Abstract
Objective
To analyze the chemical composition and to evaluate the bioactive potential of hydroalocoholic extract of propolis.
Methods
Ethanol extract of propolis was analyzed by GC-MS, HPTLC and HPLC methods and in vitro antioxidant, anticholinesterase and cytotoxicity assay were performed.
Results
GC-MS analysis revealed the presence of fatty acids, alcohols, and quercetin. Quercetin was identified and quantified by HPTLC and HPLC methods. Dose dependent DPPH and hydroxyl radical scavenging activity of hydroalcoholic extract of propolis was calculated as 16.20 and 34.33 µg/mL respectively. Inhibition of lipid peroxidation was significant and the IC50 value was calculated as 55.56µg/mL. Anticholinesterase activity was less observed. The cytotoxic activity against both breast (MCF-7) and lung cancer (A543) cell lines were significant and the IC50 value was calculated as 10 and 13 µg/mL respectively.
Conclusions
These findings showed that bioactive compounds present in propolis will alleviate many diseases and can be used for better human health.
Keywords: Propolis, Densitometry, Quercetin, Antioxidant, Anticholinesterase, Cytotoxicity
1. Introduction
Propolis is a resinous, strongly adhesive natural substance, collected by honeybees from buds and leaves of trees and plants, mixed with products of their salivary glands and wax. Its color varies from green, red to dark brown. Propolis has a characteristic smell and shows adhesive properties, because it strongly interacts with oils and proteins of the skin[1]. Chemical composition of propolis is very complex.
More than 200 compounds have been identified. Its biological activity depends on compounds from the polyphenolic fractions, mainly flavonoids, followed by aromatic acids, phenolic acid esters, triterpenes, lignans etc[2]. Flavonoids isolated from propolis reported to have bactericidal and antiprotozoal[3], antiviral[4], antioxidant[5] anti-inflammatory[6] and immunomodulatory[7] activities. Based on the various chemical composition and immense medicinal and therapeutic values, the present study on propolis was aimed to analyze the chemical composition, antioxidant, anticholinesterase and cytotoxic activities of the hydroalcoholic extract.
2. Materials and methods
2.1. Reagents and chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), tris-HCL, trichloroacetic acid (TCA), phosphate buffer, ferrozine, 1,10-phenanthroline, ferrous sulphate, thiobarbituric acid (TBA), sodium dodecyl sulphate (SDS), trypan blue solution (0.4%), RPMI 1640 medium with sodium bicarbonate without L-glutamine and phenol red, ethidium bromide, minimum essential medium (MEM), 3-(4,5-dimethylthiazolyl)-2,5- diphenyl-tetrazolium bromide (MTT), fetal calf serum (FCS) and sodium hydroxide were purchased from Hi Media Laboratories Pvt. Ltd, Mumbai, India. H2O2 was purchased from S.D. Fine Chemicals Limited, Mumbai, India. Acetylcholinesterase (AChE) type VI-S, from electric eel 349Umg/mL solid, 411U/mg protein, 5,50-dithiobis[2-nitrobenzoic acid] (DTNB) and acetylthiocholine iodide (AChI) were purchased from Sigma- Aldrich, Inc., 3050 Spruce Street, St Louis, Mo, USA.
2.2. Ethanol extract of propolis
Propolis sample was collected from Coimbatore region, Tamil nadu, India. Propolis was collected with propolis traps to minimize their contamination with foreign substances. Propolis samples were freezed at -18 °C and extracts were prepared by mixing 20 g crude propolis with 70% ethanol, with intermittent shaking and extraction was carried out at room temperature in the dark. To remove waxes and less soluble substances, the suspensions were subsequently freezed at -20 °C for 24 h, and then filtered with Whatman No. 1 filter paper. The filtrates were evaporated to near dryness on a rotary evaporator under reduced pressure at 40 °C and then freeze-dried. The resulting powder was used for further studies.
2.3. GC-MS analysis
The propolis sample was analyzed in GC- MS- Fisons instrument GC 8000 series MD 800 equipped with a DB-5MS column, 30 mm × 0.25 mm, 0.25µm film thickness. GC conditions: splitless injection mode (40s), injector temperature 300°C, temperature program: initial temperature 80 °C (1 min hold) and up to 300 °C (6 °C/min) with 15 min hold. Column interface T 280 °C and ionization source T 250 °C. Ionization voltage 70 eV.
