Abstract
Objective
To evaluate anti-inflammatory potential of leaf extract of Skimmia anquetilia by in-vitro and in-vivo anti-inflammatory models.
Methods
Acute toxicity study was carried out to determine the toxicity level of different extract using acute toxic class method as described in Organization of Economic Co-operation and Development Guidelines No.423. Carrageenan (1% w/w) was administered and inflammation was induced in rat paw. The leaf extracts of Skimmia anquetilia were evaluated for anti-inflammatory activity by in-vitro human red blood cell (HRBC) membrane stabilization method and in-vivo carrangeenan-induced rat paw edema method.
Results
The in-vitro membrane stabilizing test showed petroleum ether (PE), chloroform (CE), ethyl acetate (EE), methanol (ME) and aqueous extracts (AE) showed 49.44%, 59.39%, 60.15%, 68.40% and 52.18 % protection, respectively as compared to control groups. The in-vivo results of CE, EE and ME showed 58.20%, 60.17% and 67.53% inhibition of inflammation after 6h administration of test drugs in albino rats. The potency of the leaf extracts of Skimmia anquetilia were compared with standard diclofenac (10 mg/kg) which showed 74.18% protection in in-vitro HRBC membrane stabilization test and 71.64% inhibition in in-vivo carrangeenan-induced rat paw edema model. The ME showed a dose dependent significant (P< 0.01) anti-inflammatory activity in human red blood cell membrane stabilization test and reduction of edema in carrageenan induced rat paw edema.
Conclusions
The present investigation has confirmed the anti-inflammatory activity of Skimmia anquetilia due to presence of bioactive phytoconstitutes for the first time and provide the pharmacological evidence in favor of traditional claim of Skimmia anquetilia as an anti- inflammatory agent.
Keywords: Skimmia anquetilia, Anti-inflammatory, Carrageenan, Diclofenac, Leaf extract, Phytochemical analysis
1. Introduction
Skimmia anquetilia (S. anquetilia) is an aromatic gregarious shrub belonging to family Rutaceae. It is mostly found in Western part of Himalayas and Kashmir in India. Traditionally, the leaf infusion of S. anquetilia is taken for treatment of headache, freshness and general fever[1].The leaves of S. anquetilia are aromatic and known to contain linalool, geraniol, pinene, scopoletin, skimmianine, umbelliferone[2]. Inflammation is reaction of to infection, irritation or foreign substance. It is a part of the host defense mechanisms. It is known to be involved in the inflammatory reactions such as release of histamine, bradykinin, prostaglandins[3], fluid extravasations, cell migration, tissue breakdown and repair which are aimed at host defense and usually activated in most disease condition. The critical role of inappropriate inflammation is becoming accepted in many diseases that affect man, including cardiovascular diseases, inflammatory and autoimmune disorders, neurodegenerative conditions, infection and cancer[4],[5]. Edema formation, leukocyte infiltration and granuloma formation are main manifestiations of inflammation[6]. Oedema formation in the paw is the result of a synergism between various inflammatory mediators that increase vascular permeability or the mediators that increase blood flow[7]. Several experimental models of paw oedema have been described. Carrageenan-induced paw oedema is widely used for determining the biphasic phase of inflammation. Histamine, 5-hydroxytryptamine and bradykinin are the first detectable mediators in the early phase of carrageenan-induced inflammation[8], whereas prostaglandins are detectable in the late phase of inflammation[9]. The present research aims to investigate in-vitro and in-vivo anti-inflammatory activities of extracts of leaf of S. anquetilia.
2. Material and methods
2.1. Plant material
S. anquetilia was collected from Gulmarg area of Kashmir (J&K, India). Plant materials were properly identified by Dr.A.R.Naqshi, Taxonomist, Department of Pharmaceutical Sciences, University of Kashmir, Srinagar. The successive leaf extracts of S. anquetilia (LESA) were prepared from air dried leaves. The successive extracts were prepared in petroleum ether (PE), chloroform (CE), ethyl acetate (EE), methanol (ME) and distilled water extract (AE).
