Abstract
Objective
To evaluate the anticancer activity of the extract fraction of Polyalthia evecta (P. evecta) (Pierre) Finet & Gagnep and the synergistic anticancer effect of the extracts from P. evecta by using the ATR/FT-IR spectroscopy.
Methods
The 50% ethanol-water crude leaf extract of P. evecta (EW-L) was prepared and was further fractionated to isolate various fractions. The anticancer activity was investigated from cytotoxicity against HepG2 using a neutral red assay and apoptosis induction by evaluation of nuclei morphological changes after DAPI staining. Synergistic anticancer effects of the extracts from P. evecta were performed using the ATR/FT-IR spectroscopy.
Results
The result showed that the EW-L showed higher cytotoxicity and apoptosis induction in HepG2 cells than its fractionated extracts. The hexane extract exhibited higher cytotoxicity and apoptosis induction than the water extracts, but less than the EW-L. The combined water and hexane extracts apparently increased cytotoxicity and apoptosis induction. The %apoptotic cells induced by the extract mixture were increased about 2-fold compared to the single hexane extract.
Conclusions
The polar extract fraction is necessary for the anticancer activity of the non-polar extract fraction. The ATR/FT-IR spectra illustrates the physical interaction among the constituents in the extract mixture and reveals the presence of polyphenolic constituents in the EW-L, which might play a role for the synergistic anticancer effect.
Keywords: Polyalthia evecta, Synergistic, Apoptosis induction, Cytotoxicity, Anticancer activity, Hepatoma, ATR/FT-IR spectroscopy
1. Introduction
Polyalthia evecta (P. evecta) (Pierre) Finet & Gagnep belongs to the Family Annonaceae. It is a small shrub, young stems with hairs, flower on long pedicel with small bracteole and fruit aggregate, an umbel-like arrangement. It is widely distributed in the semi-deciduous forests of southern Indochina and is found in the Northeast region of Thailand. The local Thai name for this plant is “Nam-tou-lang” or “Tong-lang”. The water decoction of the roots is used traditionally as a galactagogue[1],[2], and a carminative[3].
The phytochemicals found in the hexane extraction of the root of P. evecta are evectic acid and furans[4]. The respective bioactive constituents from the hexane and dichloromethane extracts of the air-dried roots were shown to be active against Plasmodium falciparum and Mycobacterium tuberculosis[3]. High tannic acid content was reported in 50% ethanol-water leaf extract of P. evecta (EW-L), which was found to contribute to the antioxidant activity[5] while the EW-L crude extract showed strong antimutagenicity in TA98[5].
The plants in the genus Polyalthia exerted varied biological activities. The ethanol roots extracts of Polyalthia laui and Polyalthia rumphii were used as folk medicine by China Li ethnic minority for prevention of fever, hypertension and inhibition of cancer cells, i.e., SPC-A-1 (human lung cancer cell Line), BEL-7402 (human hepatocellular carcinoma cell line), SGC-7901 (human gastric cancer cell line) and K562 (human myelogenous leukaemia cell line) proliferation[6]. The extracts of Polyalthia longifolia is reported to have a cytotoxic effect on cancer cell lines while the extract of Polyalthia jucunda was found to have a growth inhibitory effect on tumor cell lines, possibly via apoptosis induction in NCI-H460 cells[7]–[9]. Our study has previously reported the potential anticancer effect of the extract of the P. evectic with selective cytotoxic activity in HepG2 cells compared to the Vero cells[10].
Hepatocellular carcinoma or liver cancer is the most common widespread cancer[11] and causes high mortality rates, particularly in Southeast Asia where viral hepatitis is endemic[11]. However, the main problem of chemotherapy to treat hepatocellular carcinoma is the cancer resistance mechanism[12],[13]. Recently, the human hepatoma (HepG2) cells was reported to exhibit the low gene expression of cytochrome (CYP) transcription factor which make these cell express the interactions between drugs and hepatic efflux transporter. The HepG2 can induce the interactions between food supplements and drugs that are substrates for P-glycoprotein and multidrug resistance-associated protein 2 (MRP-2) and monocarboxylic transporter 1[14]. Thus, the discovery of new compound with effective anticancer activity is needed to control liver cancer and apoptosis induction is the desirable effect for successful liver cancer treatment.
The mechanism(s) of anticancer activity of this EW-L extract has, however, not been reported; therefore, the anticancer mechanism of the EW-L crude extract via apoptosis induction was investigated against the HepG2 cells in the current study. In addition, the attenuated total reflectance (ATR)/FT-IR technique was adapted to determine the synergistic anticancer effect found in the EW-L crude extract and to convey whether further separation of the crude extract was necessary.
2. Materials and methods
2.1. Chemicals and reagents
The organic solvents used for extraction were of analytical grade from Fisher Scientific (UK) and Labscan (Thailand). cetonitrile (HPLC grade, Fisher Scientific, UK), Ortho-phosphoric acid (analytical grade, BHD, England) and ultrapure water from Milli-Q system (Millipore, Bedford, USA) were used for the mobile phase preparation. The standard agents and melphalan were provided from Sigma-Aldrich Chemie GmbH (Germany). The reagents used in the cell assay were of molecular biological grade. Dimethylsulfoxide (DMSO) was bought from United States Biological (USA). The reagent and culture media Dulbecco's modified Eagle's medium (DMEM) were bought from GIBCO®, Invitrogen Corporation (USA). Sodium bicarbonate (NaHCO3) and the fluorescence dye 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich Chemie GmbH (Germany). Neutral red and a standard anticancer drug (melphalan) were purchased from Sigma Chemical Co. (USA).
