Abstract
Objective
To investigate the antioxidant and anticancer activities of aqueous extracts of nine microalgal species.
Methods
Variable percentages of major secondary metabolites (total phenolic content, terpenoids and alkaloids) as well as phycobiliprotein pigments (phycocyanin, allophycocyanin and phycoerythrin) in the aqueous algal extracts were recorded. Antioxidant activity of the algal extracts was performed using 2, 2 diphenyl-1-picrylhydrazyl (DPPH) test and 2,2′- azino-bis (ethylbenzthiazoline-6-sulfonic acid (ABTS.+) radical cation assay. Anticancer efficiency of the algal water extracts was investigated against Ehrlich Ascites Carcinoma cell (EACC) and Human hepatocellular cancer cell line (HepG2).
Results
Antioxidant activity of the algal extracts was performed using DPPH test and ABTS.+ radical cation assays which revealed 30.1-72.4% and 32.0-75.9% respectively. Anticancer efficiency of the algal water extracts was investigated against Ehrlich Ascites Carcinoma Cell (EACC) and Human Hepatocellular cancer cell line (HepG2) with an activity ranged 87.25% and 89.4% respectively. Culturing the promising cyanobacteria species; Nostoc muscorum and Oscillatoria sp. under nitrogen stress conditions (increasing and decreasing nitrate content of the normal BG11 medium, 1.5 g/L), increased nitrate concentration (3, 6 and 9 g/L) led to a remarkable increase in phycobilin pigments followed by an increase in both antioxidant and anticancer activities in both cyanobacterial species. While the decreased nitrate concentration (0.75, 0.37 and 0.0 g/L) induced an obvious decrease in phycobilin pigments with complete absence of allophycocyanin in case of Oscillatoria sp.
Conclusions
Nitrogen starvation (0.00 g/L nitrate) induced an increase and comparable antioxidant and anticancer activities to those cultured in the highest nitrate content.
Keywords: Microalgae, Antioxidant, Anticancer, Nitrogen starvation, Cyanobacteria
1. Introduction
There is a current worldwide interest in finding new and safe antioxidants from natural sources such as plant material to prevent oxidative deterioration of food and to minimize oxidative damage to living cells The use of synthetic antioxidants has decreased due to their suspected activity as promoters of carcinogenesis as well as a general consumer rejection of synthetic food additives[1].
Microalgae are microscopic organisms capable to convert solar energy to chemical energy via photosynthesis. They contain numerous bioactive compounds that can be harnessed for commercial use. Components with antioxidant activities can be found in only a few species of algae. Although the occurrence of phenolic compounds in plants is well known and these groups of compounds possess antioxidant activity in biological systems[2], the antioxidant characteristics of algae are poorly known. Some studies reported that cancer was prevented by alga extracts, because of their antioxidant properties[3].
Several bioactive metabolites produced by cyanobacteria and algae have been discovered by screening programs, employing target organisms quite unrelated to those for which the metabolites evolved[4].
Many of these chemicals have diverse range of biological activities and chemical structures, which affect many biochemical processes within the cell. Such chemicals are presumably related to the regulation and succession of algal and bacterial populations[5]. These chemicals are expected to be synthesized under stress conditions and low growth rate and released at concentration large enough to be effective.
The medicinal value of cyanobacteria was appreciated as early as 1500 Bc, when strains of Nostoc were used to treat gout, fistula and several forms of cancer (Cyanobacteria are a rich source of potentially useful natural products)[5]. Over 40 different Nostocales species, the majority of which are Anabaena and Nostoc spp. Produce over 120 natural products (Secondary metabolites) having activities such as anti-HIV, anticancer, antifungal, antimalarial and antimicrobial.
Cyanovirin (CV-N, cyanoviorin-N), a 101 amino acid protein extracted from Nostoc ellipsosporum was found to have potent activity against all human immunodeficiency viruses such as HIV-1, M and T tropic strains of HIV-1, HIV-2, SIV (Simian), and FIV (Feline)[6]. The cosmopolitan distribution of cyanobacteria indicates that they can cope with a wide spectrum of global environmental stress, such as heat, cold, desiccation, salinity, nitrogen starvation, photooxidation, anaerobiosis and osmotic stress. They have developed a number of mechanisms by which cyanobacteria defend themselves against environmental stressors. Important among them are the production of photoprotective compounds such as mycosporine-like amino acids (MAAs) and Scytonemin[5] enzymes such as superoxide dismutase, catalase and peroxidases[7] repaire of DNA damage[8] and synthesis of shock proteins[9].
The present work aimed to investigate the antioxidant and anticancer activities of aqueous extracts for nine microalgal species including eight cyanobacteria and one green microalgal species.
2. Materials and methods
2.1. Algal species and culture conditions
Eight Cyanobacteria species (Anabaena flous-aquae, Anabaena oryzae, Nostoc humifusum, Nostoc muscorum, Oscillatoria sp., Spirulina platensis, Phormedium fragile and Wollea saccata) and the green alga strain Chlorella vulgaris were obtained from the Microbiology Department, Soils, Water and Environment Res. Inst. (SWERI), Agric. Res., Center (ARC). Cyanobacterial strains were maintained on BG11 medium[10] except Spirulina platensis which was cultivated in Zarrouk medium[11]. While, Bold medium[12] was used for culturing the green alga Chlorella vulgaris. Cultures were incubated in a growth chamber under continuous shaking (150 rpm) and illumination (40 µE/m2/s) at (25 ± 1) °C for 30 days.
Growth analysis: The analysis of different parameters like pH, electric conductivity (EC), optical density (OD) and dry weight (DW) were recorded at the time of inoculation and at the end of the experiment (stationary phase of each species).