2.2. Animal used
Albino rats (Wistar strain) of either sex (180-200 g) were obtained from the animal house of Indian Institute of Integrative Medicine, Jammu. Animals were kept under the laboratory conditions [(25±2)°C, 12 h light]. They were provided with standard rodent diet (Aashirvad Industries, Chandigarh). Food was withdrawn 12 h before the experiment and water provided ad libitum. After 7 days, animal were randomly selected for different experimental groups (6 animal/group) and used for the in vivo determination of anti-inflammatory activity.
2.3. In-vitro anti-inflammatory activity
It was tested by human red blood cell (HRBC) membrane stabilization method[4]. The blood was collected from healthy human volunteer who had not taken any NSAIDS for 2 weeks prior to the experiment and mixed with equal volume of Alsever solution(2% dextrose, 0.8% sodium citrate, 0.5% citric acid and 0.42% NaCl) and centrifuged at 3 000 rpm. The packed cells were washed with isosaline and a 10% suspension was made. PE, CE, EE, ME and AE of S. anquetilia were prepared (200 and 400 µg/mL), respectively using distilled water and to each concentration 1 m of phosphate buffer, 2 m hyposaline and 0.5 m of HRBC suspension were added. It was incubated at 370C for 30 min and centrifuged at 3 000 rpm for 20 min. The hemoglobin content of the supernatant solution was estimated spectrophotometrically at 560 nm. Diclofenac (50 and 100 µg/mL) was used as reference standard and a control was prepared by omitting the extracts[4]. The percentage of HRBC membrane stabilization or protection was calculated by using the following formula:
2.4. In-vivo anti-inflammatory activity
Acute toxicity study was carried out for different extract using acute toxic class method as described in Organization of Economic Co-operation and Development Guidelines No.423[10]. The LESA was safe up to a dose of 4 000 mg/kg/b.w. The extract of 200 mg/kg and 400 mg/kg was used as moderate dose for the evaluation.
The anti-Inflammatory activity of LESA was evaluated using the carrangeenan-induced rat paw edema. The rats were divided into five groups (n = 6). Acute inflammation was produced by sub plantar administration of 0.1 mL of 1% w/w carrageenan in normal saline to the right paw of each rat. Group I were treated with 0.5% CMC (10 mL/kg/p.o.), Group II, III, IV with CE, EE, ME of S. anquetilia at the dose of 200 and 400 mg/kg/p.o. and Group V with diclofenac 100 µg/mL as standard 1 h before the injection of carrageenan. The volume of the paw was measured at 0 h, 1 h, 3 h and 6 h after the injection of carrageenan. Edema was expressed as the increment in paw thickness due to carrageenan administration[11]. Percent inhibition of edema volume between treated and control group was calculated as follows:
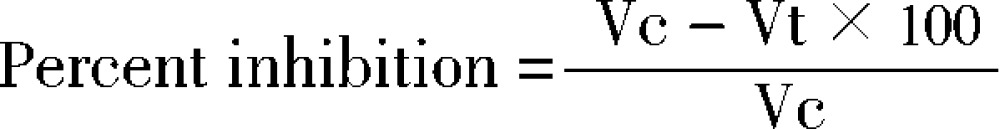 |
Where, Vc and Vt represented mean increase in paw volume in control and treated groups, respectively.
2.5. Phytochemical analysis
The phytochemical screening of PE, CE, EE, ME, and AE of S. anquetilia were performed as per described methods[12].
2.6. Statistical analysis
The values are expressed as mean±SEM. Statistical analysis was performed using ANOVA (one way) followed by Student's t-test. P < 0.05 was considered to be significant or we can say that the values are significantly different from the control or saline group at P < 0.05.
3. Results
3.1. Effect of LESA on HRBC membrane stabilization
The result of the human red blood cell membrane stabilization test were shown in Table 1. The PE, CE, EE, ME and AE of LESA showed a concentration dependent anti inflammatory activity, and the protection percent increased with increase in the concentration of the drug. Among all the extracts, ME (200 mg and 400 mg) showed statistically significant increase (P<0.01) (61.59% and 68.40%, respectively). All the results were compared with control and diclofenac (standard) which showed 74.18% protection (Table 1).