2.2. Extraction and isolation
Leaves of P. evecta (Pierre) Finet & Gagnep were collected from Khon Kaen Province in 2009. A voucher specimen (CRD-HHP-011L) was kept at the Herbarium of the Faculty of Pharmaceutical Sciences, Khon Kaen University. After drying, 1 kg of dried leaves was ground into finely powder then macerated in 8 L of 50% ethanol-water for seven days then filtered. The solvent was removed using a rotary evaporator at 40-50 °C. The residue (EW-L) was further freeze-dried (8.4% yield) and kept in amber in an air-tight container at 4 °C until used. The EW-L was further extracted with hexane to get the ‘crude hexane extract’ then further extracted with another relatively polar solvent (chloroform). Briefly, 1 g of EW-L crude extract was initially macerated in the 150 mL of hexane. The Erlenmeyer flask was shaken by hand for 5 min. The fraction was filtered by using Whatman filter paper No.1, evaporated by rotary evaporator and the % yield calculated. The remaining residue was subjected to further maceration with chloroform as the solvent until the extract was obtained. Various solvents were used consecutively; including, hexane, chloroform, dichloromethane, ethanol, methanol, ethyl acetate and water, respectively, with a %yield of 0.8%, 1.0%, 0.8%, 0.1%, 7.6%, 19.7% and 16.5%. All of the extracts were freshly prepared as a stock in DMSO and used for the study.
2.3. ATR FTIR spectroscopy
The substrate used for ATR measurement was a ZnSe crystal with a reflective index of 2.4 and an incidence angle of 45×, yielding a total of six internal reflections at the sample. Spectra were recorded with a Tensor 27 FTIR spectrometer equipped with a KBr beam splitter and an MCT detector cooled with liquid nitrogen with a measurement range of 4 000-6 000 cm−1. The samples were freshly prepared in 20% v/v DMSO-water. Each fraction of the hexane, water and combined extract fraction (1:1 ratio) was prepared to give a final concentration of 50 mg/mL in 20% v/v DMSO-water which was then applied to the ATR cell. The background spectrum was recorded for each sample. The measurements were performed with a spectral resolution of 4 cm−1 with 32 scans co-added (Bruker Optics Ltd, Germany). The relative integral area of each peak was performed using OPUS 6.5 software (Bruker optic, German).
2.4. Cell culture
The human hepatoma cell line HepG2 and the African green monkey kidney cell line Vero were maintained at the Centre for Research and Development of Medical Diagnostic Laboratories, Khon Kaen University). The HepG2 passage number 25-30 and the Vero passage number 37-40 were maintained in DMEM, supplemented with 10% fetal bovine serum and antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin) and cultured at 37 °C in a humidified atmosphere containing 5% CO2.
2.5. Cytotoxicity assay
Both the HepG2 and Vero cell lines were separately cultured in a T25 flask with medium DMEM (supplemented with 10% fetal bovine serum, 100 units/mL penicillin and 100 µg/mL streptomycin) and incubated at 37°C in a humidified 5% CO2 atmosphere.
To determine the cytotoxicity of the samples in the cell model, neutral red (NR) uptake assay was used for identification of vital cells and was performed following the method of Machana et al[15]. NR is a cationic dye which accumulates in the lysosomes of viable cells[16]. Damage to the lysosomes is induced by toxic compounds leading to a release of the NR dye from the lysosome to the medium[16]. Briefly, cells were seeded at a density of 3×105 cells in 96-well plates with medium and incubated for 24 h. The cells were then treated with plant extracts at various concentrations for a 24-h exposure time. Cells were centrifuged at 550×g for 5 min and the supernatant removed. Then, 50 µg/mL NR was added to each well and incubated for another 1 h. After NR incubation, cells were washed using media. The viable cells that accumulated NR were lyzed with 0.33% HCl in isopropanol. The absorbance of viable cells was measured as 520 nm and 650 nm (reference wavelength) using a spectromicroplate reader. The maximum concentration of the compound used in the study was 500 µg/mL in order to maintain 1% v/v DMSO with a cytotoxicity of less than 10% compared to the untreated cells. The Vero cells were used as a normal cell model for a comparison to the HepG2 cell model. The anticancer drug melphalan was used as a positive control.
2.6. Apoptosis induction assay
Apoptosis induction was evaluated by fluorescence dye staining, using DAPI to identify the condensation and fragmentation of nucleic DNA of the apoptotic cells. The cancer cells were treated with the plant extracts 500 µg/mL or melphalan 76 µg/mL for 24 h. The culture medium was removed and rinsed by using 500 µL of fresh medium without FBS and the cells were fixed by using 50 µL methanol. Then 50 µL of DAPI was added to cells with a final concentration 1 µg/mL and exposed for another 1 h. The excess dye was removed and 20 µL of PBS:Glycerin (1:1) added[17]. The stained nucleic DNA was captured in 10 views under inverted fluorescence microscopy at a magnitude of 40×. The percentage of apoptotic nuclei was calculated.