Cultures concentration was determined as optical density (OD) by spectrophotometer at 560 nm[13]. Chlorophyll-a was determined spectrophotometrically after extraction by absolute methanol as reported by Vonshak and Richmond[14], pH values and algal dry weight were estimated according to Vonshak[15]. Electric conductivity (EC) was measured by conductivity meter (systronics 304).
2.2. Phycobiliprotein pigments
The water soluble phycobiliproteins pigments including allophycocyanin (APC), phycocyanin (PC) and C-phycoerytherine (PE) were extracted from algal cells (1 g) with 10 mL distilled water. The extract was centrifuged at (30 000 ×g) at 4 °C and supernatant was separated. The pellet cells were extracted again with 10 mL distilled water and centrifuged, and supernatant was separated. The supernatants in two solutions were combined, then complete to known volume. The absorbance (A) of the solution was recorded at the following wave lengths: 650 nm, 620 nm, 565 nm[16].
2.3. Phytochemical analysis of secondary metabolites
Water soluble secondary metabolites (T. phenolic compounds, Terpenoids and alkaloids) in all the water algal extracts and their culture media were determined as the following.
2.3.1. Total alkaloids
Total alkaloids were extracted according to Sabri et al.[17]. The alkaloid extract was dissolved in 2 mL of chloroform, then 25 mL of 0.02 N H2SO4 were added, The resulting solution was warmed to driven off the chloroform, cooled and titrated back the excess acid with 0.02 N NaOH solution, using methyl red as an indicator. Each ml of H2SO4 (0.02 N) was equivalent to 5.78 mg of alkaloid.
2.3.2. Total phenols
Total phenol contents of algae were determined by the Folin-Ciocalteu method[18]. An aliquot of 0.1 g water algal extracts was dissolved in 1ml deionized water. Then, 0.1 mL of phenols extract was added to 2.8 mL of deionized water 2 mL of 2% sodium carbonate and 0.1 ml of 50% Folin-Ciocalteu reagent. After incubation at room temperature for 30 min, the absorbance of the reaction mixture was spectrophotometrically measured at 750 nm against a blank. Ferulic acid was used for preparation of standard curve.
2.3.3. Total terpenoids
Five ml extract was transferred to wide nick test tube, then placed in an oven at 100 °C for 1 h. After cooling; 5 ml freshly prepared vanillin reagent (0.7% in 65% H2SO4) was added. Then the tube was heated at (60±1)°C in a water bath for 1 h. after cooling in crushed ice bath the absorbance was measured at 473 nm within 1 min against blank prepared by using distilled water instead of terpenoid solution. saponin used for preparation of standard curve [19].
2.4. Antioxidant activity
2.4.1. DPPH method
The 2, 2 diphenyl-1-picrylhydrazyl (DPPH) test was carried out as described by Burits and Bucar[20]. One mL of algal extract (100 µg/mL) was mixed with 1ml DPPH reagent (0.002% (w/v) /methanol solution). After an incubation for 30 min in the dark at room temperature, the absorbance was measured at 517 nm (using jenway 6130 spectrophotometer). Butylated hydroxyl toluene (BHT) at 100 µg/mL was used as positive control.
2.4.2. ABTS radical cation scavenging assay
This assay was based on the ability of different fractions to scavenge 2,2′- azino-bis (ethylbenzthiazoline-6-sulfonic acid (ABTS.+) radical cation in comparison to a standard (BHT). The radical cation was prepared by mixing a 7 mM ABTS stock solution with 2.45 mM potassium persulfate (1/1, v/v) and leaving the mixture for 4-16 h until the reaction was completed and the absorbance was stable. The ABTS.+ solution was diluted with ethanol to an absorbance of (0.70±0.05) at 734 nm for measurements. The photometric assay was conducted on 0.9 mL of ABTS.+ solution and 0.1 mL of tested samples and mixed for 45 s, measurements were taken at 734 nm after 1 min. The antioxidative activity of the tested samples was calculated by determining the decrease in absorbance by using the following equation:
E%= ((Ac-At)/Ac) × 100, where:
At and Ac are the respective absorbance of tested samples and ABTS.+, respectively[21].
2.5. Screening for anticancer activity
2.5.1. Cell culture and incubation of tumor cell line
Human hepatocellular cancer cell line, HepG-2, was obtained from the Vaccera (Giza, Egypt). Cells were maintained in RPMI-1640 supplemented with 100 µg/mL streptomycin, 100 units/mL penicillin and 10% heat-inactivated fetal bovine serum in a humidified, 5% (v/v) CO2 atmosphere at 37 °C.
Female Swiss Albino mice, (kept under environmental and nutritional conditions for 2 weeks) was injected intrapertonealy (i.p.) by Ehrlich Ascites Carcinoma Cells (EACC), in order to prepare the tumor cell line.
A line of Ehrlich Ascites Carcinoma resistant to endoxan has been used. The parent line was first supplied through the coursty of Dr. G. Klein, Amestrdam, Holland. The tumor line is maintained in the National Cancer Institute, Egypt in Female Swiss Albino mice by weekly transplantation of 2.5×106 cells and centrifuged at 1 000 xg for 5 min at 4 °C. The pellet was washed with saline (0.9% NaCl) then the needed number of cells was prepared by suspending the cells in the proportional volume of saline.
2.5.2. Cytotoxicity assay against HepG-2
The cytotoxicity of crude extract was tested against HepG-2 by SRB assay as previously described [22]. Exponentially growing cells were collected using 0.25% Trypsin-EDTA and plated in 96-well plates at 1000-2000 cells/well. Cells were exposed to test extract for 72 h and subsequently fixed with TCA (10%) for 1 h at 4 °C. After several washing, cells were exposed to 0.4% SRB solution for 10 min in dark place and subsequently washed with 1% glacial acetic acid. After drying overnight, Tris-HCl was used to dissolve the SRB-stained cells and color intensity was measured at 540 nm.
