Abstract
Objective
To study risk factors, contributing factors of bacterial and fungal endophthalmitis in Upper Egypt, test the isolated species sensitive to some therapeutic agents, and to investigate the air-borne bacteria and fungi in opthalmology operating rooms.
Methods
Thirty one cases of endophthalmitis were clinically diagnosed and microbiologically studied. Indoor air-borne bacteria and fungi inside four air-conditioned operating rooms in the Ophthalmology Department at Assiut University Hospitals were also investigated. The isolated microbes from endophthalmitis cases were tested for their ability to produce some extracellular enzymes including protease, lipase, urease, phosphatase and catalase. Also the ability of 5 fungal isolates from endophthalmitis origin to produce mycotoxins and their sensitivity to some therapeutic agents were studied.
Results
Results showed that bacteria and fungi were responsihle for infection in 10 and 6 cases of endophthalmitis, respectively and only 2 cases produced a mixture of bacteria and fungi. Trauma was the most prevalent risk factor of endophthalmitis where 58.1% of the 31 cases were due to trauma. In ophthalmology operating rooms, different bacterial and fungal species were isolated. 8 bacterial and 5 fungal isolates showed their ability to produce enzymes while only 3 fungal isolates were able to produce mycotoxins. Terbinafine showed the highest effect against most isolates in vitro.
Conclusions
The ability of bacterial and fungal isolates to produce extracellular enzymes and mycotoxins may be aid in the invasion and destruction of eye tissues. Microbial contamination of operating rooms with air-borne bacteria and fungi in the present work may be a source of postoperative endophthalmitis.
Keywords: Bacterial, Fungal, Endophthalmitis, Upper Egypt, Risk factors, Trauma, Ophthalmic surgery
1. Introduction
Endophthalmitis is an inflammatory reaction of intraocular fluids or tissues. Infectious endophthalmitis is one of the most serious complications of ophthalmic surgery[1]. Endophthalmitis can occur exogenously after ophthalmic surgery, post-traumatically or endogenously[2]. Endophthalmitis, although rare, is one of the most vision-threatening complication of cataract surgery, with a frequency varying from 0.07% to 0.13%[3]. The majority of these infections are bacterial. The occurrence of fungal endophthalmitis after cataract surgery is rare[4]. Fungal endophthalmitis is usually seen as cluster infections caused by the use of contaminated intraocular irrigating solutions, intraocular lenses, ventilation system[5], and hospital construction activity[6]. While postoperative fungal endophthalmitis may be rare in the Western world, apparently it is not as infrequent in developing countries. In recent studies from India, fungi accounted for up to 21.8% of all the culture-positive postoperative endophthalmitis cases[7]. Operating theatres in developing countries often do not adhere to standards for physical parameters. Most conventional operating theatres in hospitals of such countries are equipped with window-mounted air conditioning units, mainly installed for comfort rather than for the delivery of clean air. Fungi that can cause healthcare-associated infections include Aspergillus spp., members of the orders Mucorales and Moniliales. Many of these fungi have the potential to proliferate in air filtration devices and air conditioning units[8]. The lack of information about endophthalmitis in Upper Egypt and its possible reasons were the motivation of this study. This work was designed to study bacterial and fungal species associated with endophthalmitis, different risk factors that predispose patients to this infection, contributing factors, and to test the isolated species sensitive to some therapeutic agents. Also the contamination of air in opthalmology operating rooms by bacteria and fungi was studied.
2. Materials and methods
2.1. Clinical diagnosis and sampling of endophthalmitis specimens
A total of 31 patients clinically diagnosed to have endophthalmitis were investigated. Patients were from Upper Egypt (El-Minya, Assiut, The New Valley, Sohag, Qena, Aswan) and their informed consent for vitreous sampling was obtained. All patients were admitted and treated in the Department of Ophthalmology of Assiut University Hospitals. A history was taken of circumstances in which the eye became infected, of the predisposing factors. The patients were thoroughly examined using slit-lamp biomicroscope by an ophthalmologist. Vitreous sampling was done at the pars plana, 3 mm from the limbus with 22- to 26-Gauge needle and 2 mL disposable syringe[9]. From each endophthalmitis case, a vitreous sample was taken for direct microscopic examination and culturing. The potassium hydroxide wet mount 10% and two stains were used in examining the direct smears, lactophenol cotton blue stain and Gram stain. The vitreous samples were divided into four parts and inoculated directly into the following 4 media: two for bacteria (Blood agar and Endo agar) and two for fungi (Sabouraud dextrose agar and Czapek glucose agar). The standard microbiological methods were used for identification of bacterial and fungal genera and species[10]. Bacterial and fungal isolates were given numbers and deposited in the Assuit University Mycological center.
