Abstract
Objective
To explore and identify the most potent antihyperglycemic fraction from the ethanol extract of Rhododendron arboreum (R. arboreum) flowers.
Methods
Normal and streptozotocin induced diabetic rats were treated with all four fractions of R. arboreum flowers for short term and with fraction 3 for long term study. On completion of the treatment, a range of indicators were tested including fasting blood glucose, plasma protein, haemoglobin A1C, insulin secretion, body weight, blood lipid profile and carbohydrate metabolism regulating enzymes of liver.
Results
In short term study, the fraction 3 (Active fraction) produced a significant (P<0.000 1) reduction (73.6%) in blood glucose level at a dose of 200 mg/kg after the treatment in the diabetic rats. Administration of active fraction (200 and 400 mg/kg) once daily for 30 d in streptozotocin diabetic rats resulted in a significant (P<0.001 to P<0.000 1) fall in blood glucose level, hemoglobin A1C, serum urea and creatinine with significant but a increase in insulin level similar to standard drug glybenclamide. Further, the active fraction showed antihyperlipidemic activity as evidenced by significant (P<0.001 to P<0.000 1) decreases in serum serum total cholesterol, triglycerides, low density lipoprotein cholesterol and very low density cholesterol levels coupled together with elevation of high density lipoprotein cholesterol in the diabetic rats.
Conclusions
The active fraction of R. arboreum flowers decreases streptozotocin induced hyperglycemia by promoting insulin secretion and glycolysis and by decreasing gluconeogenesis.
Keywords: Antihyperglycemic, Antihyperlipidemic, Ethanol, Fraction, Rhododendron arboreum, Streptozotocin
1. Introduction
Diabetes is a chronic disorder of carbohydrate, fat and protein metabolism characterized by elevation of both fasting and postprandial blood sugar levels. The peroxidation of cellular membrane lipids can lead to cell necrosis and is purportedly associated with various chronic disorders including carcinogenesis and hyperglycemia[1]. This pathological condition occurs as a result of the loss of insulin-producing pancreatic beta-cells by an environmentally triggered autoimmune reaction. Currently available oral antidiabetic agents have a number of serious adverse effects. Errors in the choice of drug can also occur; some sulphonylureas like chlorpropamide and glybenclamide are more commonly associated with hypoglycaemia[2]. Thus, the management of diabetes without any side effects is still a challenge. Rhododendron arboreum Sm. (R. arboreum) (family: Ericaceae) is commonly known as “laligurans”, a small evergreen tree. The flowers of the plant are used traditionally in the treatment of diabetes by rural Nepalese people[3]. Flowers are used in acute inflammation[4] and also showed antinociceptive and anti-inflmmatory activities[5]. The flowers were reported for hypolipidemic activity of the ayurvedic herbal formulation Arborium Plus (Hyppophae ramnoides and R. arboreum) in experimentally induced hypercholestermic rabbits[6] and hepatoprotective activity[7]. However, the 50% (v/v) methanolic extract of R. arboreum showed significant in-vitro antidiabetic activity[3] and no study is reported on the antihyperlipidemic effects of R. arboreum. Therefore, the present study was undertaken to investigate the effect of chronic (long term) treatment with different fractions of R. arboreum flowers extract on experimental animals.
2. Materials and methods
2.1. Collection and extraction of plant material
The flowers of R. arboreum were collected from Rudraprayag, Uttaranchal and authenticated with the existed specimen (NBRI/CIF/83/2009) at National Botanical Research Institute (NBRI), Lucknow, UP, India. The prepared herbarium was deposited in the laboratory of NBRI for future reference. The air dried powdered flowers (200 g) were extracted with 50% (v/v) aqueous ethanol using soxhlet apparatus, which was then evaporated under vacuum and afforded the ethanolic extract of flowers (56 g). With organic solvents, concentrated ethanol extract was fractionated by column chromatography on silica gel (100-200 mesh size) and afforded the n-hexane (fraction 1), chloroform (fraction 2), ethyl acetate (fraction 3) and n-butanol (fraction 4)[5]. All the fractions were screened for short term antihyperglycemic activity in diabetic rats.
2.2. Chemicals
Streptozotocin, glybenclamide and glucose-6-posphate were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Silica gel (100-200 mesh) for column chromatography was purchased from E-Merck (Darmstadt, Germany). Rat insulin ELISA kit was purchased from Crystal Chem Inc (Downers Grove, US). All reagents were of analytical grade.