2.5.3. Cytotoxicity assay against EACC
The viability percentage of tumor cells was measured after incubation with the tested algal extracts as well as DMSO as control. Two ml of cells (4×106 cells) were transferred to a set of tubes, then different algal extracts (200 µg/mL) were added to the propriate tubes as well as DMSO. The tubes were incubated at 37 °C for 2 h. Then, in a test tube containing 80 µL saline and 10 µL trypan blue and 10 µL of cell suspension were added and mixed then the number of living cells was calculated using a hemocytometer[23].
2.6. Nitrogen stress experiments
The promising algal species; Nostoc muscorum and Oscillatoria sp. which recorded the greatest antioxidant and anticancer activities were cultured in culture media with increasing (1.5 (control), 3, 6, 9 g/L) or decreasing (1.5 (control), 0.75, 0.375, 0.0 g/L) nitrate nitrogen contents. Experiments were carried out in the previously used culture conditions of light intensity, temperature and experiment duration. Filtration of the culture media was followed by addition of distilled water, freezing, then aqueous extraction of pigments (Chl a, phycobiliproteins) was performed and antioxidant and anticancer activities were carried out on these extracts by the same methods used in the normal cultured algal species.
2.7. Statistical analysis
Data were subjected to an analysis of variance, and the means were compared using the “Least Significant Difference (LSD)” test at 0.05 and 0.01 levels, as recommended by Snedecor and Cochran[24].
3. Results
Table 1 recorded the growth rate of the tested algal species represented as optical density, chlorophyll a content and as dry weight (mg/L). Also the hydrogen ion concentrations (pH) of the algal cultures were recorded. The obtained data showed variability of the growth rates of the investigated microalgal species (8 cyanobacteria+1 green alga) under the same experimental conditions.
Table 1. The growth parameters of the microalgal cultures.
| Parameters | Nostoc muscorum | Anabaena flous aquae | Chlorella vulgaris | Oscillatoria sp | Spirulina platensis | Anabaena oryzae | Wollea saccata | Nostoc humifusum | Phormedium fragile |
| pH | 7.630 | 6.610 | 8.110 | 5.820 | 10.480 | 6.270 | 6.110 | 8.700 | 9.330 |
| Optical density at 650 (nm) | 1.163 | 1.155 | 1.611 | 0.219 | 2.500 | 1.755 | 1.981 | 1.200 | 1.660 |
| Chl-a (mg/L) | 4.335 | 1.776 | 5.876 | 4.800 | 23.450 | 5.874 | 14.250 | 5.230 | 2.860 |
| Dry weight (mg/L) | 744.320 | 727.650 | 1052.160 | 140.160 | 2622.400 | 1123.200 | 1267.840 | 760.800 | 1062.400 |
Spirulina platensis recorded the highest growth rate (2.5, 23.45 mg/L and 2 622.4 mg/L as optical density, chl a content and dry weight respectively). It was followed in descending order by those of Wollea saccata and Anabaena oryzae, where the different growth criteria went parallel and have the same trend. The other algal species behaved differently.
Table 2 recorded the algal culture filtrate contained the phytochemically determined major secondary metabolites (Total phenolic content, terpenoids and alkaloids). The extractracellular released metabolites (%) in the algal cultures show large variability, Anabaena oryzae, Phormedium fragile and Wallea saccata recorded, the highest total phenolic compounds (0.0085%, 0.0078% and 0.0074% respectively), while Nostoc muscorum (0.0019%) showed the lowest content.
Table 2. Secondary metabolites (as mg/100 g) in algal filtrate (extracellular).
| Metabolites | Nostoc muscorum | Anabaena flous aquae | Chlorella vulgaris | Oscillatoria sp | Spirulina platensis | Anabaena oryzae | Wollea saccata | Nostoc humifusum | Phormedium fragile |
| T. phenolic | 1.91±0..02 | 4.10±0.51 | 2.90±0.05 | 4.40±0.65 | 4.80±0.74 | 8.50±1.87 | 7.40±0.84 | 3.10±0.055 | 7.80±0.96 |
| Terpenoids | 1.80±0.01 | 2.10±0.16 | 2.10±0.27 | 2.50±0.68 | 5.00±0.52 | 8.10±0.65 | 4.90±0.64 | 2.10±0.0 | 5.50±0.32 |
| Alkaloids | 35.00±1.65 | 39.00±2.98 | 36.00±1.64 | 49.00±2.65 | 68.00±3.61 | 75.00±4.95 | 59.00±3.94 | 42.00±1.66 | 68.00±3.74 |
| LSD 0.05 | 2.60 | 2.61 | 2.61 | 2.64 | 2.60 | 1.423 | 1.36 | 2.60 | 3.93 |
Each value is presented as mean of triplet treatments, LSD: Least different significantly at P≤ 0.05 according to Duncan's multiple range test.
The intracellular algal species contained in general larger percentage of these metabolities complaining with the released extracellularly metabolities, Spirulina platensis, Nostoc muscorum and Oscillatoria sp. recorded the greatest total phenolic compounds (0.71, 0.61 and 0.55% respectively), while Wollea saccata showed the least content (0.1%) as shown in Table 3. Aqueous extracts of the tested eight algal species (except that of Chlorella vulgaris, the green alga) recorded no detectable phycoerytherin pigments, while phycocyanins and allophycocyanins show variable contents in the tested cyanobacteria species (Figure 1, Table 4).