2.7. Statistical analysis
All of the tests were done in triplicate. The results were expressed as a mean ± standard deviation. A probability level less than 5% (P < 0.05) was considered significant. The t-test, one-way ANOVA and Tukey-Kramer multiple comparisons were performed using Microsoft Excel.
3. Results
3.1. Cytotoxicity effects of P. evecta extracts in HepG2 cells
The cytotoxicity of the crude extract of P. evecta (EW-L) and the extracts obtained from the fractionation were determined vis-à-vis the anticancer drug melphalan (Table 1). The results showed that the strong cytotoxic compound was melphalan and EW-L crude extract. The moderate level cytotoxic compound was the extract from the hexane extract. The inactive extracts were the extracts from fractions F2-F7.
Table 1. Percentage of cytotoxicity and apoptotic cells induced in malignant human hepatoma (HepG2) by P. evecta extract and a combination of extract fractions compared to melphalan.
| Samples | Final concentration(µg/mL) | % Cytotoxicity |
% Apoptotic cells in HepG2 | |
| HepG2 | Vero | |||
| Hexane fraction (F1) | 500 | 74.6±1.6 | 54.2±15.4 | 46.4±2.6 |
| Chloroform fraction (F2) | 500 | 24.3±9.3 | 2.2±3.8 | 9.8±4.7 |
| Ethylacetate fraction (F3) | 500 | 29.6±8.8 | 32.5±8.1 | 3.2±1.6 |
| Dichloromethane fraction(F4) | 500 | 24.0±7.4 | 25.5±8.1 | 2.9±1.1 |
| Ethanol fraction (F5) | 500 | 22.7±8.8 | 7.6±8.3 | 2.4±1.9 |
| Methanol fraction (F6) | 500 | 9.9±2.5 | 12.3±1.3 | 4.6±1.6 |
| Water fraction (F7) | 500 | 7.8±2.5 | 10.8±0.8 | 9.2±2.1 |
| Hexane:water | 500:100 | 98.9±1.8 | 4.0±2.9 | n.d. |
| Hexane:water | 500:250 | 81.8±10.6 | 43.9±3.3 | n.d. |
| Hexane:water | 500:500 | 100.0±1.0 | 45.1±4.1 | 72.7±13.6 |
| Hexane:methanol | 500:100 | 91.0±6.2 | 26.9±5.6 | n.d. |
| Hexane:methanol | 500:250 | 100.0±5.7 | 36.1±8.0 | n.d. |
| Hexane:methanol | 500:500 | 100.0±8.9 | 39.9±3.2 | 54.9±10.8 |
| P. evecta crude extract (EW-L) | 140 | 60.0±4.8 | 30.2±9.3 | 46.4±2.6 |
| P. evecta crude extract (EW-L) | 500 | 100.0±4.1 | 37.2±4.2 | 92.8±10.8 |
| Melphalan | 76 | 67.2±3.1 | 70.3±3.1 | 41.6±2.1 |
n.d. = not be determined.
The EW-L crude extract of P. evecta exhibited the highest significant cytotoxicity to HepG2 cells (P < 0.05). Interestingly, the EW-L crude extract showed significant cytotoxicity and high selectivity than melphalan based on relative high cytotoxicity in the HepG2 cells than the Vero cells. The EW-L crude extract showed significant cytotoxicity and a high selective against HepG2 cells after 24 h exposure as dose dependent manner (Table 1). Thus, fractionation with various solvents was performed to determine the fraction contributing to the cytotoxicity. It was found that the extracts from the polar fractions F5-F7 (i.e., ethanol, methanol and water) were inactive because at a maximum concentration of 500 µg/mL they showed no cytotoxicity to either HepG2 or Vero cells.
The percent cytotoxicity of 100, 250 and 500 µg/mL EW-L crude extract and the extracts from each fraction were also determined in HepG2 cells (Figure 1). The percent cytotoxicity in the HepG2 cells was found to be the highest when the cancer cells were exposed to EW-L crude extract at 100, 250 and 500 µg/mL while percent cytotoxicity was less when the HepG2 cells were exposed to the extracts which had been further fractionated by using relatively more polar solvent (F2-F7). At 100 µg/mL none of the fractionated extracts demonstrated any cytotoxicity. The hexane extract fraction began to exhibit cytotoxicity at 250 µg/mL (Figure 1). Among the fractionated extracts, the hexane fraction exhibited the highest cytotoxicity but less than that of the EW-L crude extract of P. evecta. The extracts from water, methanol, ethanol, and dichloromethane and the ethylacetate fractions showed a cytotoxicity less than 30% at the maximum concentration of 500 µg/mL. The cytotoxicity was found to be diminished in the crude extract obtained from the polar solvent extraction compared to the relatively non-polar solvent extraction. The solvent polarity extracted different constituents and thus contributed to different cytotoxicities. The cytotoxicity of a single extract from the hexane fraction was found to be concentration dependent. The highest cytotoxicity of the EW-L crude extract indicated that the chemical constituents in the whole crude extract were necessary for the specific cytotoxicity against the HepG2 cells.
Figure 1. Percent cytotoxicity of a single treatment after 24-h incubation by neutral red assay.