2.2. Sampling for bacterial and fungal airospora in Ophthalmology operating rooms
Settle (gravity) plate method[11] was used to catch air-borne bacterial and fungal species after surgery in the atmospheres of four air-conditioned operating rooms at Ophthalmology Departement, Assiut University Hospitals. Four types of agar media were used: two for isolation of bacteria (Blood agar and Endo agar) and two for isolation of fungi (Sabouraud dextrose agar and Czapek glucose agar). Total bacterial catch for each bacterium and Total fungal catch for each fungus in 10 exposures was calculated per 160 plates, 30 minutes exposure each.
2.3. Screening for extracellular enzyme production by bacterial and fungal isolates of endophthalmitis origin
Eight bacterial and five fungal isolates of endophthalmitis origin were screened for their ability to produce extracelluar enzymes including protease, lipase, urease, phosphatase and catalase in solid media using different substrates and reagents[12]. The ability of these organisms to produce enzymes was measured by the clear zone around the colony (protease), the depth of visible precipitate (lipase), the color intensity (urease), the color zone around the colony (phosphatase) and O2 bubbles evolution (catalse).
2.4. Determination of mycotoxins produced by some fungal isolates of keratitis and endophthalmitis origin
Mycotoxins were extracted using chloroform then the thin-layer chromatographic technique was applied for semi quantitative analysis of mycotoxins[13],[14]. Five fungal isolates of endophthalmitis origin were tested for their ability to produce different mycotoxins (Aflatoxins, sterigmatocystin, zearalenone and trichothecene toxins). Thin layer of silica gel (type 60- F254) of about 0.3 mm thickness was prepared and poured on glass plates using an applicator. The samples were added to the plates as spots and for the purpose of separation of the different mycotoxins, solvent systems of reagents grade of the following composition were used:
Chloroform: methanol (97:3 v/v) and chloroform: acetone (9:1, v/v) for aflatoxins[13].
Toluene: acetone: methanol (50:30:20 v/v/v) for zearalenone and other fusarium toxins as described previously[15].
Benzene: methanol: acetic acid (90:1:15, v/v/v) for sterigmtocystin as described previously[14].
The plates were detected before and after spraying with the different reagents using shortwave (254 nm) and longwave UV light (365 nm). Mycotoxins were identified by comparison with appropriate reference standards[13],[14],[16]–[18].
2.5. Determination of antifungal activity
The disc susceptibility test[19] was employed using filter paper discs fully saturated with the antifungal agent (∼10 µL). The antifungal activity of four types of antifungal therapeutic agents [amphotericin B(50 mg), cetrimide (powder), ketoconazole (200 mg) and terbinafine (250 mg)] against five fungal isolates of endophthalmitis origin were tested using three concentrations (0.1%, 0.5% and 1%). Amphotericin B at 0.005% concentration which is recommended for endophthalmitis therapy also used to find out if this concentration is effective in-vitro or not. Discs were placed on the surface of Sabouraud dextrose agar seeded with the test organism. Cultures were incubated at 28 °C for 48 h after which the zone of inhibition of fungal growth around discs was measured in mm and the data were recorded as the mean of three replicates. Dimethyl sulphoxide was used to dissolve the antifungal agents and also as a control in this test.
