethnopharmacol. 2002;82:83–87. doi: 10.1016/s0378-8741(02)00137-x. [DOI] [PubMed] [Google Scholar]
- 29.Ultee A, Bennik MHJ, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002;68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdollahi M, Salehnia A, Mortazavi HR, Ebrahimi M, Shafiee A, Fouladian F, et al. et al. Antioxidant, antidiabetic, antihyperlipidemic, reproduction stimulatory properties and safety of essential oil of Satureja khuzistanica in rat in vivo: a toxicopharmacological study. Med Sci Monit. 2003;9:331–335. [PubMed] [Google Scholar]