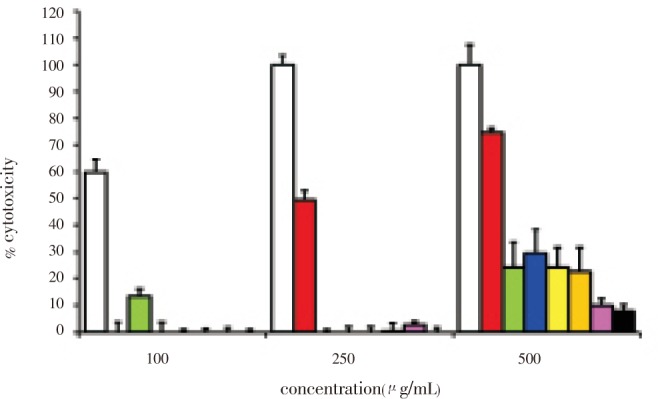
The HepG2 cells were incubated with 100, 250 and 500 µg/mL EW-L crude extract (white), single extract from fraction hexane (red), chloroform (green), ethylacetate (blue), dichloromethane (yellow), ethanol (orange), methanol (pink), and water (black), respectively.
3.2. Enhancement cytotoxicity effects of the combined extract fraction from P. evecta in HepG2 cells
An enhancement of cytotoxicity of the hexane extract fraction compared to the EW-L crude extract was observed particularly when it was combined with the polar extract fraction (i.e., from hexane+water, hexane+methanol, hexane+ethanol) (Figure 2). An enhanced activity of the combined extracts-between a fixed final concentration of 500 µg/mL hexane extract and the other extracts-were initially observed when the final concentrations of those relative polar extracts were 100 µg/mL. It should be noted that this enhanced cytotoxicity of the combined extract fraction was higher than that of 100 µg/mL of EW-P crude extract. To exceed the cytotoxicity of the combined extract fraction, the EW-P crude extract concentration had to be higher than 250 µg/mL. These results indicated that the polar extract fraction of P. evecta enhanced the cytotoxicity of the hexane extract fraction.
Figure 2. The cytotoxic effect of hexane extract enhanced by the polar extract fraction after 24 h incubation by neutral red assay.
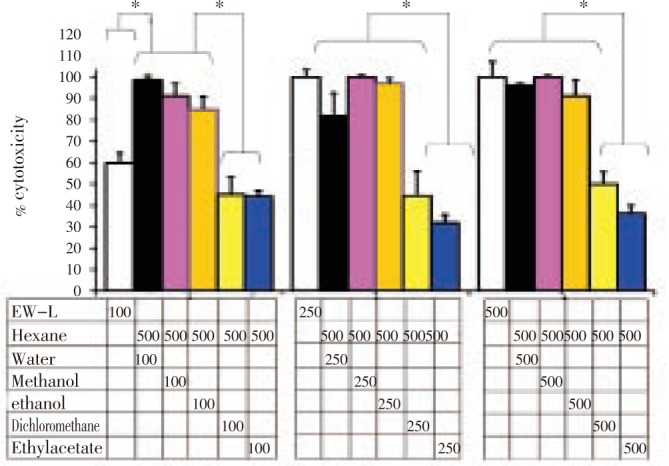
HepG2 cells were incubated with EW-L (white) and the mixture of 500 µg/mL hexane extract and 100, 250, and 500 µg/mL extract from the water (black), methanol (pink), ethanol (orange), dichloromethane (yellow), or ethyl acetate (blue) fraction, respectively. *Significantly different with P < 0.05 using a one-way ANOVA, Tukey-Kramer multiple comparison.
3.3. Apoptosis effects of the combined extract fraction from P. evecta in HepG2 cells
Programmed cell death or apoptosis performs an essential role for an effective cancer therapy and becomes the pharmaco-dynamic endpoint for anti-cancer treatment[17]. To confirm the anticancer potential of the EW-L crude extract and the combined extracts fraction, apoptosis induction in cancer cells was tested with the DAPI staining assay. The morphological changes of the nuclei DNA-after being treated with EW-L extract of P. evecta, hexane fraction (non-polar), water fraction (polar) and combined extract from hexane and water fraction-are shown in Figure 3.