Table 3. Secondary metabolites (as %) in different microalgae cells.
| Metabolites | Nostoc muscorum | Anabaena flous aquae | Chlorella vulgaris | Oscillatoria sp | Spirulina platensis | Anabaena oryzae | Wollea saccata | Nostoc humifusum | Phormedium fragile |
| T. phenolic | 0.61±0.06 | 0.32±0.05 | 0.20±0.00 | 0.55±0.0 | 0.71±0.14 | 0.40±0.00 | 0.10±0.00 | 0.34±0.0 | 0.36±0.00 |
| Terpenoids | 0.10±0.01 | 0.15±0.02 | 0.09±0.00 | 0.20±0.0 | 0.14±0.00 | 0.12±0.00 | 0.14±0.00 | 0.10±0.0 | 0.08±0.00 |
| Alkaloids | 2.30±0.20 | 1.9±0.03 | 2.45±0.05 | 2.62±0.15 | 3.02±0.06 | 1.60±0.14 | 1.50±0.40 | 1.65±0.16 | 1.80±0.25 |
| LSD 0.05 | 0.066 | 0.105 | 0.0036 | 0.018 | 0.029 | 0.067 | 0.031 | 0.031 | 0.066 |
Each value is presented as mean of triplet treatments, LSD: Least different significantly at P≤ 0.05 according to Duncan's multiple range test.
Figure 1. Color of aqueous extracts of the different microalgae under investigation.

1. Nostoc muscorum; 2. Anabaena flous-aquae; 3. Chlorella vulgaris; 4. Oscillatoria sp.; 5. Spirulina platensis; 6.Anabaena oryzae; 7. Wollea saccata; 8. Nostoc humifusum; 9. Phormedium fragile
Table 4. Phycobilins pigments in different aqueous filtrate of tested microalgal species (mg/mL).
| Algal species | CPC | APC | CPE | Total phycobilins |
| Nostoc muscorum | 0.089±0.010 | 0.140±0.020 | 0.0±0.0 | 0.229±0.020 |
| Anabaena flous aquae | 0.000±0.000 | 0.017±0.000 | 0.0±0.0 | 0.017±0.000 |
| Chlorella vulgaris | ND | ND | ND | ND |
| Oscillatoria sp | 0.021±0.000 | 0.001±0.000 | 0.0±0.0 | 0.022±0.000 |
| Spirulina platensis | 0.030±0.000 | 0.113±0.000 | 0.0±0.0 | 0.143 ±0.000 |
| Anabaena oryzae | 0.009±0.000 | 0.002±0.000 | 0.0±0.0 | 0.011±0.000 |
| Wollea saccata | 0.002±0.000 | 0.033±0.000 | 0.0±0.0 | 0.035±0.000 |
| Nostoc humifusum | 0.012±0.000 | 0.019±0.000 | 0.0±0.0 | 0.031±0.000 |
| Phormedium fragile | 0.130±0.010 | 0.030±0.000 | 0.0±0.0 | 0.160±0.030 |
| LSD 0.05 | 0.005 | 0.0057 | -- | 0.003 |
Each value is presented as mean of triplet treatments, LSD: Least different significantly at P≤ 0.05 according to Duncan's multiple range test.
Antioxidant activity of the tested algal extracts was determined by both DPPH and ABTS methods (Figure 2). The obtained data illustrated that, ABTS method showed higher antioxidant activity than DPPH method. Concerning DPPH, the antioxidant activity of the nine tested algal species ranged between 30.1 and 72.4% comparing with the standard antioxidant BHT (80.2%). Using ABTS method, which is more sensitive than the DPPH method, the antioxidant activity ranged between 31.2 and 75.9% (Standard BHT showed 85.6%). S. platensis, Oscillatoria sp, Anabaena flous-aquae and Nostoc muscorum recorded the highest antioxidant activities (75.9, 75.6, 73.6 and 72.8%).
Figure 2. Antioxidant activity of algal water extracts at 100 µg/ mL against DPPH (a) and ABTS (b) radicals.
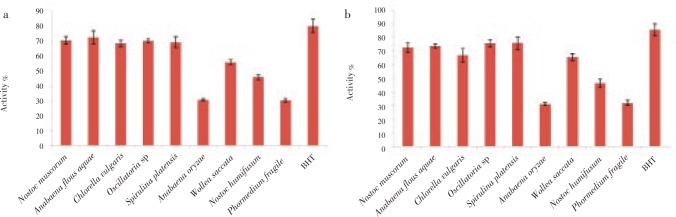
The anticancer efficiency of the algal aqueous extracts illustrated in Figure 3 and using EACC and HepG2 cell lines, recorded that the anticancer activity ranged between 15.68% to 87.25 % in case of EACC cell line and from 9.5% to 89.4% using HepG2 cell line. Nostoc muscorum aqueous extracts recorded the highest anticancer activity in both cell lines (87.25% in case of EACC and 89.4% in case of HepG2), followed by Oscillatoria sp. (67.40% and 77.8% in EACC and HepG2 respectively).
Figure 3. (a, b). Anticancer activity of algal water extracts at 100 µg/ ml against EACC (a) and HepG2 (b) cell lines.