3. Results
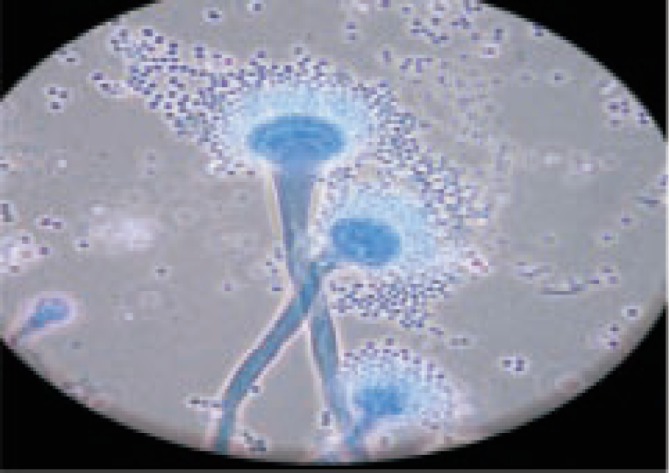
Results showed that bacteria and fungi were responsihle for infection in 10 and 6 cases of endophthalmitis, respectively. Only 2 cases produced a mixture of bacteria and fungi whereas 13 were negative for microbial cultures (Table 1). Enterobacter species was the most common bacterial agent involved in 3 (30%) out of the 10 bacterial endophthalmitis cases followed by Staphylococcus species which was reported from 2 cases (20%), while Aspergillus fumigatus (A. fumigatus) (Figure 1,2) and A. terreus (A. terreus) were the dominant species associated with fungal endophthalmitis involved in 3 and 2 out of the 6 fungal cases, respectively (Table 1).
Table 1. Collective data of endophthalmitis due to bacterial and/or fungal species.
| Case type | Isolated organism | Number of cases of isolations (n) | Percent of isolations (%) |
| Bacterial | Enterobacter sp. | 3 | 30.00 |
| Staphylococcus sp. | 2 | 20.00 | |
| M. luteus (Schroeter) Cohn | 1 | 10.00 | |
| Staphylococcus aureus Rosenbach | 1 | 10.00 | |
| Escherichia coli (Migula) Castellani and Chalmers | 1 | 10.00 | |
| Streptococcus pneumoniae (Klein) Chester | 1 | 10.00 | |
| Actinomycete sp. | 1 | 10.00 | |
| Klebsiella pneumoniae (Schroeter) Trevisan | 1 | 10.00 | |
| Fungal | A. fumigatus Fresenius | 3 | 50.00 |
| A. terreus Thom | 2 | 33.33 | |
| A. niger Van Tieghem | 1 | 16.67 | |
| Mixed | Staphylococcus sp. | 1 | 50.00 |
| Actinomycete sp. | 1 | 50.00 | |
| P. chrysogenum Thom | 1 | 50.00 | |
| Eurotium amstelodami Mangin | 1 | 50.00 | |
| Negative | No fungi and/or bacteria | 13 | 100.00 |
Figure 1. A case of fungal endophthalmitis due to A. fumigatus in a 5 year old girl.
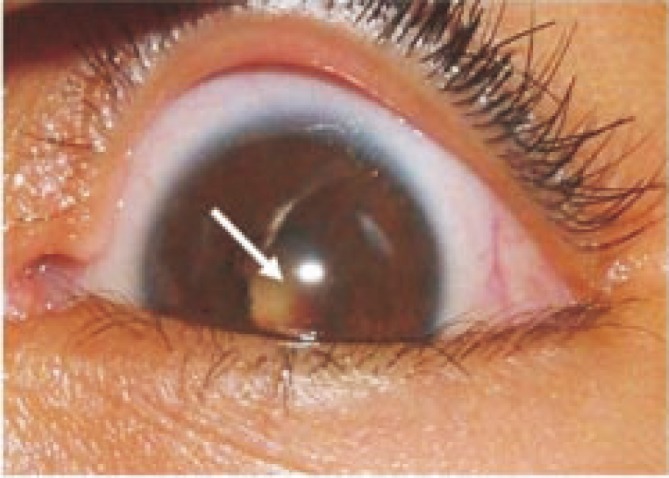
Figure 2. Microscopic appearance of A. fumigatus stained by lactophenol cotton blue (100×).
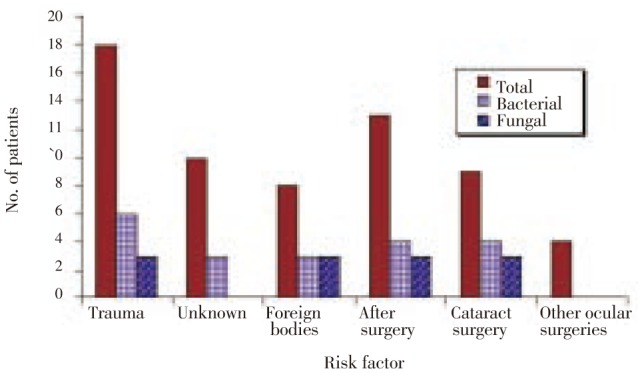
Trauma was the most prevalent risk factor of endophthalmitis where 58.1% of the 31 cases were due to trauma. Cases of trauma due to unknown factors and foreign bodies were represented by 55.6%, 44.4% of trauma cases and 32.3%, 25.8% of the total cases, respectively. Previous ocular surgery came second as a risk factor with 41.9% of the 31 cases, including cataract surgery which accounted 29.0% of total cases (Figure 3).
