Abstract
This report describes the clinical and pathological aspects of an apocrine sweat gland carcinoma with distant metastasis in an aged dog. A 7-year-old male terrier dog was referred to small animal hospital of Shahid Bahonar University of Kerman with a 5.5×3.5 centimeter pedunculated mass on its head near left auricular region which had been progressively growing since three months ago. The radiography showed no local and distant metastasis. Surgical excision and histological evaluation was done. Histologically, the mass was composed of epithelial cells arranged in glandular and solid patterns. The morphologic findings suggested either a primary or metastatic apocrine-gland carcinoma. Immunohistochemically, the tumor cells were intensely positive for cytokeratin 7 and 20 and negative for S100 protein. On the basis of histopathological and clinical findings, the tumor was diagnosed as a malignant apocrine gland tumor, arising from apocrine sweat glands of the skin. Local tumor recurrence with anorexia and weight loss was reported by the owner nine month later. Severe submandibular and prescapular lymphadenomegaly was noted in clinical examination. Several large pulmonary nodules were noted in chest radiographs resembling mediastinal lymph node metastasis. Second surgery and chemotherapy was rejected by the owner due to grave prognosis of the patient. The animal was died 45 days later due to respiratory complications. Tumors of apocrine sweat glands are relatively uncommon in dogs whereas apocrine gland adenocarcinoma with distant metastasis is extremely rare.
Keywords: Apocrine sweat gland adenocarcinoma, Dog, Metastasis, Tumor recurrence, Diagnosis
1. Introduction
Apocrine glands are the major type of sweat gland in dogs, and the distribution of eccrine sweat glands is limited to the footpads. Apocrine sweat gland carcinomas comprise a group of rare malignant skin tumors and tend to occur on the head, neck, and limb[1]. These tumors are solid, poorly circumscribed, subcutaneous nodules with ulceration of the overlying skin in some cases[2]. The prevalence of apocrine carcinomas were reported to be about 1.7% of epithelial tumors in dogs by Souza et al and golden retriever is the breed that shows a predisposition to these tumors[3],[4]. The inguinal and axillary areas are the favored sites for the occurrence of apocrine carcinomas whereas perioral region is another reported site in cats[5]. Approximately 70% of canine apocrine tumors are benign in nature, but malignant ones tend to recur locally and metastasize to regional lymph nodes and the lung[6]. The recent World Health Organization classification categorizes apocrine sweat gland tumors in domestic animals into adenoma/carcinoma, complex or mixed adenoma/carcinoma (or both), and ductal adenoma/carcinoma[7]–[9]. Literature reviews showed the occurrence of mentioned tumor in other animal species as fallow, 3 mixed tumors on the ventral surface of the tail in cattle[10]–[12], 2 cases each of adenoma and carcinoma in aged ponies[13], 1 case of carcinoma in a golden hamster[14] and 1 case of apocrine adenoma in a hare[15]. Apocrine adenocarcinomas are also very rare in human[16].
Apocrine gland carcinoma spreads via both lymphatic and vascular routes, however metastatic disease found in regional lymph nodes, lungs, liver, and bone. Approximately one-third of patients have regional lymph node involvement at diagnosis, and the incidence is higher in patients with higher grade tumors in humans[17]. The present case report describes the morphologic features of a malignant metastatic canine apocrine adenocarcinoma in an aged dog.
2. Case report
A pedunculated lobulated mass which was approximately 3.5 × 5.5 cm in diameter was found on the dermis of peri-auricular region in a 7-year-old, male terrier dog. The mass was well-circumscribed and bipartite (Figure 1).
Figure 1. A pedunculate erosive bipartite mass on the head of the affected dog (arrow).