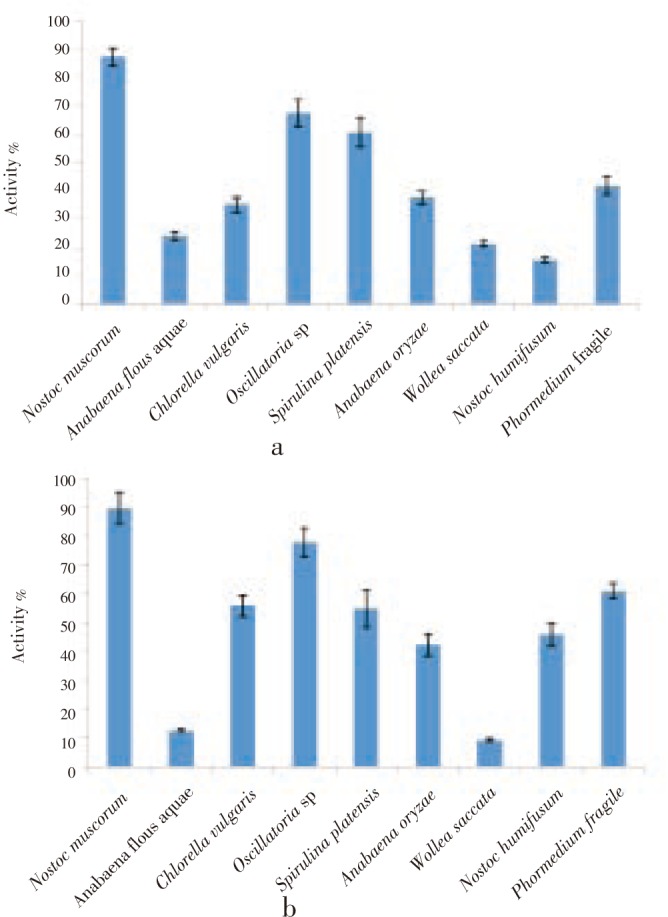
Table 5 recorded the phycobilin pigments of the nitrogen stressed N. muscorum and Oscillatoria sp. Increasing (3, 6, 9 g/L) or decreasing (0.75, 0.37, 0.00 g/L) nitrate nitrogen concentration induced a remarkable change in phycocyanin and allophycocyanin pigment contents compared to the control (1.5 g/L). Increasing nitrate conc. led to an obvious increase in both phycocyanin and allophycocyanin pigments of both the promising cyanobacterial species; and Oscillatoria sp. On the same time, the decrease in nitrate conc. in the growth media (BG11) led to a decrease in phycobilin pigments compared to the control.
Table 5. Phycobilins pigments (mg/mL) in the promising microalgae cultivated under nitrate stress conditions.
| Treatment |
Nostoc muscorum |
Oscillatoria sp |
||
| CPC | APC | CPC | APC | |
| Control (1.5 g/L NaNO3) | 0.095±0.000 | 0.13±0.02 | 0.20±0.01 | 0.001±0.000 |
| 3g/L | 0.110±0.000 | 0.14±0.00 | 0.23±0.05 | 0.001±0.000 |
| 6 | 0.140±0.020 | 0.16±0.00 | 0.24±0.03 | 0.001±0.000 |
| 9 | 0.150±0.010 | 0.19±0.05 | 0.26±0.04 | 0.001±0.000 |
| 0.75 | 0.090±0.000 | 0.13±0.01 | 0.16±0.01 | 0.000±0.000 |
| 0.37 | 0.086±0.000 | 0.11±0.00 | 0.16±0.02 | 0.000±0.000 |
| 0.0 | 0.075±0.000 | 0.10±0.00 | 0.14±0.01 | 0.000±0.000 |
| LSD 0.05 | 0.021 | 0.015 | 0.0227 | 8.95E.05 |
Each value is presented as mean of triplet treatments, LSD: Least different significantly at P≤ 0.05 according to Duncan's multiple range test.
In case of N. muscorum, doubling nitrate conc. led to an increase in phycocyanin and allophycocyanin contents[1.5 (control), 3, 6, 9 g/L induced 0.095, 0.11, 0.14, 0.15 mg/mL respectively in case of phycocyanin and 0.13, 0.14, 0.16 and 0.19 mg/mL respectively of allophycocyanin at the same nitrate-concentrations]. Decreasing nitrate conc. caused a marked decrease in both phycobilin pigments (0.75, 0.37 and 0.0 g/L nitrate) give rise to 0.090, 0.086 and 0.075 mg/mL phycocyanin and 0.13, 0.11 and 0.095 mg/mL allophycocyanin compared to the control (1.5 g/L nitrate) which induced 0.095 and 0.13 mg/mL phycocyanin and allophycocyanin respectively.
In N. muscorum, maximum phycobilin production was marked at the maximum nitrate conc. used (0.15 and 0.19 mg/mL phycocyanin and allophycocyanin at 9 g/L nitrate) and the least phycobilin content was produced in the absence or starvation of nitrate (0.075, 0.095 mg/mL phycocyanin and allophycocyanin at 0.00 g/L nitrate conc).
In case of Oscillatoria sp. the same trend was mostly recorded, but on decreasing nitrate conc. no allophycocyanin production was observed. Also, maximum phycobilin production was recorded at higher nitrate conc. (9 g/L) 0.26 and 0.0011 mg/mL phycocyanin and allophycocyanin while at nitrate starvation (0.75, 0.37 and 0.00 mg/mL of reduced content of phycocyanin pigments was produced and no allophycocyanin was recorded.
Table 6 illustrated the antioxidant activity of the nitrate-stressed cyanobacterial species (N. muscorum and Oscillatoria sp.) using both DPPH and ABTS methods. The obtained data showed that increasing nitrate conc. led to an increase in antioxidant activity (by both methods) and the decreased nitrate conc primarily decreased the antioxidant activity which then progressively increased at the lowest nitrate conc or starvation. ABTS method recorded lower antioxidant activity than DPPH method in both cyanobacterial species.