2.3. Rats
Male albino Wistar rats (150-200 g) were kept in the departmental animal house of NBRI, Lucknow at (25±2) °C and relative humidity of 42%-54%, light and dark cycles of 10 and 14 h respectively for 1 week before and during the experiments. All the studies were performed in accordance with the guidelines for the care and use of laboratory animals, as adopted and promulgated by the Institutional Animal Care Committee, CPCSEA, India (Reg. No.222/2000/CPCSEA).
2.4. Acute toxicity assay
Acute oral toxicity for fractions 1, 2, 3 and 4 was carried out using OECD/OCED guidelines 425. The adult albino Wistar rats (200-250 g) were randomly selected and kept in their cages for at least 5 d prior to the start of dosing to allow for acclimatisation to the laboratory conditions. Animals were fasted prior to dosing (food but not water was withheld over-night).
2.4.1. Limit test at 2 000 mg/kg
The test fractions (1, 2, 3 and 4) were individually administered at the dose of 2 000 mg/kg body mass orally to one animal. This animal was survived and then four additional animals were dosed sequentially, so that a total of five animals were tested. Animals were observed individually at least once during the first 30 min after dosing, periodically during the first 24 h (with special attention during the first 4 h), and daily thereafter, for a total of 14 d[8].
2.5. Induction of diabetes
To the overnight fasted rats, streptozotocin (60 mg/kg body mass) dissolved in ice cold citrate buffer (0.1 M, pH 4.5) was administered intraperitoneally[9]. After a fortnight, rats with marked hyperglycemia (blood glucose level>16 mM/L) were selected and used for the study. All the animals were allowed free access to water and pellet diet and maintained at room temperature (25±2) °C in plastic cages.
2.6. Screening of different fractions of R. arboreum ethanol extract for antihyperglycemic activities
The rats were divided into seven groups and each group consisted of six rats. Group 1 (NC), normal control rats; Group 2 (DC), Diabetic control rats; Group 3 (DF1), Diabetic rats treated with 200 mg/kg of fraction 1; Group 4 (DF2), Diabetic rats treated with 200 mg/kg of fraction 2; Group 5 (DF3), Diabetic rats treated with 200 mg/kg of fraction 3; Group 6 (DF4), Diabetic rats treated with 200 mg/kg of fraction 4; Group 7 (DG), Diabetic rats treated with 20 mg/kg of glybenclamide.
After an overnight fasting, fractions 1, 2, 3 and 4 at a dose of 200 mg/kg were fed to the normal and diabetic animals in distilled water by using orogastric cannula. Normal control and diabetic control rats were fed with distilled water alone. Blood samples were collected by clipping the tail vein for the measurement of blood glucose, from 0 (before administration) to 6 h at an interval of 1 h after the treatment. Blood glucose was measured by glucose oxidase method[10], and the results were compared with normal control and diabetic control groups. The fraction with maximum antihyperglycemic activity was selected as the active fraction.
2.7. Effect of long term treatment with the active fraction in streptozotocin induced diabetic rats
The rats were divided into five groups and each group consisted of six rats. Group 1 (NC), normal control rats; Group 2 (DC), Diabetic control rats; Group 3 (DAF1), Diabetic rats treated with active fraction 200 mg/kg per day; Group 4 (DAF2), Diabetic rats treated with active fraction 400 mg/kg per day; Group 5 (DG), Diabetic rats treated with glybenclamide 20 mg/kg per day.
The active fraction and glybenclamide were administered to the animals of the respective groups every day morning for 30 d by using orogastric cannula. All the five groups were sacrificed on the day 31 after an overnight fasting by cervical dislocation and then blood samples were collected. Body weights of all the animals were recorded prior to and after the treatment.
2.8. Biochemical measurements
Fasting blood glucose was estimated by glucose oxidase method[10]. Glycosylated haemoglobin (haemoglobin A1c) was estimated following the methods of Eross et al[11]. Plasma insulin level was measured by using rat insulin ELISA kit. The estimation of protein was performed by the method of Lowry et al[12]. Activities of hepatic hexokinase, glucose-6-phosphatase and fructose-1,6-bisphosphatase were estimated according to standard methods[13]. Hepatic glycogen levels were measured according to the anthrone-H2SO4 method, with glucose as standard[14]. Serum total cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides (TG) were were assayed according to standard methods using a spectrophotometric assay kit. Low-density lipoprotein (LDL) cholesterol and very low-density lipoprotein (VLDL) cholesterol were calculated by using Friedewald formula[15]. Serum urea and creatinine were measured by assay kits.