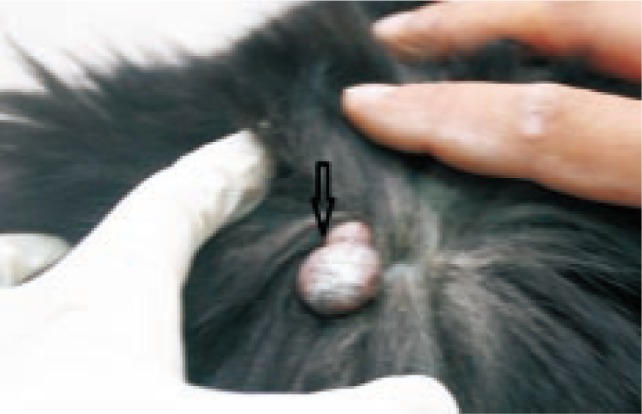
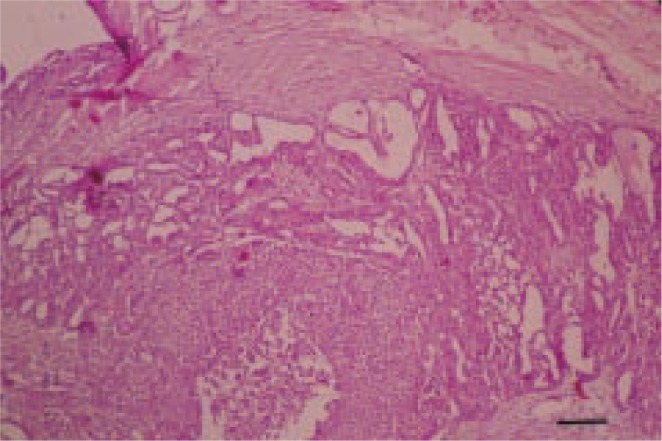
The owner had been aware of the mass for at least 3 months before the dog was referred to the veterinary hospital and during this period progressive enlargement of the mass was noted but there had been no other clinical symptom. Complete physical examination, hematological tests and radiography was done. No abnormality was detected in clinical examination and laboratory findings were within normal limits. Radiographic surveys showed no local and distant metastasis. Surgical excision was done. The surgical margin at the deep subcutis was separated by a thin capsule, but the border between the tumor and dermis was unclear. The cut surface was lobulated in appearance with white and partly grey color. The mass was sectioned and fixed in buffered formalin and routinely processed to paraffin wax method, sectioned of 5 µm thickness, and stained with hematoxylin and eosin. Immunohistochemical staining of additional sections was performed with avidin–biotin complex (ABC) method using antibodies against pancytokeratin (1/500), cytokeratin 7 (1/500), cytokeratin 20 (1/500), and S100 protein (1/400; all from Dakocytomation, Denmark). Histopathologic examination revealed an invasive tumor located into the dermis. The mass was subdivided into multiple lobules by connective tissue. The tumor was composed of large, cuboidal to polygonal cells with abundant eosinophilic cytoplasm, round nuclei, and central prominent nucleoli arranged in glandular and solid sheets (Figure 2).
Figure 2. Cuboidal and polygonal cells are arranged in glandular and solid sheets. H &E. Bar=100 µm.
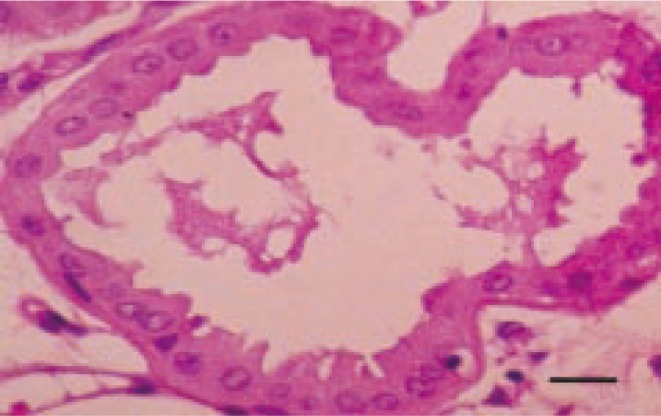
These cells showed decapitation secretion of apocrine glands and low mitotic index (Figure 3).