2.4. High Performance Thin Layer Chromatography (HPTLC)
One hundred milligram of the extract was dissolved in 70% ethanol and made up to 1 mL. This solution was centrifuged and supernatant was collected. This solution was used as test solution for HPTLC analysis. 3 µL of test solution and 5 µL of standard solution (quercetin) was loaded as 6mm band length in the 4 × 10 Silica gel 60 F254 TLC plate using Hamilton syringe and CAMAG LINOMAT 5 instrument. The samples loaded plate was kept in TLC twin trough developing chamber with respective mobile phase (Phenolics) and the plate was developed in the respective mobile phase up to 90 mm. The developed plate was dried by hot air to evaporate solvents from the plate. The plate was kept in photo documentation chamber (CAMAG REPROSTAR 3) and captured the image at UV254 nm. The developed plate was sprayed with respective spray reagent (Phenolics) and dried at 120 °C in hot air oven. The plate was photo documented in visible light mode using photo documentation chamber. Mobile phase were toluene-acetone-formic acid (4.5: 4.5: 1) and the spray reagent was 5% ferric chloride reagent and dried at 120 °C for 10 min.
2.5. High Performance Liquid Chromatograph (HPLC)
A HPLC unit equipped with Photo Diode Array detector (PDA) and with LC solution software was operated under the following suggestive parameters. Column: C18 Phenomenex (250×4.6) mm SS, 5 micron particle size. Flow rate: 0.8mL/min. Mobile phase used was methanol: acetonitrile: water (40:15:45v/v/v), Detection: PDA detector 368nm, Micro syringe: 20 µL capacity. Quercetin was used as standard. Weighed accurately 3 mg of the quercetin standard into a 10 mL standard flask and dissolved with methanol up to the mark (0.3 µg/mL). 20 µL of the above standard solution was injected in HPLC (6 µg/20µL). 20 mg of the propolis sample was accurately weighed, dissolved the content using 70% ethanol and made up to 1 mL (394 µg/20µL). 20µL of the standard quercetin and sample solution was injected respectively to get area reproducibility for two consecutive injections. The area of two consecutive injections should not vary more than 2 percent. From the HPLC chromatogram the percentage of quercetin in the sample was calculated as follows;
Where,
A1 = peak area of quercetin in reference standard solution
A2 = peak area of quercetin in sample solution
M1 = mass, in µg of the reference standard quercetin
M2 = mass, in µg, of the sample and
P = Purity of reference standard
2.6. Antioxidant studies
2.6.1. DPPH radical scavenging activity
Scavenging activity on DPPH free radicals by the test compound was assessed according to Blois[8]. Different concentrations (15, 30, 45, 60, and 75 µg/mL) of the propolis extract was dissolved in DMSO and mixed individually with 1 mL of 0.1 mM DPPH in ethanol solution and 450µL of 50 mM Tris-HCl buffer (pH 7.4) was added. The solution was incubated at 37 °C for 30 min and reduction of DPPH free radicals was measured by reading the absorbance at 517 nm (Shimadzu-1601). This activity is given as % DPPH scavenging and calculated according to the following equation:
% Inhibition = [(AB - AA)/AB] × 100
where AB, absorption of blank sample; AA, absorption of test sample.
2.6.2. Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity of the test compound was measured by the method of Zhao[9]. Reaction mixture contained 0.5 mL of 7.5 mM FeSO4, 0.5 mL of 7.5 mM 1, 10-phenanthroline, 2.5 mL of 0.2 M phosphate buffer (pH 7.8), 0.5 mL of 30 mM H2O2 and test compound at different concentrations (50, 100, 150, 200 and 250 µg/mL). The reaction was initiated by adding H2O2. After incubation at room temperature for 5 min, the absorbance of the mixture at 536 nm was measured.
% Inhibition = [(AB - AA)/AB] × 100
where AB, absorption of blank sample; AA, absorption of test sample.
2.6.3. Inhibition of lipid peroxidation
A modified thiobarbituric acid reactive species (TBARS) assay[10] was used to measure the lipid peroxide formed using egg-yolk homogenates as lipid-rich media[11]. Egg homogenate (0.5 mL, 10% in distilled water, v/v) and different concentrations (50, 100, 150, 200 and 250µg/mL) of the propolis extract was mixed in a test tube and the volume was made up to 1 mL, by adding distilled water. Finally, 0.05 mL FeSO4 (0.07 M) was added to the above mixture and incubated for 30 min, to induce lipid peroxidation. Thereafter, 1.5 mL of 20% acetic acid (pH adjusted to 3.5 with NaOH) 1.5 mL of 0.8% TBA (w/v) (prepared in 1.1% sodium dodecyl sulphate) and 0.05 mL 20% TCA were added, vortexed and then heated in a boiling water bath for 60 min. After cooling, 5.0 mL of 1-butanol was added to each tube and centrifuged at 3000 rpm for 10 min. The absorbance of the organic upper layer was measured at 532 nm using spectrophotometer (Shimadzu-1601).