2.9. Statistical analysis
The experimental results were expressed as mean of six replicates±S.D. Statistical comparison was done using one-way ANOVA followed by Duncan's multiple range test when more than two groups were involved. P values<0.01 were considered significant. Value “*” (P<0.01) represents the significant change as compared to normal control and values “**” (P<0.001) and “***” (P<0.000 1) represent the significant change as compared to diabetic control respectively.
3. Results
3.1. Phytochemical constituents
The ethyl acetate fraction was tested and showed presence of carbohydrates, flavanoids, anthocyanins and tannins.
3.2. Acute toxicity of fractions
Acute toxicity assay revealed the non-toxic nature of all the fractions of R. arboreum flowers. There was no grass evidence of any abnormalities or mortality in rat up to the end of the observation period.
3.3. Antihyperglycemic activity
3.3.1. Antihyperglycemic activity of different fractions of R. arboreum ethanol extract
Table 1 shows the effect of the fraction 1, fraction 2, fraction 3 and fraction 4 on fasting blood glucose levels at different time intervals in the normal and diabetic rats. None of these fractions had produced any hypoglycemic effect in the normal rats at a dose of 200 mg/kg. Among the four fractions of R. arboreum ethanol extract, only the fraction 3 has produced a significant (P<0.000 1) antihyperglycemic activity with 73.6% reduction in blood glucose at a dose of 200 mg/kg after 5 h post the treatment in the streptozotocin diabetic rats. The fraction 2 did not show any antihyperglycemic activity at the same dose in the diabetic treated groups. However, the fraction 1 and fraction 4 had shown a reduction of 13.4% and 21.9% in the blood glucose of diabetic rats treated with 200 mg/kg at 4 and 5 h respectively. Treatment of diabetic rats with glybenclamide had resulted in a 33.6% reduction in their blood glucose after 5 h, indicating the presence of residual pancreatic beta-cells. Since the fraction 3 at a dose of 200 mg/kg had produced maximum antihyperglycemic activity, it was considered as the active fraction containing the active principle(s) for antihyperglycemic action.
Table 1. Screening of different fractions of ethanol extract of R. arboreum flowers for antihyperglycemic activity (mM/L).
| Group | Blood glucose level after different time post the treatment with different fractions at dose 200 mg/kg |
||||||
| 0 h | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | |
| Group 1 (NC) | 4.8±0.2 | 4.7±0.4 | 4.9±0.2 | 4.6±0.4 | 4.5±0.3 | 4.5±0.4 | 4.6±0.3 |
| Group 2 ( DC) | 20.2±1.8* | 18.7±2.4* | 18.3±2.0* | 20.7±2.5* | 19.4±2.1* | 20.5±2.5* | 20.9±1.0* |
| Group 3 (DF1) | 21.0±2.3 | 20.1±1.7 | 18.9±2.2 | 17.7±1.5 | 16.8±1.6 | 17.1±1.0 | 17.9±1.1 |
| Group 4 (DF2) | 20.3±1.2 | 20.4±2.1 | 19.9±1.8 | 19.3±1.7 | 18.6±2.3 | 19.1±0.9 | 19.4±1.3 |
| Group 5 (DF3) | 20.4±2.6 | 17.8±1.8 | 16.6±1.5 | 12.9±2.0 | 11.0±1.8 | 5.4±1.1*** | 6.7±0.7** |
| Group 6 (DF4) | 20.3±1.7 | 20.3±1.6 | 19.8±1.4 | 18.7±2.2 | 17.7±1.3 | 16.0±1.5 | 16.5±1.7 |
| Group 7 (DG) | 20.9±2.1 | 18.4±2.1 | 18.0±1.8 | 16.9±1.8 | 15.9±1.7 | 13.6±1.9*** | 15.9±1.1** |
Values are given as mean±SD for six rats in each group.
*P<0.01 compared with the normal control (NC).