Table 6. Antioxidant activity of the nitrogen stressed promising algal species using DPPH and ABTS radicals (%).
| Treatment |
Oscillatoria sp. |
Nostoc muscorum |
||
| DPPH | ABTS | DPPH | ABTS | |
| Control (1.5 g/L NaNO3) | 59.80±0.95 | 69.80±1.45 | 69.80±1.22 | 72.60 |
| 3 g/L | 60.20±1.58 | 70.00±0.95 | 70.60±1.00 | 75.30±2.30 |
| 6 | 61.50±0.88 | 71.60±0.63 | 70.20±0.98 | 76.10±3.00 |
| 9 | 68.00±3.60 | 73.60±2.80 | 72.90±0.51 | 80.30±1.65 |
| 0.75 | 60.30±1.50 | 69.80±4.31 | 71.50±0.64 | 70.00±0.58 |
| 0.37 | 62.50±2.45 | 73.60±2.55 | 70.00±1.60 | 74.30±0.47 |
| 0.0 | 66.80±1.67 | 73.00±1.60 | 74.00±2.78 | 76.80±0.61 |
| LSD 0.05 | 0.968 | 0.956 | 0.987 | 1.001 |
Each value is presented as mean of triplet treatments, LSD: Least different significantly at P≤ 0.05 according to Duncan's multiple range test.
Table 7 recorded the anticancer efficiency of nitrate stressed N. muscorum and Oscillatoria sp. against EACC and HepG2 cell lines. The promising cyanobacterial species N. muscorum and Oscillatoria sp. induced both the highest antioxidant (by DPPH and ABTS methods) and anticancer activities (using EACC and HepG2 cell lines) which may be attributed to their large content in total phycobiliprotein pigments together with the higher secondary metabolites content (phenolic compounds, terpenoids, alkaloids).
Table 7. Anticancer activity of the nitrate stressed promising algal species using EACC and HepG2 cell lines (%).
| Treatment |
Oscillatoria sp. |
Nostoc muscorum |
||
| HepG2 | EACC | HepG2 | EACC | |
| Control (1.5 g/L NaNO3) | 70.40±1.87 | 68.30±0.58 | 88.60±2.96 | 85.90±3.60 |
| 3 g/L | 72.10±2.60 | 69.70±1.64 | 85.60±2.30 | 84.60±4.00 |
| 6 | 70.60±0.80 | 72.60±2.34 | 86.90±1.80 | 85.60±2.98 |
| 9 | 75.90±2.61 | 82.60±4.65 | 88.70±0.95 | 90.40±4.82 |
| 0.75 | 72.60±3.05 | 68.30±0.72 | 86.70±1.00 | 83.00±1.64 |
| 0.37 | 74.80±2.50 | 78.30±5.96 | 88.00±2.70 | 84.00±0.50 |
| 0.0 | 82.00±4.85 | 82.90±3.40 | 92.30±1.65 | 89.90±1.96 |
| LSD 0.05 | 0.575 | 0.907 | 1.63 | 0.505 |
Each value is presented as mean of triplet treatments, LSD: Least different significantly at P≤ 0.05 according to Duncan's multiple range test.
4. Discussion
The cyanobacteria S. platensis showed higher antioxidant activity (69.3%, 75.9% by DPPH and ABTS methods respectively) and moderate anticancer efficiency (60.67 and 54.8% against EACC and HepG2 respectively) which may be due to its content of total phycobiliprotein pigments, and highly produced secondary metabolites.
S. platensis was widely investigated and its variable biological activities were previously recorded in many literatures[2],[25]–[29]. So the data concerning this species is not highlighted in this work, nitrogen stress conditions of the promising N. muscorum and Oscillatoria sp. induced a variable content of the produced phycobiliprotein pigments depending on the nitrogen content in the algal culture media (BG11).
Increasing nitrate concentrations in the culture media of both cyanobacteria species (N. muscorum and Oscillatoria sp.) led to a marked enhancement in phycobiliprotein production which was translated in an obvious increase in antioxidant activity (by DPPH and ABTS) in both species under study while decreasing the nitrate content, phycobilin pigments production were consequently decreased in both species and its complete absence was recorded on nitrogen starvation especially in case of Oscillatoria sp., and no allophycocyanin pigments were produced.
Phycobiliprotein pigments were known by its antioxidant activity[5], increasing of these pigments production as a result of doubling nitrate concentration in the growth culture media, led to a progressive increase in the antioxidant activity recorded by both DPPH and ABTS assays in the two cyanobacteria under investigation.
Keeping in mind that, synergetic effect occurred between the polar secondary metabolites especially the phenolic compounds and the polysaccharides in antioxidant activity. Increasing nitrate conc. and the consequent increase in phycobilin pigments production, have the major role in enhancing the antioxidant activity may be attributed.
The decrease in nitrate conc. was followed by an obvious decrease in phycobilin pigment and even an absence of one of its constituents on nitrate starvation. The antioxidant activity in both species (by both assays) was apparently not affected comparing with the control (1.5 g/L nitrate). Under stress conditions, it was known that, deviation in metabolic pathways may occur. In presence of nitrate, nitrogenous compounds, including the phycobilin pigments were increasingly produced leading, together with other antioxidant active secondary metabolites (as phenolics), to a marked increase in biological activity.
The decrease in nitrate content induced a stress condition and not only a decrease in nitrogen skeleton compounds as phycobilin pigment production, but an increase in the carbon skeleton compounds (as phenolics) as a result of metabolic alterations under these stress conditions.
So on decreasing nitrate content, the antioxidant activity remain at a level comparable or even higher than the control due to the synergistic effect of the phycobilin pigment and the phenolic compounds produced in excess under stress nitrate condition which have high redox potentials[30].
On nitrogen starvation the recorded antioxidant activity (Comparable to those in presence of high nitrate content (6-9 g/L) was largely due to the high production of the carbon skeleton compounds (phenolic compounds) which show potent antioxidant activity
Our results were confirmed by those reported by Glazer[31], who mentioned that, the cultured cyanobacteria in presence of abundant nutrients, the phycobiliprotein pigments can make up as much as 40% of the protein of the cell. The author added that these proteins have numerous surface functions (at groups NH2, p-or COO-) per molecule which can be readily coupled to a variety of small molecules or proteins. The potent antioxidant activity of polar algal extracts was reported by Shalaby[5], who found that the aqueous and ethanol extracts of brown algae especially Sargassum species showed high antioxidant activity and could act as electron or hydrogen donors for DPPH radicals.