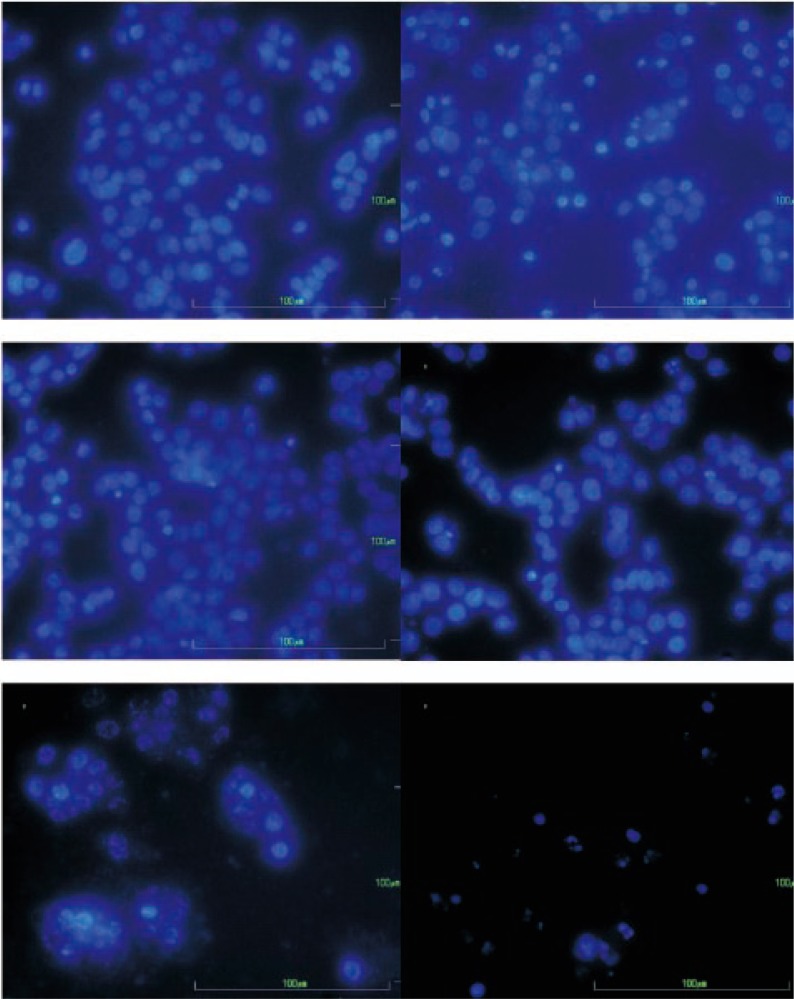
Figure 3. Nuclei morphological changes based on apoptosis induction in HepG2 cells after staining with DAPI and observed under inverted fluorescent microscopy with 40× magnification.
The HepG2 cells were incubated as: (A) untreated HepG2 cells (control); (B) EW-L extract 140 µg/mL; (C) hexane fraction 500 µg/mL; (D) water fraction 500 µg/mL of P. evecta; (E) combined extracts from the hexane and water fractions (500:500 µg/mL); and (F) melphalan alone (76 µg/mL).
The results indicated that the fraction that possessed a low cytotoxicity also induced fewer %apoptotic cells (Table 1). The extracts classified into the inactive group (F2-F7) exhibited less than 10% apoptotic cells. The strong cytotoxic compounds-such as EW-L crude extract, and melphalan-exhibited a moderate percentage of apoptotic cells (40–50%). The percentage of apoptotic cells induced in human hepatoma (HepG2) cells-by combining non-polar and the low cytotoxic extract (hexane) and polar inactive cytotoxic extracts (e.g., water or methanol)-were (72.7±13.6)% and (54.9±10.8)%, respectively (Table 1). These results confirmed that the polar extract fraction was necessary for the apoptosis induction of the non-polar extract fraction. The EW-L crude extract demonstrated an anticancer activity based on cytotoxicity and apoptosis induction effect which was reduced when it was fractionated. Thus, the anticancer activity occurred when the opposite polarity of each fraction was combined, which indicates a synergistic effect among the constituents of the whole EW-L crude extract.
3.4. Physical interaction by ATR/FT-IR spectroscopy
Attenuated Total Reflectance (ATR)/FT-IR spectroscopy is a useful technique for simple, direct, and sensitive quantitative study of the liquid sample for investigation of specific interaction between molecules[18]. The ATR-FT-IR was adapted to identify the chemical functional groups and the interaction type between the non-polar (hexane) and polar (water) extract fractions (Figure 4). All the assigned bands are indicated in Table 2.
Figure 4. ATR/FT-IR chromatograms.
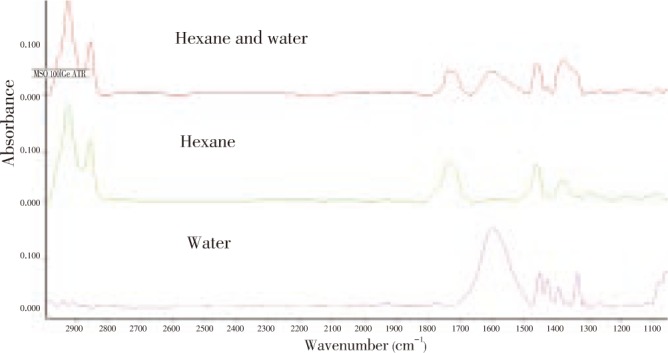
ATR/FT-IR chromatograms of 50 mg/mL of the hexane fraction (green), 50 mg/mL of the water fraction (pink) and the combined hexane and water extract fraction at a 1:1 ratio (red); to give a final concentration of 50 mg/mL in 20%v/v DMSO-water.
Table 2. Infrared absorption bands and vibrational modes of observed species on crude extracts collected from each fraction.
| Absorbance bands (cm−1) | Vibration mode | Species |
| 3 000-2 800 | C-H stretch | aliphatic carbons(alkanes, alkenes, alkynes |
| 1 760-1 700 | C=O stretch | esters, aldehydes, ketones, carboxylic acids |
| 1 650-1 500 | C=C | aromatics, alkenes |
| 1 500-1 400 | C-H bending | CH2 and CH3 bending |
| 1 400-1 300 | C-O-H bending | phenolic C-O-H bending |
| 1 300-1 000 | C-O stretch | alcohols, ethers, esters, carboxylic acids |
The relative integral area (%) of the infrared absorption bands of the average representative spectra of the crude extract from each fraction are presented in Table 3. The functional groups of the extract from the hexane fraction showed predominantly (about 58.7%) C-H stretching aliphatic carbons (3 000-2 800 cm−1)[19]. The integral area of C=O (1 760-1 700 cm−1) groups was approximately 20.2%. By contrast, the water extract fraction clearly showed a high relative integral area (79.2%) of aromatic C=C (1 650-1 500 cm−1) absorption bands. The relative integral area of this water extract fraction showed the lowest integral area of C=O species of 0.164%. In addition, there was no peak of C-H stretching in this fraction. Interestingly, the combination fractions of hexane and water showed the appearance of C-H stretching, C=O, aromatic C=C, C-H bending, C-O-H bending and C-O stretching with a respective relative integral area of 50.4, 10.1, 15.8, 7.4, 16.3 and 2.52[20].