Figure 3. The tumor cells show decapitation secretion of apocrine glands. H &E. Bar=10 µm.
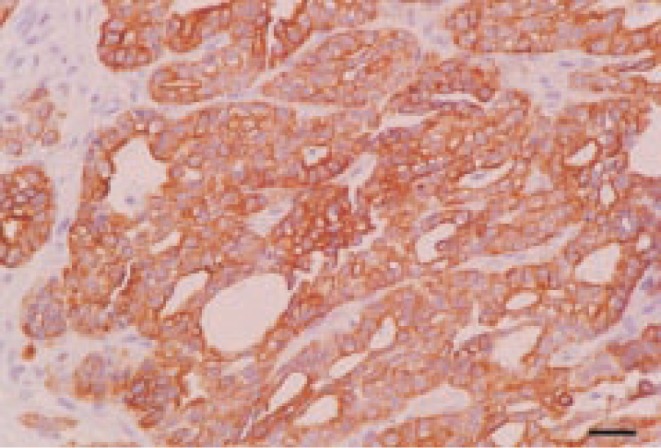
Immunohistochemically, the tumor cells were intensely positive for cytokeratin 7 and 20, and negative for S100 protein (Figure 4).
Figure 4. Neoplastic cells are characterized by intense pancytokeratin expression. ABC. Bar=25 µm.
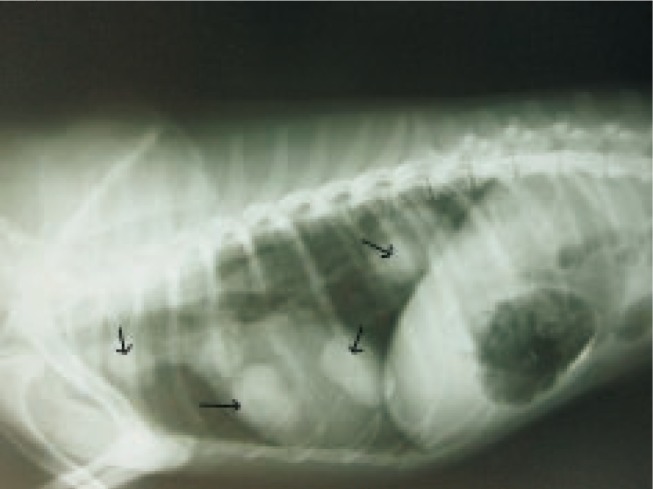
Unfortunately the owner did not follow the monthly post- excisional check-ups until local tumor recurrence with anorexia and weight loss in affected animal has been reported nine month later. Severe submandibular and prescapular lymphadenomegaly and dyspnea was noted in clinical examination. Several large pulmonary well-defined nodules were noted in chest radiographs resembling mediastinal lymph node metastasis. Moderate interstitial infiltrate in the cranioventral lung field and enlargement of the right ventricle were other radiogeraphical findings (Figure 5).
Figure 5. Large pulmonary well-defined nodules in the mediastinal area was obvious because of apocrine gland adenocarsinoma metastasis.
Second surgery and chemotherapy was rejected by the owner due to grave prognosis. Palliative therapy with theophline G elixir, 10 mg/kg every 8 hours (200 mg, Razak, Iran) and oral tramadole 2mg/kg/day (50 mg, Rooz daru, Iran) was prescribed but he animal was died 45 days later due to respiratory complications.
The diagnosis of primary apocrine-gland carcinoma was made based on the characteristic morphologic and immunohistochemical findings, and the lack of clinical or radiogheraphical features to support metastatic adenocarcinoma originating from other sites such as lung, prostate, or gastrointestinal system.