Figure 3. Incidence of endophthalmitis in relation to risk factor (out of 31 cases).
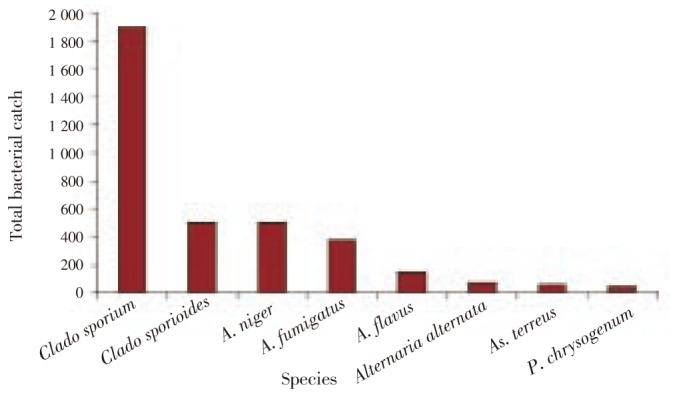
In the environment of ophthalmology operating rooms, Micrococcus luteus (M. luteus) and unidentified species of Staphylococcus, Actinomycetes and Gram negative Bacilli were the predominant bacteria (Figure 4), whereas Alternaria alternata, Aspergillus flavus, Aspergillus niger (A. niger), A. fumigatus, A. terreus, Penicillium chrysogenum (P. chrysogenum) and Cladosporium cladosporioides were the most common indoor airborne fungi (Figure 5).
Figure 4. Total catch of bacterial species isolated from air of ophthalmology operating rooms.
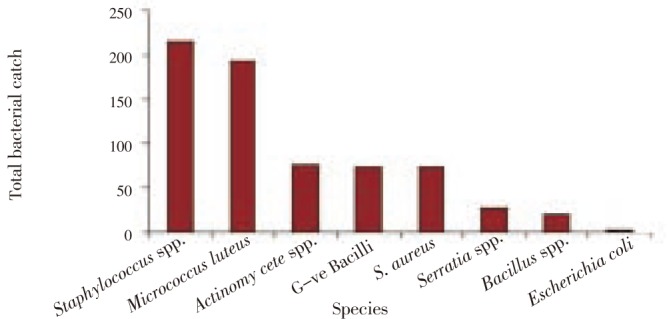
Figure 5. Total catch of most common fungal species isolated from air of ophthalmology operating rooms.
Out of 8 bacterial isolates tested for enzymes production during the present study, 12.5% were positive for protease, lipase and urease, 37.5% for phosphatase and 100% for catalase enzymes (Figure 6) while out of 5 fungal isolates tested, 80% were positive for protease and lipase where 100% for urease, phosphatase and catalase enzymes (Figure 6). Of 5 fungal isolates tested for toxin production only 3 were able to produce detectable amounts of toxins in culture medium (Table 2). The minimal inhibitory concentration (MIC) of amphotericin B, cetrimide and ketoconazole for most isolates was at 0.5% concentration while terbinafine showed the highest effect against most isolates at the three concentrations used (Table 3).
Figure 6. Extracellular enzymes produced by 8 bacterial and 5 fungal isolates of endophthalmitis origin.
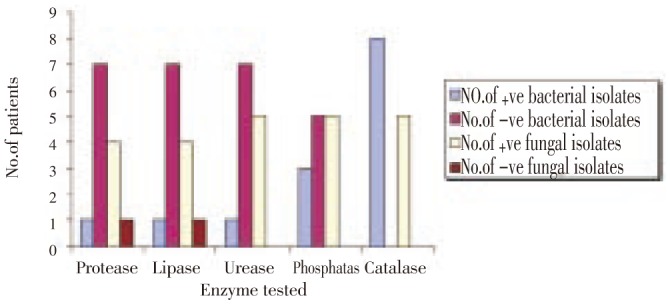
Table 2. Mycotoxins produced by fungal isolates of endophthalmitis origin.
| Fungi tested | Mycotoxin detected | Toxin level |
| A. fumigatus | Sterigmatocystin | High (> 500 mg/50 mL medium) |
| Versiclorin | Moderate (from 2-10 mg/50 mL medium) | |
| A. fumigatus | Sterigmatocystin | Low (< 2 mg/mL medium) |
| A. terreus | - | - |
| Eurotium amstelodami | - | - |
| P. chrysogenum | Patulin | Moderate (from 2-10 mg/mL medium) |
-: negative for mycotoxins.