Table 3. Relative integral area (%) of infrared absorption bands of averaged representative spectra of crude extract from each fraction.
| Crude extract | % relative integral area |
|||||
| C-H stretch | C=O stretch | C=C | C-H bending | C-O-H bending | C-O stretch | |
| Hexane | 58.7 | 20.20 | 0.35 | 10.40 | 6.40 | 3.90 |
| Water | - | 0.16 | 79.20 | 12.90 | 6.00 | 1.20 |
| Hexane & water | 50.4 | 10.10 | 15.80 | 7.40 | 16.30 | 2.52 |
The ATR/FT-IR results of the mixture between the hexane extract fraction and water extract fraction showed an increase of the integral area of C-H and C=O that was absent in the crude fraction of water and showed an increase of the integral area of C-O-H bending; from either the hexane or water extracts spectra (Table 3). These functional groups might be affected by the physical interaction between the non-polar and polar extracts[21]–[23].
In the combined extract fraction of water and hexane, an increment of C-O-H bending-assigning the polyphenolic functional group-was more discernible. The lipophilic functional groups of mixture (i.e., C-H stretch, C-H bending, C=C) and hydrophilic functional groups (C=O stretch, C-O-H bending, C-O stretch) were also changed from both the original hexane and water extract fractions. This might result in optimal hydrophilic-lipophilic properties, which would enhance the permeability of the active components.
4. Discussion
Anticancer drugs have been extensively discovered from natural compounds. Several anticancer activity studies have focused on the ability of single pure compounds; however, there have been reports that the total contents of a whole herb show a significantly better effect than a single isolated active ingredient (i.e., synergistic action); that is, the potential anticancer effects of herbal combinations are more effective than using any single constituent alone[24]–[26]. The synergistic combination of bioactive fractions presented highly potential effect in Barleria prionitis[24]. Moreover, the combination of two or more agents can overcome the toxicities or other side effects of a single pure compound[25]. For instance, the glycyrrhizin and saponin fractions of ginsenosides were individually reported to be ineffective but collectively useful for reducing ulcerative colitis in male Wistar/ST rats caused by 2,4,6-trinitrobenzene sulfonic acid[27].
In addition, the existence of phenolic compounds in plants was reported to be responsible for chemopreventive properties such as antioxidant, anticarcinogenic, or antimutagenic and anti-inflammatory effects. Phenolic compounds are also contribute to their inducing apoptosis by arresting cell cycle, regulating carcinogen metabolism and ontogenesis expression, inhibiting DNA binding and cell adhesion, migration, proliferation or differentiation, and blocking signalling pathways[28]–[33]. The hydrolysable tannin which is commonly found in the polar extract was previously reported to enhance the activity of the other phytochemicals by inhibition of the efflux mechanism, and decrease expression of P-glycoprotein, MRP1 and MRP2 membrane efflux transporter proteins in human cholangiocarcinoma[34]. Previously, the 50% ethanol and water extract of leaves of P. evecta was reported to contain high tannic acid content -(198.8±10.4) mg tannic acid equivalents/g dried extract[5], which agrees with our study which was based upon ATR/FT-IR techniques.
The known synergistic anticancer effect in the crude extract of P. evecta might be due to the polyphenolic functional group present in the polar fraction, which was confirmed by ATR/FT-IR techniques. An overexpression of the cancer drug efflux in the HepG2 cells was previously reported[13],[35]. This polyphenolic functional group might act by inhibiting the efflux mechanism existed on HepG2 cells or facilitating the permeability of the active compound in the extract mixture. Moreover, the apparent anticancer activity of the EW-L extract was also possible based on the inactive functional group in the non-polar fraction's being blocked; hence, the undesirable activity was lessened. As a consequence, the functional group that possesses the higher anticancer activity is dominant. Our observation confirmed a plausible molecular basis for the synergistic anticancer effect of whole crude leaf extract of P. evecta by which the total contents of a whole plant show a significantly better anticancer effect than each isolated extract fraction singly.
In conclusion, our study clearly show a cytotoxicity and apoptosis inductive effect indicative of an anticancer activity of the 50% ethanol-water leaf extract of P. evecta on the human hepatoma (HepG2) cell line. The synergistic anticancer effect of the extracts from P. evecta caused apoptosis in HepG2 cells. The combination of the extracts from the hexane and water fraction confirmed an enhanced anticancer activity with a synergistic anticancer effect found in the whole crude extract of P. evecta.