3. Disscussion
Apocrine gland carcinoma is a rare subtype of sweat gland carcinoma[18]. Patients usually present with a slow-growing, purple or red skin mass, which can be either firm or cystic in consistency. Characteristic histopathologic findings include decapitation secretion, a feature considered pathognomonic for apocrine differentiation, periodic-acid–Schiff-positive material in the cells or lumen, and immunoreactivity with gross cystic disease fluid protein. Normal apocrine glands and apocrine-gland adenomas are often found alongside the tumor and are occasionally infiltrated by carcinoma cells[19].
In dogs, the eccrine gland is located only in the foot pad, while the apocrine gland distributed in all sites of the skin. Since the present tumor occurred in the periauricular region, the tumor must be originated from the apocrine gland.
The pathological distinction between benign and malignant neoplasms is not easy in apocrine tumors[20]. In the present case, despite the relatively small size of the primary tumor, the patient already had metastasis to the local and distant lymph nodes at the time of tumor recurrence. This phenomenon is rarely reported in canine medicine and only 2% of dogs with apocrine gland adenocarsinoma showed distant metastasis due to intravascular invasion in the study of Simiko et al[8]. In the opposite of that, lymphatic invasion seems to be responsible for tumor metastasis in the present case because of mediastinal lymph node involvement. In veterinary medicine, a similar case was reported in a free-living mouflon along with kidney metastasis[21].
The only curative therapy for localized apocrine-gland carcinoma involves wide local excision with regional lymph node dissection and consideration of postoperative radiotherapy in patients with moderately or poorly differentiated tumors which was applied in human and veterinary medicine[22]. One of the agents reported to produce good chemotherapy result is 5-Fluorouracil and a case with complete response to this drug was reported by Swanson et al[23].
Although the post excisional fallow up of the present case was partially done but rejection of second surgery, chemotherapy and necropsy by the owner were the major limitation of this case study. The rarity of this type of tumor precludes clinical trials in this field so there are no particular guidelines for the treatment of recurrent tumors. Accordingly, reporting similar cases could yield useful information about the diagnosis and treatment of canine apocrine gland adenocarcinoma.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Rütten A, Requena L. Sweat gland carcinomas of the skin. Hautarzt. 2008;59(2):151–160. doi: 10.1007/s00105-007-1448-0. [DOI] [PubMed] [Google Scholar]
- 2.Pai RR, Kini JR, Achar C, Rau A, Kini H. Apocrine (cutaneous) sweat gland carcinoma of axilla with signet ring cells: a diagnostic dilemma on fine-needle aspiration cytology. Diagn Cytopathol. 2008;36:739–741. doi: 10.1002/dc.20889. [DOI] [PubMed] [Google Scholar]
- 3.Souza TM, Fighera RA, Irigoyen LF, Barros CSL. Estudo retrospectivo de 761 tumores cutâneos em cães. Cienc Rural. 2006;36(2):555–560. [Google Scholar]
- 4.Kalaher KM, Anderson WI, Scott DW. Neoplasms of the apocrine sweat glands in 44 dogs and 10 cats. Vet Rec. 1990;127:400–403. [PubMed] [Google Scholar]
- 5.Meuten Donald J., editor. Tumors in Domestic Animals. 5th ed. United States: John Wiley & Sons Publishing; 2008. [Google Scholar]