The obtained results in this investigation go parallel with the results of Abd El-Baky et al[32], who studied Chlorella ellipsoid and Spirulina maxima, recorded that under nitrogen limitation and starvation conditions, a decrease in algal proteins and an increase in secondary metabolites were noticed with an accumulation of antioxidant molecules for bimolecular protection against the formed ROS under stress conditions.
Shalaby et al[2] reported that Spirulina platensis under salt stress conditions; the algal polar extracts showed the highest antioxidant activity include phycobilin pigments, sulfated polysaccharides and phenolic compounds.
Water extracts of the tested promising algal species demonstrated higher anticancer efficiencies against both EACC and HepG2 cell lines (87.25 and 89.4% respectively in case of N. muscorum and 67.40 and 77.8% in case of Oscillatoria sp.). Under stress nitrogen conditions, these two cyanobacteria species recorded higher anticancer activities on exess limitation or starvation of nitrate comparing with its normal content in growth media
The recorded maximum activity in both species against both cell lines at the highest nitrate content (9 g/L) may be attributed mainly to the higher content of the phycobiliprotein pigments produced under excess nitrate contents.
Nitrate limitation and starvation, in spite of the caused decrease in phycobilin pigment production due to metabolic alteration expected under stress conditions, the carbon skeleton compounds as phenolic may replace phycobilin shortage in inducing similar anticancer activity of or even higher efficiency caused by great phycobilin contents at higher nitrate supplementation.
These results demonstrated that the compounds responsible for anticarcinogenic activity was highly polar as the phycobilins, phenolic compounds and polysaccharides which induced apoptosis of the cancer cells as reported by Aboul-Enein et al[33], which go parallel with our results
Our results coincides with the results obtained by Wang et al[34] who reported that the aqueous extract of red algae mainly contain c-phycocyanin, exhibited higher antipraleferation inducing apoptosis body formation. The authors explained that phycocyanin interact with membrane associated B-tubulin and glyceraldehydes-3-phosphate dehydrogenase (GAPDH), caused polymerization of microtubules and actins filaments leading to arrested the cell cycle at G0/G1 phase
Shalaby[35] reported that the water extract of the red alga Asparagopsis taxiformis (red alga) showed high anticancer activity against EACC (82.4%) which may be attributed to the algal content of phycobiliprotein pigments, sulfated polysaccharides and phenolic contents, which were comparable to the results obtained by the promising cyanobacteria species in this work.
Also, recently Zandi et al[36] and Aboul-Enein et al[37]reported that the water extract of Gracilaria corticata (red alga) and Egyptian wild plant was effective against Jurkat, molt-4, EACC and Hep-G2 cell lines, respectively, which may be attributed to the same previously mentioned compounds.
The aqueous extract of the tested algal species (8 cyanobacteria and one green alga) have variable color ranging from green, violet, blue, light blue and pink color, which can be used as an additive coloring agents to different food products (natural, non toxic) instead of the synthetic coloring substances which may be carcinogenic.
As these aqueous extracts exhibited antioxidant and anticancer activities, its effect as coloring agent is amplified by these biological efficiencies which are very important for human health. Also, it can be used for the manufacture of pharmaceutical drugs (antioxidant and anticancer).
Footnotes
Foundation Project: Supported by a grant from STDF, Cairo, Egypt (Project No. 312).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Sanab SMM, Shalaby EA, El-Fayoumy EA. Enteromorpha compressa exhibits potent antioxidant activity. J Biomed Biotechnol. 2011;726405:1–11. doi: 10.1155/2011/726405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shalaby EA, Shanab SMM, Singh V. Salt stress enhancement of antioxidant and antiviral efficiency of Spirulina platensis. J Med Plants Res. 2010;4(24):2622–2632. [Google Scholar]
- 3.Shanab SMM, Ameer MA, Fekry AM, Ghoneim AA, Shalaby EA. Corrosion resistance of magnesium alloy (AZ31E) as orthopaedic biomaterials in sodium chloride containing antioxidantly active compounds from Eichhornia crassipes. Int J Electrochem Sci. 2011;6:3017–3035. [Google Scholar]
- 4.Zhang J, Zhan B, Yao X, Gao Y, Shong J. Evaluation of 28 marine algae from the Qingdao coast for antioxidative capacity, determination of antioxidant efficiency, total phenolic content of fractions and subfractions derived from Symphyocladia latiuscula (Rhodomelaceae) J Appl Phycol. 2007;19:97–108. [Google Scholar]
- 5.Shalaby EA. Algae as promising organisms for environment and health. Plant Signaling and Behavior. 2011;6(9):1338–1350. doi: 10.4161/psb.6.9.16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burja AM, Banaigs B, Abou-Mansour E, Burgess JG, Wright PC. Marine cyanobacteria - a prolific source of natural products. Tetrahedron. 2001;57:9347–9377. [Google Scholar]
- 7.Canini A, Leonardi D, Grilli Caiola M. Superoxide dismutase activity in the cyanobacterium Microcystis aeruginosa after surface bloom formation. New Phytol. 2001;152:107–116. doi: 10.1046/j.0028-646x.2001.00244.x. [DOI] [PubMed] [Google Scholar]
- 8.Aboul-Enein AM, Al-Abd AM, Shalaby EA, Abul-Ela F, Nasr-Allah AA. Eichhornia crassipes (Mart) solms: from Water parasite to potential medicinal remedy. Plant Signaling and Behavior. 2011;6(6):118. doi: 10.4161/psb.6.6.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha RP, Hader DP. Response of a rice field cyanobacterium Anabaena sp. to physiological stressors. Environ Exp Bot. 2001;36:147–155. [Google Scholar]
- 10.Rippka R. Isolation and purification of cyanobacteria. Methods Enzymol. 1988;167:3–27. doi: 10.1016/0076-6879(88)67004-2. [DOI] [PubMed] [Google Scholar]
- 11.Zarrouk C. Contribution á l′étude d'une cyanophycée. Influence de divers facteurs physiques et chimiques sur la croissance et la photosynthése de Spirulina maxima (Setch. Et Gardner) Geitler. Ph. D.Thesis. University of Paris, France. 1966.