% Inhibition = [(AB - AA)/AB] × 100,
where AB, absorption of blank sample, AA, absorption of test sample.
2.7. Antiacetylcholinesterase activity
The enzymatic activity was measured using an adaptation of the method described by Ingkaninan[12]. Different concentrations of propolis extract like 300-500 µg/mL were used. 500 µL of DTNB 3mM, 100 µL of AChI 15 mM, 275 µL of tris-HCl buffer 50 mM, pH 8 and different concentration of the test compound were added to a 1mL cuvette. This cuvette served as blank. In the reaction cuvette, 25 µL of buffer was replaced by the same volume of an enzyme solution containing 0.28U/mL. The reaction was monitored for 5 min at 405 nm.
2.8. Cytotoxicity - MTT assay [13]
The breast and lung cancer cell lines were grown and maintained in a humidified incubator at 37 °C and in a 5% CO2 atmosphere. MEM supplemented with 10% FBS, 100 units/mL of penicillin and 100µg/mL of streptomycin were used for cell culture of both cell lines. Breast cancer (MCF-7) cells and Lung cancer (A543) were incubated in 96-well plates containing 100µL of the growth medium per well. Cells were permitted to adhere for 24 h and then treated with various concentrations (2-20 µg/mL) of propolis extract dissolved in medium for 48 h; 20µL of 5 mg/mL MTT in phosphate buffered saline (PBS) were added to each well and the plate was incubated at 37 °C for 4 h. After incubation, absorbance at 570 nm of the dissolved solutions was measured. The absorbance of control cells (treated with 0.1% DMSO) was considered as 100%.
2.9. Statistical analysis
The data obtained from in vitro experiments like antioxidant, anticholinesterase and cytotoxic activities were expressed as mean (n=5) and the IC50 values were calculated using SPSS (16.0).
3. Results
Chemical composition analysis of the hydroalcoholic extract of propolis collected from Coimbatore region, Tamil Nadu, India has been determined by GC-MS analysis. A total of ten compounds have been identified. Major compounds present in the propolis were fatty acids such as 9- octadecenoic acid (3.2%), decanoic acid (2.12%) 9,12 hexadecanoic acid (1.29%), octadecadienoic acid methyl ester (0.49%) and alcohols such as 1-tetradecanol (0.89%), octadecanol (0.69%), 1-dotricontanol (0.48%) and 2, 3 epoxy-5, 8-hectadecadien-1- ol (0.6%). In addition trace amount of quercetin and cyclopentadiene was detected. Further, HPTLC and HPLC analysis were performed to identify and quantify the amount of quercetin present in the propolis.
HPTLC spectrum revealed the presence of phenolics in hydroalcoholic extract of propolis. Further, derivatization process confirmed the presence of quercetin. The compound quercetin was identified based on the retention frequency, peak height and peak area. The retention frequency (Rf) was observed as 0.58, height and area were observed as 321.2 and 11089.0 respectively. The corresponding Rf value, height and area of the HPTLC spectrum of hydroalcoholic extract of propolis confirmed the presence of bioactive flavonoid quercetin when compared with standard. Quercetin was detected at 254 nm using UV light. The densitogram of hydroalcoholic extract of propolis produced same densitogram as displayed by standard quercetin at 254 nm (Figure 1). This densitogram display also confirmed the presence of quercetin in the hydroalcoholic extract of propolis (Figure 1).
Figure 1. HPTLC densitogram of quercetin (A) and ethanolic extract of propolis (B).