Table 1. Anti inflammatory activity of leaf extracts of S. anquetilia on HRBC membrane stabilization.
| Groups | Concentration (mg/kg) | Activity (%Protection) |
| Control | - | - |
| PE | 200 | 40.18 ± 0.61 |
| 400 | 49.44 ± 1.52 | |
| CE | 200 | 54.31 ± 1.27 |
| 400 | 59.39 ± 2.85b | |
| EE | 200 | 58.08 ± 1.36b |
| 400 | 60.15 ± 1.48b | |
| ME | 200 | 61.59 ± 1.20b |
| 400 | 68.40 ± 1.57a | |
| AE | 200 | 49.58 ± 1.81 |
| 400 | 52.18 ± 1.33 | |
| Diclofenac | 10 | 74.18 ± 1.86a |
Symbols represent statistical significance: a P< 0.01, b P< 0.05.
3.2. Effects of LESA on carrageenan-induced paw edema in rats
Treatment with 400 mg/kg of CE, EA, ME and Diclofenac showed 58.22%, 60.17%, 67.53% and 71.64% inhibition in albino rats. Among all the extracts, methanol was found to be the most potent (67.53%, P<0.01) when compared with control group after 5 hours (Table 2). The data demonstrate that ME has higher anti-inflammatory activity than CE and EE extract in vivo.
Table 2. Anti-inflammatory activity of leaf extracts of S. anquetilia on carrageenan induced paw edema in rats.
| Group | Treatment (mg/kg) | Paw volume (mL) at hours |
% Inhibition after at time (hours) |
|||
| 1 h | 6 h | 1 h | 3 h | 6 h | ||
| Control | 0.5% CMC | 0.22 ± 0.04 | 0.46 ± 0.02 | - | - | - |
| CE | 200 | 0.13 ±0.01 | 0.22 ± 0.01 | 38.71 | 44.62 | 52.16 |
| 400 | 0.12 ± 0.01 | 0.19 ± 0.02 | 42.85 | 51.10 | 58.22b | |
| EE | 200 | 0.13 ±0.01 | 0.20 ± 0.02 | 41.47 | 50.02 | 56.27 |
| 400 | 0.12 ± 0.01 | 0.18 ± 0.02 | 45.16 | 50.72 | 60.17b | |
| ME | 200 | 0.12 ± 0.02 | 0.17 ± 0.02 | 42.85 | 52.65 | 63.85b |
| 400 | 0.11 ± 0.01 | 0.15 ± 0.01 | 47.92 | 56.46 | 67.53a | |
| Diclofenac | 10 | 0.10 ± 0.02 | 0.13 ± 0.02 | 52.99 | 68.18 | 71.64a |
Symbols represent statistical significance: aP < 0.01, bP< 0.05.
3.3. Phytochemical analysis
The phytochemical screening showed that leaves extracts of S. anquetilia had different secondary metabolites such as alkaloids, glycosides, tannins, flavonoids, saponins and carbohydrates( Table 3).
Table 3. Preliminary phytochemicals screening of leaves extracts of S. anquetilia.
| Test | PE | CE | EAE | ME | AE |
| Carbohydrates | + | + | + | + | + |
| Alkaloids | - | + | + | + | + |
| Saponins | - | - | + | + | + |
| Steroids | + | - | - | + | + |
| Proteins | - | - | - | + | + |
| Starch | - | - | - | - | + |
| Tannins | - | - | + | + | + |
| Flavonoids | - | - | + | + | + |
+: Presence; -: Absence (Based on color reaction).