- 6.Goldschmidt MH, Hendrick MJ. Tumors of the skin and soft tissues. In: Meuten DJ, editor. Tumors in domestic animals. 4th Ed. Ames, Iowa, USA: Iowa State Press; 2008. p. 52. [Google Scholar]
- 7.Gross Tl, Ihrke PJ, Walder E, Affoltter VK. Skin disease of the dog and cat, clinical and histopathologic diagnosis. 2nd ed. London, UK: Blackwell Publishing; 2005. pp. 566–569. [Google Scholar]
- 8.Simko E, Wilcock BP, Yager JA. A retrospective study of 44 canine apocrine sweat gland adenocarcinomas. Can Vet J. 2003;44:38–42. [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes KH, Werner AH. Blackwell's five-minute veterinary consult clinical companion: small animal dermatology. 2nd ed. London, UK: Blackwell Publishing; 2011. pp. 359–340. [Google Scholar]
- 10.Garma-Avina A, Valli VE. Mixed sweat gland tumor in a bull (a case report) Vet Med Small Anim Clin. 1981;76:557–559. [PubMed] [Google Scholar]
- 11.Gulbahar MY, Alkan I, Aslan L, Golen I. Mixed apocrine sweat gland tumor of the tail in a cow. Vet Pathol. 2002;39:281–285. doi: 10.1354/vp.39-2-281. [DOI] [PubMed] [Google Scholar]
- 12.Piercy DWT, Cranwell MP, Collins AJ. Mixed apocrine (sweat gland) adenocarcinoma in the tail of cow. Vet Rec. 1994;134:473–474. doi: 10.1136/vr.134.18.473. [DOI] [PubMed] [Google Scholar]
- 13.Anderson WI, Scott DW, Crameri FM. Two rare cutaneous neoplasms in horses: apocrine gland adenocarcinoma and carcinosarcoma. Cornell Vet. 1990;80:339–345. [PubMed] [Google Scholar]
- 14.Urayama F, Sato T, Shibuya H, Shirai W, Matsutani M, Yamazaki R. Apocrine adenocarcinoma in a golden hamster. J Vet Med Sci. 2001;63:1249–1252. doi: 10.1292/jvms.63.1249. [DOI] [PubMed] [Google Scholar]
- 15.Karpenko A, Bukovjan K. Tumors in wildlife. Cesk Patol. 1996;32:78–83. [PubMed] [Google Scholar]
- 16.Yugueros P, Kane WJ, Goellner JR. Sweat gland carcinoma: a clinicopathologic analysis of an expanded series in a single institution. Plast Reconstr Surg. 1998;102:705–710. doi: 10.1097/00006534-199809030-00014. [DOI] [PubMed] [Google Scholar]
- 17.Calonje JE, Brenn T, Lazar AJF, McKee PH, editors. McKee's pathology of the skin. 4th ed. Vol 2. Philadelphia: Elsevier (Mosby); 2012. Tumors of the sweat glands. [Google Scholar]
- 18.Jacobson YG, Rees TD, Grant R, Fitchett VH. Metastasizing sweat gland carcinoma: notes on surgical therapy. Arch Surg. 1959;784:574–581. doi: 10.1001/archsurg.1959.04320040070018. [DOI] [PubMed] [Google Scholar]
- 19.Chintamani, Sharma RD, Badran R, Singhal V, Saxena S, Bansal A. Metastatic sweat gland adenocarcinoma: a clinico-pathological dilemma. World J Surg Oncol. 2003;1:13. doi: 10.1186/1477-7819-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shintaku M, Tsuta K, Yoshida H, Tsubura A, Nakashima Y, Noda K. Apocrine adenocarcinoma of the eyelid with aggressive biological behavior. Pathol International. 2002;52:169–173. doi: 10.1046/j.1440-1827.2002.01323.x. [DOI] [PubMed] [Google Scholar]
- 21.Morandi F, Benazzi C, Simoni P. Adenocarcinoma of apocrine sweat glands in a mouflon (Ovis musimon) J Vet Diagn Invest. 2005;17(4):389–392. doi: 10.1177/104063870501700417. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain RS, Huber K, White JC, Travaglino-Parda R. Apocrine gland carcinoma of the axilla: review of the literature and recommendations for treatment. Am J Clin Oncol. 1999;22:131–135. doi: 10.1097/00000421-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Swanson JD, Jr, Pazdur R, Sykes E. Metastatic sweat gland carcinoma: Response to 5-fluorouracil infusion. J Surg Oncol. 1989;42:69–72. doi: 10.1002/jso.2930420114. [DOI] [PubMed] [Google Scholar]