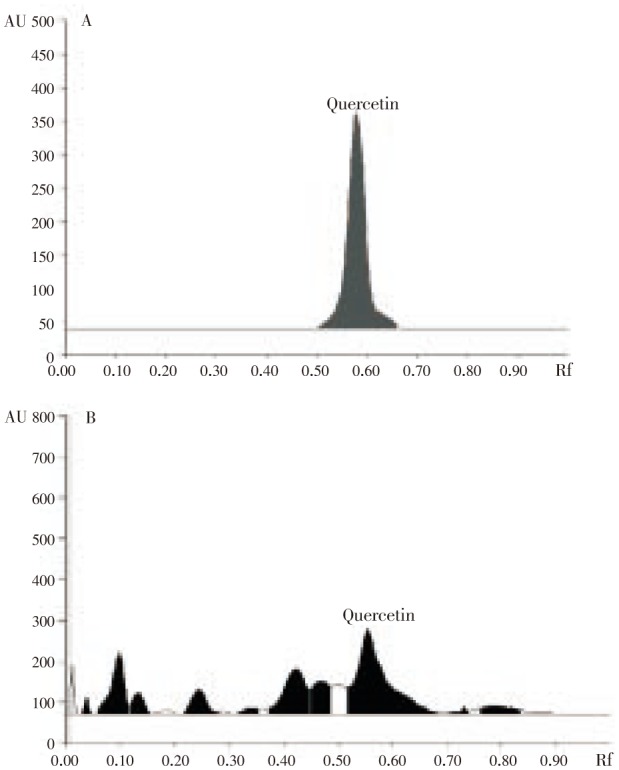
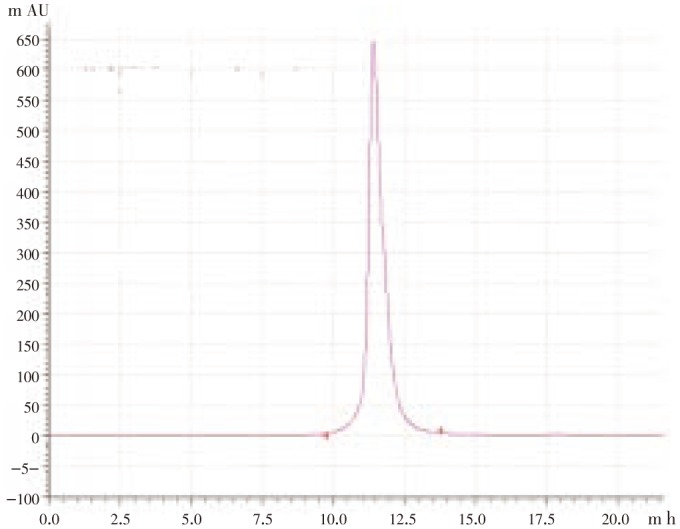
Based on the observations obtained from HPTLC, further the sample was taken into HPLC analysis for quantification of quercetin. For quantification, methanol: acetonitrile: water (40:15:45 v/v/v) were used as mobile system. The active ingredients separated and adhered to different areas of the separation column. Among the various compounds, quercetin has been observed and quantified based on the spectrum correlation with standard (quercetin) at 368 nm (Figure 2). The concentration of quercetin was calculated as 0.01%.
Figure 2. HPLC spectrum of quercetin in propolis.
DPPH free radical and hydroxyl radical scavenging activity of the hydroalcoholic extract of propolis was calculated as 16.20 and 34.33 µg/mL respectively. Hydroalcoholic extract of propolis inhibited lipid peroxidation induced by ferrous sulfate in egg-yolk homogenate. The inhibitory percentage was recorded in the range of 43.54 to 76.34. The concentration of extract needed to scavenge the free radicals by 50% was calculated as 55.56 µg/mL.
In the present study, hydroalcoholic extract of propolis showed inhibitory activity against acetylcholinesterase enzyme and the percentage of inhibition was ranges between 33.06 to 53.22. IC50 value was calculated as 43.46 µg/mL. The cytotoxic activity of hydroalcoholic extract of propolis was tested against lung (A549) and breast cancer (MCF-7) cell lines using MTT assay. The cytotoxicity of the test sample was significant against both cell lines tested. The activity of the test sample on cell death was time and concentration dependent. After 48 h treatment cell death rate reached to almost 50% and the IC50 value was calculated as 10 and 13 µg/mL respectively.
4. Discussion
In the present study, GC-MS analysis of the hydroalcoholic extract of propolis showed the presence of fatty acids and phenolic substances. The presence of fatty acids such as 1- octadecenoic acid, decanoic acid and hexadecanoic acid are compared to the fatty acids of propolis collected from Canada[14] and Iran[15]. Flavonoids are synthesized by plants as a response to environmental stress and microbial infections and are known to have antioxidant, anti inflammatory, antimicrobial properties[16]. The known flavonoid, quercetin identified in the hydroalcoholic extract of propolis in the present study are similar with the results of propolis collected from Greece and Cyprus[17]. The presence of quercetin in the hydroalcoholic extract of propolis also supported by the results of Tosi et al[18].
The data obtained from DPPH and hydroxyl radical scavenging activity of propolis may be attributed to the presence of electron donating groups. The similar result was reported by Wang et al[19]. The significant lipid peroxidation inhibitory of propolis may be either due to chelation of iron or by free radical trapping[20]. The results obtained are in agreement with the results of propolis collected from China[21]. Ethanolic extract of Brazilian propolis showed potent cytotoxicity and apoptosis inducing ability against prostate cancer cell lines also supported our results[22].