4. Discussion
The incredible development in the field of synthetic drugs during present era is accompanied by numerous undesirable side effects. Whereas plants still hold their own unique place, with lesser side effects. Therefore, a systematic approach should be made to find out the efficacy of plants against inflammation as herbal anti-inflammatory agents. The enzyme, phospholipase A2, is known to be responsible for the formation of mediators of inflammation such as prostaglandins and leukotrienes. By attracting polymorphonuclear leucocytes to the site of inflammation, they can lead to tissue damage probably by the release of free radicals. Phospholipase A2 converts phospholipids in the cell membrane into arachidonic acid, which is highly reactive and is rapidly metabolized by cyclooxygenase (prostaglandin synthesis) to prostaglandins. Prostaglandins are major components that induce pain and inflammation[13],[14]. It is well known that carrageenan induced paw edema is characterized by biphasic event with involvement of different inflammatory mediators. In the first phase (during the first 2 h after carrageenan injection), chemical mediators such as histamine and serotonin play role, while in second phase (3-4 h after carrageenan injection), Kinin and prostaglandins are involved[15]–[18]. Our results revealed that administration of methanolic extract inhibited the oedema starting from the first hour and during all phases of inflammation, which is probably due to inhibition of different aspects and chemical mediators of inflammation.
The successive leaf extracts of S. anquetilia exhibited membrane stabilization effect by inhibiting hypotonicity induced lysis of erythrocyte membrane. The erythrocyte membrane is analogous to the lysosomal membrane and its stabilization implies that the extract may as well stabilize lysosomal membranes[4]. Stabilization of lysosomal membrane is important in limiting the inflammatory response by preventing the release of lysosomal constituents of activated neutrophil such as bactericidal enzymes and proteases, which cause further tissue inflammation and damage upon extra cellular release[19]. Though the exact mechanism of the membrane stabilization by the extract is not known yet, hypotonicity-induced hemolysis may arise from shrinkage of the cells due to osmotic loss of intracellular electrolyte and fluid components. The extract may inhibit the processes, which may stimulate or enhance the efflux of these intracellular components[15],[20]. The methanol extract (400 mg/kg) has shown significant anti-inflammatory activity (68.40%). On the basis of in-vitro evaluated results, in vivo evaluation was also estimated and methanol extract (400 mg/kg) showed significant anti-inflammatory activity (67.53%) as compared to control, whereas diclofenac showed 71.64% inhibition percentage in carrageenan induced paw edema. The ability of the extract to inhibit carrageenan induced paw edema is suggestive of its anti-inflammatory potential. Anti-inflammatory effects have been observed in flavonoids as well as tannins. Flavonoids such as quercetin are known to be effective in reducing acute inflammation. Certain flavonoids possess potent inhibitory activity against a variety of enzymes such as protein kinase C, protein tyrosine kinases, phospholipase A2, phosphodiesterases and others. The anti-inflammatory effect of the extract may be due to the presence in the extract of flavonoids, tannins etc. either singly or in combination[21]. Both in-vitro and in-vivo models results showed that the leaf extracts of S. anquetilia possess potential anti-inflammatory activity.
It is concluded that ME of leaves of S. anquetilia possess significant anti-inflammatory activity in HRBC membrane stabilization test and carrageenan induced paw edema in rats. This may be due to the presence of active phytoconstituents i.e. flavonoids, and due to their effect on the prostaglandins pathway. Further research, to isolate anti-inflammatory principle/principles & exact mechanism involved, is currently in progress.