Table 3. In-vitro antifungal activity of some antifungal theraputic agents (mm).
| Fungi tested | Control (solvent) | Amphotericin B |
Cetrimide |
Ketoconazole |
Terbinafine |
|||||||||
| 1% | 0.5% | 0.1% | 0.005% | 1% | 0.5% | 0.1% | 1% | 0.5% | 0.1% | 1% | 0.5% | 0.1% | ||
| A. fumigatus | 0 | 8.0 | 7.0 | 0.0 | 0.0 | 10.0 | 7.0 | 0.0 | 11.3 | 8.3 | 0.0 | 26.0 | 20.0 | 13.0 |
| A. fumigatus | 0 | 7.0 | 6.0 | 0.0 | 0.0 | 10.0 | 8.0 | 0.0 | 9.3 | 7.3 | 0.0 | 31.0 | 22.0 | 14.0 |
| A. terreus | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 18.0 | 11.0 | 0.0 | 18.7 | 15.0 | 0.0 | 33.0 | 28.0 | 21.0 |
| Eurotium amstelodami | 0 | 30.0 | 25.0 | 20.0 | 15.0 | 10.0 | 0.0 | 0.0 | 38.0 | 32.3 | 24.0 | 0.0 | 0.0 | 0.0 |
| P. chrysogenum | 0 | 11.0 | 10.0 | 7.0 | 0.0 | 13.0 | 10.0 | 0.0 | 10.7 | 8.7 | 0.0 | 38.0 | 35.0 | 27.0 |
Solvent: dimethyl sulphoxide (DMSO).
4. Discussion
Species of Enterobacter and Staphylococcus were the common etiologic agents of bacterial endophthalmitis. Staphylococcus species were reported as the most common causative agents of bacterial endophthalmitis previously with 26%[20], and 35% of the cases[21], while Enterobacter species was isolated from one out of 34 cases of endophthalmitis[21]. From the available literatures it is worthy to mention that M. luteus (reported from one out of 10 bacterial endophthalmitis cases studied) was not reported earlier[20],[22].
The current results revealed that Aspergillus species were the most common agents of fungal endophthalmitis. Earlier study also reported Aspergillus species from 9 out of 27 fungal endophthalmitis cases studied and these species were A. fumigatus, Aspergillus glaucus, A. terreus and A. niger[23].
Eye trauma was the most common risk factor for endophthalmitis followed by previous ocular surgeries. Earlier studies have shown that previous ocular surgery especially cataract surgery was the most predisposing factor of endophthalmitis followed by systemic diseases, trauma and corticosteroid therapy[20],[22].
It is worthy to mention that contamination of operating rooms with air-borne bacteria and fungi in the present work may be a source of postoperative endophthalmitis as suggested by others. Earlier study postulated that microbial contamination of air in operating rooms is generally considered to be a risk factor of postoperative infections[24]. Postoperative fungal infection may be caused by discharge of spores from contaminated air conditioning units. The findings of many authors are in agreement with our findings where the filters of such units may be act as a nidus for the growth of fungi[9],[25],[26]. In this respect, and a trial to solve that problem originating from air fungal spores, the frequent change of air filters is very necessary to reduce airborne spores of Aspergillus species.
The ability of bacterial and fungal isolates of endophthalmitis origin to produce extracellular enzymes and mycotoxins may be aid in the invasion and destruction of eye tissues as reported earlier[27],[28]. In an expermintal study carried out in the United States[29] the ocular lesions developed in most rats after 7-14 days of feeding on the fungal diet (rice cultures or fungal mats of Penicillium viridicatum), according to these findings mycotoxins maybe have a role as contributing factors in eye infections.
The in-vitro sensitivity test using different antifungal agents is greatly recommended to choose the most effective drug for therapy. Noteworthy to mention that amphotericin B showed antifungal activity at 0.005% (the recommended concentration for fungal endophthalmitis therapy) only against one isolate out of five tested while Terbinafine at the three concentrations used showed high activity against most isolates tested and this revealed that terbinafine was more effective in vitro than the other three antifungal therapeutic agents used in this study. Terbinafine has a broad spectrum and is considered a fungicidal in vitro whereas the azoles (e.g.; ketoconazole) are primarily fungistatic and this may partially account for the greater efficacy of terbinafine[30].