Acknowledgments
SM was scholarly supported by The Office of the Higher Education Commission, Thailand, under the Strategic Scholarships for Thai Doctoral Degree Programs (CHE-PhD-THA-RG 3/2549). This research was partially supported by the Plant Genetics Conservation Project under the Royal Initiation of Her Royal Highness Princess Maha Chakri Sirindhorn and research funding from Khon Kaen University (520123). The authors would like to thank (a) the Center for Research and Development of the Herbal Health Product, Khon Kaen University, for facilities (b) Miss Kansinee Wachiratanarom and Mr. Taveepong Nowarat for laboratory assistances and (c) Mr. Bryan Roderick Hamman and Mrs. Janice Loewen-Hamman for assistance with the English-language presentation of the manuscript.
Footnotes
Foundation project: It is scholarly supported by The Office of the Higher Education Commission, Thailand, under the Strategic Scholarships for Thai Doctoral Degree Programs (CHE-PhD-THA-RG 3/2549), and partially supported by the Plant Genetics Conservation Project under the Royal Initiation of Her Royal Highness Princess Maha Chakri Sirindhorn and research funding from Khon Kaen University (520123).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Finet A, Gagnepin F. Contributions à l′étude de la Flore de l′Asie orientale. Fam. 5. Annonacées. Bulletin de la Société Botanique de France. 1906;53:91–92. [Google Scholar]
- 2.Mahidol University . Pharmaceutical sciences. Bangkok: Saim-Phi-Chacha-Ya-Prug, Amarin Printing and Publishing; 1996. p. 190. [Google Scholar]
- 3.Kanokmedhakul S, Kanokmedhakul K, Ohtani II, Isobe M. A diynoic acid from Polyalthia evecta. Phytochemistry. 1998;47:131–133. [Google Scholar]
- 4.Kanokmedhakul S, Kanokmedhakul K, Kantikeaw I, Phonkerd N. 2-substituted furans from the roots of Polyalthia evecta. J Nat Prod. 2006;69:68–72. doi: 10.1021/np0503202. [DOI] [PubMed] [Google Scholar]
- 5.Sripanidkulchai B, Fangkratok N, Junlatat J, Sripanidkulchai K. Antioxidative activity and antimutagenicity of three plants in Annonaceae in plant genetics conservation at Khok Phutaka, Amphur Phuwiang, Khon Kaen. Isan J Pharm Sci. 2008;4:104–112. [Google Scholar]
- 6.Yuan Y, Huang GJ, Wang TS, Chen GY. In vitro screening of five Hainan plants of Polyalthia (Annonaceae) against human cancer cell lines with MTT assay. J Med Plants Res. 2011;5:837–841. [Google Scholar]
- 7.Sashidhara KV, Singh SP, Sarkar J, Sinha S. Polyalthia longifolia was found in India and had cytotoxicity based on the clerodane diterpenoids from the leaves. Nat Prod Res. 2010;24:1687–1694. doi: 10.1080/10236240902765301. [DOI] [PubMed] [Google Scholar]
- 8.Sashidhara KV, Singh SP, Kant R, Maulik PR, Sarkar J, Kanojiya S, et al. et al. Cytotoxic cycloartane triterpene and rare isomeric bisclerodane diterpenes from the leaves of Polyalthia longifolia var. pendula. Bioorg Med Chem Lett. 2010;20:5767–5771. doi: 10.1016/j.bmcl.2010.07.141. [DOI] [PubMed] [Google Scholar]
- 9.Suedee A, Mondranondra I, Kijjoa A, Pinto M, Nazareth N, Nascimento MSJ, et al. et al. Constituents of Polyalthia jucunda and their cytotoxic effect on human cancer cell lines. Pharma Biol. 2007;45:575–579. [Google Scholar]
- 10.Prayong P, Barusrux S, Weerapreeyakul N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia. 2008;79:598–601. doi: 10.1016/j.fitote.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Newell P, Villanueva A, Friedman SL, Koike K, Llovet JM. Experimental models of hepatocellular carcinoma. J Hepatol. 2008;48:858–879. doi: 10.1016/j.jhep.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hui KM. Human hepatocellular carcinoma: expression profiles-based molecular interpretations and clinical applications. Cancer Lett. 2009;286:96–102. doi: 10.1016/j.canlet.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Chen YB, Yan ML, Gong JP, Xia RP, Liu LX, Lu SC, et al. et al. Establishment of hepatocellular carcinoma multidrug resistant monoclone cell line HepG2/mdr1. Chinese Med J (Engl) 2007;120:703–707. [PubMed] [Google Scholar]
- 14.Berginc K, Kristl A. Transwell-grown HepG2 cell monolayers as in vitro permeability model to study drug-drug or drug-food interactions. J Med Food. 2011;14:135–139. doi: 10.1089/jmf.2010.0041. [DOI] [PubMed] [Google Scholar]
- 15.Machana S, Weerapreeyakul N, Barusrux S, Nonpunya A, Sripanidkulchai B, Thitimetharoch T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG2) cell line. Chin Med. 2011;6:39. doi: 10.1186/1749-8546-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumantran VN. Cellular chemosensitivity assays: an overview. Methods Mol Biol. 2011;731:219–236. doi: 10.1007/978-1-61779-080-5_19. [DOI] [PubMed] [Google Scholar]