Acknowledgments
We would like to acknowledge that this research was financial supported by the University of Kashmir, Jammu & Kashmir, India and authors also thankful to Prof. (Dr.) M.Y. Shah, Head, Department of Pharmaceutical Sciences, by providing the necessary lab facility for this work.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Bhattarai NK. Medical ethno botany in Karnali zone, Nepal. Economic Botany. 1992;46(3):257–261. [Google Scholar]
- 2.Kunwar RM, Shrestha KP, Bussmann RW. Traditional herbal medicine in Far-west Nepal: a pharmacological appraisal. J Ethnobiol & Ethnomed. 2010;6:35. doi: 10.1186/1746-4269-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dandiya PC, Kulkarni SK. 5th edition. Delhi: Vallabh Prakashan; 1995. Introduction to pharmacology-including toxicology & practicals; pp. p.131–132. [Google Scholar]
- 4.Kumar V, Bhat ZA, Kumar D, Bohra P, Sheela S. In-vitro anti-inflammatory activity of leaf extracts of Basella alba linn. Var. alba. Int J Drug Dev & Res. 2011;3(2):124–127. [Google Scholar]
- 5.Azeem AK, Dilip C, Prasanth SS, Junise V, Hanan Shahima. Anti-inflammatory activity of the glandular extracts of Thunnus alalunga. Asia Pac J Med. 2010;3(10):412–20. [Google Scholar]
- 6.Mitchell RN, Cotran RS. Robinsons basic pathology. 7th edition. New Delhi, India: Harcourt Pvt. Ltd; 2000. pp. 33–42. [Google Scholar]
- 7.Tian YQ, Kou JP, Li LZ, Yu BY. Anti-inflammatory effects of aqueous extract from radix Liriope muscari and its major active fraction and component. Chin J Nat Med. 2011;9(3):222–226. [Google Scholar]
- 8.Ramachandran S, Rajinikanth B, Rajasekaran A, Manisenthil KKT. Evaluations of anti inflammatory and analgesic potential of methanol extract of Tectona grandis flowers. Asian Pac J Trop Biomed. 2011:S155–S158. doi: 10.1016/S1995-7645(11)60160-0. [DOI] [PubMed] [Google Scholar]
- 9.Sakat S, Juvekar AR, Gambhire MN. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharma & Pharmacol Sci. 2010;2(1):146–155. [Google Scholar]
- 10.Ecobichon DJ. The basics of toxicology testing. New York: CRC Press; 1997. pp. 43–60. [Google Scholar]
- 11.Patra P, Jha S, Murthy PN, Vaibhav DA, Chattopadhyay P, Panigrahi G, et al. et al. Anti- inflammatory and antipyretic activities of Hygrophila spinosa T. Anders leaves (Acanthaceae) Trop J Pharma Res. 2009;8:133–137. [Google Scholar]
- 12.Kokate CK. Practical pharmacognosy. New Delhi: Vallabh Prakashan; 1994. pp. 105–107. [Google Scholar]
- 13.Vane JR, Botting RM. New insight into the mode of action of anti-inflammatory drugs. Inflamm Res. 1995;44:1–10. doi: 10.1007/BF01630479. [DOI] [PubMed] [Google Scholar]
- 14.Smith GR, Sotiris M. Cancer, inflammation and the AT1 and AT2 receptors. J Inflamm. 2004;1:3. doi: 10.1186/1476-9255-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang GM, Wang D, Tang W, Chen X, Fan LQ, Zhang FF, et al. et al. Anti-inflammatory and antioxidant activities of Oxytropis falcata fractions and its possible anti-inflammatory mechanism. Chin J Nat Med. 2010;8(4):285–292. [Google Scholar]
- 16.Emamuzo ED, Miniakiri SI, Tedwin EJO, Ufouma O, Lucky M. Analgesic and anti-inflammatory activities of the ethanol extract of the leaves of Helianthus Annus in Wistar rats. Asian Pac J Trop Med. 2010;3(5):341–347. [Google Scholar]
- 17.Shenoy S, Shwetha K, Prabhu K, Maradi R, Bairy KL, Shanbhag T. Evaluation of antiinflammatory activity of Tephrosia purpurea in rats. Asian Pac J Trop Med. 2010;3(3):193–195. [Google Scholar]
- 18.Georgewill OA, Georgewill UO, Nwankwoala RNP. Anti-inflammatory effects of Morninga oleifera lam extract in rats. Asian Pac J Trop Med. 2010;3(2):133–135. [Google Scholar]
- 19.Yurugasan N, Vember S, Damodharan C. Studies on erythrocyte membrane IV: In vitro haemolytic activity of Oleander extract. Toxicol Lett. 1981;8:33–38. doi: 10.1016/0378-4274(81)90134-x. [DOI] [PubMed] [Google Scholar]
- 20.Vadivu R, Lakshmi KS. In vitro and in vivo anti inflammatory activity of leaves of Symplocos cochinchinensis (Lour) Moore ssp Laurina. Bangladesh J Pharmacol. 2008;3:121–124. [Google Scholar]
- 21.Sudharshan SJ, Prashith KTR, Sujatha ML. Anti-inflammatory activity of Curcuma aromatica Salisb and Coscinium fenestratum Colebr: A comparative study. J Pharm Res. 2010;3(1):24–25. [Google Scholar]