The present study showed that previous ocular surgery came second as a risk factor of postoperative endophthalmitis infections, thus using of ultraclean air conditioning units in Ophthalmology operating rooms is very essential and necessary to reduce the risks of such infections.
Acknowledgments
We thank all the members of Ophthalmology Department and Assuit University Mycological center for assistance in samples collection and identification. The authors declare that there is no conflict of interests.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Kresloff MS, Castellarin AA, Zarbin MA. Endophthalmitis: Major review. Surv Ophthalmol. 1998;43:193–224. doi: 10.1016/s0039-6257(98)00036-8. [DOI] [PubMed] [Google Scholar]
- 2.Allen HF, Mangiaracine AB. Bacterial endophthalmitis after cataract extraction. II. Incidence in 36 000 consecutive operations with special reference to preoperative topical antibiotics. Arch Ophthalmol. 1974;91:3–7. doi: 10.1001/archopht.1974.03900060007002. [DOI] [PubMed] [Google Scholar]
- 3.Aaberg TM, Flynn HW, Schiffman J, Newton J. Nosocomial acute onset postoperative endophthalmitis survey: A ten year review of incidence and outcome. Ophthalmol. 1998;105:1004–1010. doi: 10.1016/S0161-6420(98)96000-6. [DOI] [PubMed] [Google Scholar]
- 4.Boldt HC, Mieler WF. Endopthalmitis. In: Tabbara KF, Hynduik RA, editors. Infections of eye. 2nd ed. Boston, New York, Toronto, London: Little Brown and Co; 1996. pp. 571–594. [Google Scholar]
- 5.Fridkin S, Kremer FB, Bland LA, Padhye A, McNeil MM, Jarvis WR. Acremonium kiliense endophthalmitis that occurred after cataract extraction in an ambulatory surgical center and was traced to an environmental reservoir. Clin Infect Dis. 1996;22:222–227. doi: 10.1093/clinids/22.2.222. [DOI] [PubMed] [Google Scholar]
- 6.Tabbara KF, Jabarti AA. Hospital construction-associated outbreak of ocular aspergillosis after cataract surgery. Ophthalmol. 1998;105:522–526. doi: 10.1016/s0161-6420(98)93037-8. [DOI] [PubMed] [Google Scholar]
- 7.Anand AR, Therese KL, Madhavan HN. Spectrum of etiological agents of postoperative endophthalmitis and antibiotic susceptibility of bacterial isolates. Indian J Ophthalmol. 2000;48:123–128. [PubMed] [Google Scholar]
- 8.Simmons RB, Price DL, Noble JA, Crow SA, Ahearn DG. Fungal colonization of air filters from hospitals. Am Ind Hyg Assoc J. 1997;58:900–904. doi: 10.1080/15428119791012252. [DOI] [PubMed] [Google Scholar]
- 9.Narang S, Gupta A, Gupta V, Dogra MR, Ram J, Pandav SS, et al. et al. Fungal endophthalmitis following cataract surgery: clinical presentation, microbiological spectrum and outcome. Am J Ophthalmol. 2001;132:609–617. doi: 10.1016/s0002-9394(01)01180-1. [DOI] [PubMed] [Google Scholar]
- 10.Collins GH, Lyne PM, Grange JM. Microbiological methods. 7th ed. Cambridge, UK: Butterworth / Heinemann; 1995. [Google Scholar]
- 11.Hoekstra ES, Samson RA, Summerbell RC. Methods for the detection and isolation of fungi in the indoor environments. In: Samson RA, Hoekstra ES, Frusvad JC, Filtenborg O, editors. Introduction to food and airborne fungi. Utrecht-The Netherlands: Centraalbureau Voor Schimmelcultures; 2004. pp. 298–305. [Google Scholar]
- 12.Paterson RRM, Bridge PD. Biochemical techniques for filamentous fungi. Surrey, UK: CAB International; 1994. [Google Scholar]
- 13.Horwitz W, Sanzel A, Reynolds H. Methods of analysis of the association official chemists. 12th ed. Washington, USA: George Banta Comp. Inc; 1975. [Google Scholar]
- 14.Josefsson BGE, Möller TE. Screening method for the detection of aflatoxins, ochratoxin, patulin, sterigmatocystin and zearalenone in cereals. JAOAC. 1977;60:1369–2371. [PubMed] [Google Scholar]