- 12.Nichols HW, Bold HC. Trichosarcina polymorpha gen. et sp. Nov J Phycol. 1965;1:34–38. [Google Scholar]
- 13.Leduy A, Therien N. An improved method for optical density measurement of the semimicroscopic blue algal Spirulina maxima. Biotechnol Bioeng. 2008;19:1219–1224. [Google Scholar]
- 14.Vonshak A, Richmond A. Mass production of the blue green alga Spirulina. An overview. Biomass. 1988;15:233–247. [Google Scholar]
- 15.Vonshak A. Handbook of microalgal mass culture. Boca Raton: CRC Press; 1986. Laboratory techniques for the cultivation of microalgae. In: A Richmond. (ed) pp. 117–145. [Google Scholar]
- 16.Bryant DA. Phycoerythrin and phycocyanin properties and occurance in cyanobacteria. J Gen Microbiol. 1979;128:835–844. [Google Scholar]
- 17.Sabri NN, El-Masry S, Khafagy SM. Phytochemical investigation of Hyoscyamus desertoru. Planta Med. 1973;23(1):4–9. doi: 10.1055/s-0028-1099408. [DOI] [PubMed] [Google Scholar]
- 18.Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. [Google Scholar]
- 19.Ebrahimzadeh H, Niknam V. A revised spectophotometric method for determination of triterpenoid saponins. Indian Drugs. 1998;32(6):379–381. [Google Scholar]
- 20.Burits M, Bucar F. Antioxidant activity of Nigella sativa esserntial oil. Phtother Res. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 22.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 23.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 24.Snedecor GW, Cochran WG. Statistical methods. Ames., Iowa: The Iowa State Univ. Press; 1982. p. 507. [Google Scholar]
- 25.Shalaby EAA. Chemical and biological studies on Spirulina species. 2004. pp. 115–122. MSc. Thesis, Department of Biochemistry, Faculty of Agriculture, Cairo University.
- 26.Basma AA, Zakaria Z, Latha LY, Sasidharan S. Antioxidant activity and phytochemical screening of the methanol extracts of Euphorbia hirta L. Asian Pac J Trop Med. 2011;4(9):386–390. doi: 10.1016/S1995-7645(11)60109-0. [DOI] [PubMed] [Google Scholar]
- 27.Patel DK, Kumar R, Laloo D, Hemalatha S. Evaluation of phytochemical and antioxidant activities of the different fractions of Hybanthus enneaspermus (Linn.) F. Muell. (Violaceae) Asian Pac J Trop Med. 2011;4(9):391–396. doi: 10.1016/S1995-7645(11)60110-7. [DOI] [PubMed] [Google Scholar]
- 28.Kannan RRR, Arumugam R, Anantharaman P. In vitro antioxidant activities of ethanol extract from Enhalus acoroides (L.F.) Royle. Asian Pac J Trop Med. 2010;3(11):898–901. [Google Scholar]
- 29.Kumar BSA, Lakshman K, Jayaveera KN, Shekar DS, Kumar AA, Manoj B. Antioxidant and antipyretic properties of methanolic extract of Amaranthus spinosus leaves. Asian Pac J Trop Med. 2010;3(9):702–706. [Google Scholar]
- 30.Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22(4):375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 31.Glazer AN. Phycobiliproteins: a family of valuable, widely used fluorophores. J Appl Phycol. 1994;6:105–112. [Google Scholar]
- 32.Abd El-Baky HH, Hussein MM, El-Baroty GS. Algal extracts improve antioxidant defense abilities and salt tolerance of wheat plant irrigated with sea water. Afr J Biochem Res. 2008;2(7):151–164. [Google Scholar]
- 33.Aboul-Enein AM, Shalaby EA, Abul-Ela F, Nasr-Allah AA, Mahmoud AM, El-Shemy HA, et al. et al. Back to nature: Spotlight on cancer therapeutics. Vital Signs. 2011;10:8–9. [Google Scholar]
- 34.Wang H, Liu Y, Gao X, Carter CL, Liu ZR. The recombinant beta subunit of C-phycocyanin inhibits cell proliferation and induces apoptosis. Cancer Letters. 2007;247(1):150–158. doi: 10.1016/j.canlet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Shalaby EAA. Biochemical and Biotechnological studies on some marine algae. 2008. p. 206. Ph.D. Thesis, Department of Biochemistry, Faculty of Agriculture, Cairo University.
- 36.Zandi K, Tajbakhsh S, Nabipour I, Rastian Z, Yousefi F, Sharafian S, et al. et al. In vitro antitumor activity of Gracilaria corticata (a red alga) against Jurkat and molt-4 human cancer cell lines. Afr J Biotechnol. 2010;9(40):6787–6790. [Google Scholar]
- 37.Aboul-Enein AM, Shalaby EA, Abul-Ela F, El-Shemy HA. Traditional medicinal plants research in egypt: studies of antioxidant and anticancer activities. J Med Plant Res. 2012;6(5):689–703. [Google Scholar]