Acknowledgments
Authors are thankful to Kongunadu Arts and Science College Research and Development Council for financial support.
Footnotes
Foundation Project: Supported by the Kongunadu Arts and Science College Research and Development Council.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Marcucci MC. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. [Google Scholar]
- 2.Bankova V, de Castro SL, Marcucci MC. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31:3–15. [Google Scholar]
- 3.Pepeljnjak S, Jalsenjak I, Maysinger D. Growth inhibition of Bacillus subtilis and composition of various propolis extracts. Pharmazie. 1982;37:864–865. [PubMed] [Google Scholar]
- 4.Kujumgiev A, Tsvetkova I, Serkedjieva V, Bankova R, Christov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol. 1999;64:235–240. doi: 10.1016/s0378-8741(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 5.Russo A, Longo R, Vanella A. Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia. 2002;73:21–29. doi: 10.1016/s0367-326x(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 6.Borelli F, Maffia F, Pinto L, Ianaro A, Russo A, Capaso F, et al. et al. Phytochemical compounds involved in the anti-inflammatory effect of propolis extract. Fitoterapia. 2002;73:53–63. doi: 10.1016/s0367-326x(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 7.Orsolic N, Basic I. Immunomodulation by water-soluble derivative of propolis: a factor of antitumor reactivity. J Ethnopharmacol. 2003;84:265–273. doi: 10.1016/s0378-8741(02)00329-x. [DOI] [PubMed] [Google Scholar]
- 8.Blois MS. Antioxidant determination by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 9.Zhao GR, Xiang ZJ, Ye TX, Yuan YJ, Guo ZX. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng. Food Chem. 2006;99:767–774. [Google Scholar]
- 10.Ohkowa H, Ohisi N, Yagi K. Assay for lipid peroxides in animals tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 11.Ruberto G, Baratta MT, Deans SG, Dorman HJD. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000;66:687–693. doi: 10.1055/s-2000-9773. [DOI] [PubMed] [Google Scholar]
- 12.Ingkaninan K, Temkitthawon P, Chuenchon K, Yuyaem T, Thongnoi W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J Ethnopharmacol. 2003;89:261–264. doi: 10.1016/j.jep.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Lau CBS, Ho CY, Kim CF, Leung KN, Fung KP, Tse TF. Cytotoxic activities of Coriolus versicolor (Yunzhi) extract on human leukemia and lymphoma cells by induction of apoptosis. Life Sci. 2004;75:797–808. doi: 10.1016/j.lfs.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Christov R, Trusheva B, Popova M, Bankova V, Bertrand M. Chemical composition of propolis from Canada, its antiradical activity and plant origin. Nat Prod Res. 2006;20:531–536. doi: 10.1080/14786410500056918. [DOI] [PubMed] [Google Scholar]
- 15.Mohammadzadeh S, Shariatpa M, Hamedi M, Ahmad R, Samadi N, Nasser S. Chemical composition, oral toxicity and antimicrobial activity of Iranian propolis. Food Chem. 2007;103:1097–1103. [Google Scholar]
- 16.Bankova V. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol. 2005;100:114–117. doi: 10.1016/j.jep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Kalogeropoulos N, Konteles SJ, Troullidou E, Mourtzinos I. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009;116:452–461. [Google Scholar]
- 18.Tosi E, Re E, Ortega ME, Cazzoli AF. Food preservative based on propolis: Bacteriostatic activity of propolis polyphenols and flavonoids upon Escherichia coli. Food Chem. 2007;104:1025–1029. [Google Scholar]
- 19.Wang BJ, Lien YH, Yu ZR. Superficial fluid extractive fractionation - study of the antioxidative activity of propolis. Food Chem. 2004;86:237–243. [Google Scholar]
- 20.Pandey N, Chaurasia J, Tiwari OP, Tripathi YB. Antioxidant properties of different fractions of tubers from Pueraria tuberosa Linn. Food Chem. 2007;105:219–222. [Google Scholar]
- 21.Kumazawa S, Ryeon M, Usai Y, Nakamira T, Matsuka M, Zhu F, et al. et al. Antioxidan activity and constituents collected in various area of China. Food Chem. 2007;101:1383–1392. [Google Scholar]
- 22.Li H, Kapur A, Yang JX, Srivastava S, McLeod DG, Paredes-Guzman JF, et al. et al. Antiproliferation of human prostate cancer cells by ethanolic extracts of brazilian propolis and its biological origin. Int J Oncol. 2007;31:601–606. [PubMed] [Google Scholar]