- 15.Kamimura H, Nishijama M, Yasuda K, Saito K, Ibe A, Nagayama T, et al. et al. Simultaneous detection of several Fusarium mycotoxins in cereals, grains and foods stuffs. JAOAC. 1981;64:1067–1073. [PubMed] [Google Scholar]
- 16.Eppley RM. Screening method for zearalenone, aflatoxin and ochratoxin. JAOAC. 1986;51:74–78. [Google Scholar]
- 17.Scott PM, Lawarence JW, Van Walbeek W. Detection of mycotoxins by thin layer chromatography. Application to screening of fungal extracts. Appl Microbiol. 1970;20:839–842. doi: 10.1128/am.20.5.839-842.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takitani S, Asabe U, Kato I, Suzuki M, Ueno Y. Spectrodensitometric determination of trichothecene mycotoxins with 4-p-nitrobenzyle pyridine on silica gel thin layer chromatograms. J Chromatogr. 1979;172:335–342. doi: 10.1016/s0021-9673(00)90970-1. [DOI] [PubMed] [Google Scholar]
- 19.Boyle VJ, Faucher ME, Ross RW. Rapid modified kirby bauer susceptibility test with single high concentration antimicrobial discs. Antimicrob Agents Chemother. 1973;3:415–424. doi: 10.1128/aac.3.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montan P, Lundström M, Stenevi U, Thorburn W. Endophthalmitis following cataract surgery in Sweden. The 1998 national prospective survey. Acta Ophthalmol Scand. 2002;80:258–261. doi: 10.1034/j.1600-0420.2002.800305.x. [DOI] [PubMed] [Google Scholar]
- 21.Wong TY, Chee S. The Epidemiology of acute endophthalmitis after cataract surgery in an Asian Population. Ophthalmol. 2004;111:699–705. doi: 10.1016/j.ophtha.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Wong JS, Chan TK, Lee HM, Chee SP. Endogenous bacterial endophthalmitis: an east Asian experience and a reappraisal of a severe ocular affliction. Ophthalmol. 2000;107:1483–1491. doi: 10.1016/s0161-6420(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 23.Benz MS, Scott IU, Flynn JRHW, Unonius N, Miller D. Endophthalmitis isolates and antibiotic sensitivities: A 6-year review of culture-proven cases. Am J Ophthalmol. 2004;137:38–42. doi: 10.1016/s0002-9394(03)00896-1. [DOI] [PubMed] [Google Scholar]
- 24.Landrin A, Bissery A, Kac G. Monitoring air sampling in operating theatres: can particle counting replace microbiological sampling? J Hosp Infect. 2005;61:27–29. doi: 10.1016/j.jhin.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Tarkkanen A, Raivio V, Anttila VJ. Fungal endophthalmitis caused by Paecilomyces variotii following cataract surgery: a presumed operating room air-conditioning system contamination. Acta Ophthalmol Scand. 2004;82:232–235. doi: 10.1111/j.1600-0420.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- 26.Kelkar U, Bal AM, Kulkarni S. Fungal contamination of air conditioning units in operating theatres in India. J Hosp Infect. 2005;60:81–84. doi: 10.1016/j.jhin.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Dorner JW. Chromatographic analysis of mycotoxins. In: Shibamoto T, editor. Chromatographic analysis of environmental and food toxicants. New York, USA: Marcel Dekker Inc; 1998. p. 113. [Google Scholar]
- 28.Ali R, Raja AMN, Mohtar I. Endogenous endophthalmitis and Horner's syndrome secondary to brain abscess in HIV patient. Asian Pac J Trop Dis. 2011;1(3):251–252. [Google Scholar]
- 29.McCracken MD, Carlton WW, Tuite J. Penicillium viridicatum mycotoxicosis in the rat. I: Ocular lesions. Food Cosmet Toxicol. 1974;12:79–82. doi: 10.1016/0015-6264(74)90323-x. [DOI] [PubMed] [Google Scholar]
- 30.Evans EG, Sigurgeirsson B. Double blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. Br Med J. 1999;318:1031–1035. doi: 10.1136/bmj.318.7190.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]