- 17.Ward TH, Cummings J, Dean E, Greystoke A, Hou JM, Backen A, et al. et al. Biomarkers of apoptosis. Br J Cancer. 2008;99:841–846. doi: 10.1038/sj.bjc.6604519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan KL, Govada L, Bill RM, Chayen NE, Kazarian SG. Attenuated total reflection-FT-IR spectroscopic imaging of protein crystallization. Anal Chem. 2009;81:3769–3775. doi: 10.1021/ac900455y. [DOI] [PubMed] [Google Scholar]
- 19.Dirwono W, Park JS, Agustin-Camacho MR, Kim J, Park HM, Lee Y, et al. et al. Application of micro-attenuated total reflectance FTIR spectroscopy in the forensic study of questioned documents involving red seal inks. Forensic Sci Int. 2010;199:6–8. doi: 10.1016/j.forsciint.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Markovic J, Radovic J, Strbanovic R, Bajic D, Vrvic M. Changes in the infrared attenuated total reflectance (ATR) spectra of lignins from alfalfa stem. J Serb Chem Soc. 2009;74:885–892. [Google Scholar]
- 21.Wang S, Meckling KA, Marcone MF, Kakuda Y, Tsao R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J Agric Food Chem. 2011;59:960–968. doi: 10.1021/jf1040977. [DOI] [PubMed] [Google Scholar]
- 22.Bullen HA, Oehrle SA, Bennett AF, Taylor NM, Barton HA. Use of attenuated total reflectance Fourier transform infrared spectroscopy to identify microbial metabolic products on carbonate mineral surfaces. Appl Environ Microbiol. 2008;74:4553–4559. doi: 10.1128/AEM.02936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan XH, Chen GH, Shang C. ATR-FTIR and XPS study on the structure of complexes formed upon the adsorption of simple organic acids on aluminum hydroxide. J Environ Sci (China) 2007;19:438–443. doi: 10.1016/s1001-0742(07)60073-4. [DOI] [PubMed] [Google Scholar]
- 24.Anand AS, Banerjee SK, Chandan BK, Handa SS, Jaggi BS, Prabhakar A, et al. et al. Synergistic composition of bioactive fraction isolated from Barleria prionitis Linn and a method of treatment for hepatotoxicity, immuno-deficiency and fatigue and a process thereof. 2002 Patent No. 6664236. [Google Scholar]
- 25.Ma XH, Zheng CJ, Han LY, Xie B, Jia J, Cao ZW, et al. et al. Synergistic therapeutic actions of herbal ingredients and their mechanisms from molecular interaction and network perspectives. Drug Discov Today. 2009;14:11–12. doi: 10.1016/j.drudis.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Wagner H, Ulrich-Merzenich G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16:97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Kawashima K, Nomura A, Makino T, Saito K, Kano Y. Pharmacological properties of traditional medicine (XXIX): effect of Hange-shashin-to and the combinations of its herbal constituents on rat experimental colitis. Biol Pharm Bull. 2004;27:1599–1603. doi: 10.1248/bpb.27.1599. [DOI] [PubMed] [Google Scholar]
- 28.Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- 29.Li ZY, Y Wang, WT Shen, P Zhou. Content determination of benzyl glucosinolate and anti-cancer activity of its hydrolysis product in Carica papaya L. Asian Pac J Trop Med. 2012;5(3):231–233. doi: 10.1016/S1995-7645(12)60030-3. [DOI] [PubMed] [Google Scholar]
- 30.Baravalia Y, Vaghasiya Y, Chanda S. Hepatoprotective effect of Woodfordia fruticosa Kurz flowers on diclofenac sodium induced liver toxicity in rats. Asian Pac J Trop Med. 2011;4(9):342–346. doi: 10.1016/S1995-7645(11)60100-4. [DOI] [PubMed] [Google Scholar]
- 31.Madhumitha G, Saral A Mary , Senthilkumar B, Sivaraj A. Hepatoprotective potential of petroleum ether leaf extract of Crossandra infundibuliformis on CCl4 induced liver toxicity in albino mice. Asian Pac J Trop Med. 2010;3(10):788–790. [Google Scholar]
- 32.Nwaehujor CO, Udeh NE. Screening of ethyl acetate extract of Bridelia micrantha for hepatoprotective and anti-oxidant activities on Wistar rats. Asian Pac J Trop Med. 2011;4(10):796–798. doi: 10.1016/S1995-7645(11)60196-X. [DOI] [PubMed] [Google Scholar]
- 33.Karthikeyan R, Somasundaram ST, Manivasagam T, Balasubramanian T, Anantharaman P. Hepatoprotective activity of brown alga Padina boergesenii against CCl4 induced oxidative damage in Wistar rats. Asian Pac J Trop Med. 2010;3(9):696–701. [Google Scholar]
- 34.Naus P, Henson R, Bleeker G, Wehbe H, Meng F, Patel T. Tannic acid synergizes the cytotoxicity of chemotherapeutic drugs in human cholangiocarcinoma by modulating drug efflux pathways. J Hepatol. 2007;46:222–229. doi: 10.1016/j.jhep.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawami M, Yumoto R, Nagai J, Junyaprasert VB, Soonthornchareonnon N, Patanasethanont D, et al. et al. Effect of Thai plant extracts on P-glycoprotein function and viability in paclitaxel-resistant HepG2 cells. Drug Metab Pharmacokinet. 2010;25:155–162. doi: 10.2133/dmpk.25.155. [DOI] [PubMed] [Google Scholar]