**P<0.001 and ***P<0.000 1 compared with diabetic control (DC).
3.3.2. Effect of long term treatment with the active fraction in streptozotocin-induced diabetic rats
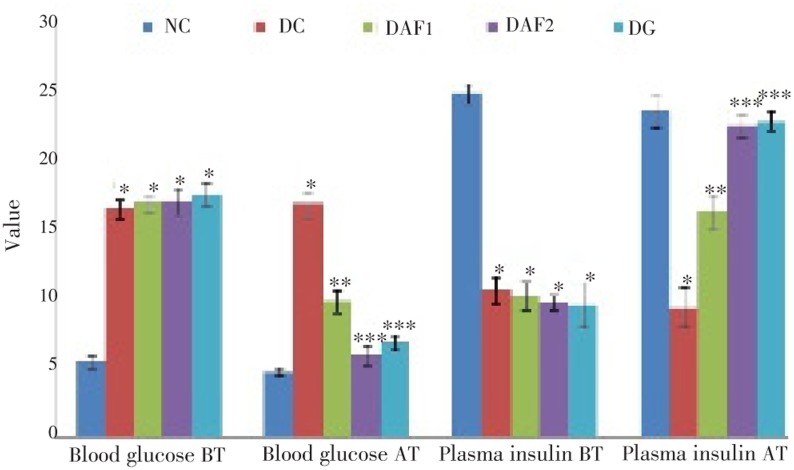
The effects of long term treatment with active fraction on blood glucose and plasma insulin levels in the normal and diabetic rats are shown in Figure 1. Fasting blood glucose levels of the diabetic control rats were significantly (P<0.01) higher than those of normal rats. A significant (P<0.001 to P<0.000 1) dose dependent decrease in blood glucose levels was observed in the diabetic treated group from an initial level of (16.9±0.6) mM/L to the level of (9.8±0.8) mM/L and from (17.0±0.9) mM/L to (5.9±0.7) mM/L after the treatment at a dose of 200 mg/kg and 400 mg/kg respectively for 30 d. No change in plasma glucose level was observed in the normal rats at a dose of 200 and 400 mg/kg till the end of study. There was a significant (P<0.01) decrease in plasma insulin level in the diabetic control group compared to the normal rats. After the treatment with active fraction at a dose of 200 and 400 mg/kg, the insulin level was significantly (P<0.001 to P<0.000 1) increased in the diabetic rats, which is similar to that of the glybenclamide treated diabetic rats. The diabetic rats showed a marked reduction in their body weights when compared to the normal control rats. After treatment with active fraction, the body weights of diabetic rats were increased significantly (P<0.001 to P<0.0001) but not near to those of the normal rats.
Figure 1. Effect of the active fraction of ethanol extract of R. arboreum flowers on blood glucose (mM/L) and plasma insulin (µU/mL). NC, normal control; DC, diabetic control; DAF1, diabetic rats administered active fraction (200 mg/kg); DAF2, diabetic rats administered active fraction (400 mg/kg); DG, diabetic rats administered glibenclamide; “*” represents the significant (P<0.01) change as compared to NC and “**” represents the significant (P<0.001) and “***” represents the significant (P<0.000 1) change as compared to DC.
The higher glycosylated haemoglobin was significantly (P<0.001 to P<0.000 1) decreased in the diabetic control group on treatment with active fraction. The hepatic glycogen and plasma protein levels of the diabetic rats were lower [(6.3±3.9) mg glucose equivalents/g wet tissue and (4.7±3.6) mg/dL] than those of the normal rats and they were significantly (P<0.000 1) increased upon treatment with active fraction or glybenclamide. The diabetic rats showed an increase in serum urea [(76.7±3.9) mg/dL] and creatinine [(0.77±0.06) mg/dL) compared with the normal control rats. A significant (P<0.001 to P<0.000 1) decrease in serum urea and creatinine levels were observed in the diabetic treated group after the treatment at a dose of 200 and 400 mg/kg with active fraction. Treatment with glybenclamide at a dose of 20 mg/kg also significantly (P<0.000 1) decreased serum urea and creatinine level in the diabetic rats. The activity of hexokinase was found to be decreased, while the activities of gluconeogenic enzymes: glucose-6-phosphatase and fructose-1,6-bisphosphatase were significantly (P<0.01) increased in the diabetic rats compared to those in normal rats. Treatment with active fraction in diabetic rats reversed the above changes significantly (Table 2).
Table 2. Effect of the active fraction of ethanol extract of R. arboreum flowers on blood biochemical indicators on day 31.
| Group | Hepatic glycogena | Plasma protein (mg/dL) | Haemoglobin A1c (%) | Urea (mg/dL) | Creatinine (mg/dL) | Glucose-6- phosphatase (U/mg protein) | Hexokinase (U/g protein) | Fructose-1,6- bisphosphatase (U/mg protein) |
| Group 1 (NC) | 12.1±3.8 | 7.3±2.7 | 6.7±1.4 | 32.1±1.8 | 0.45±0.08 | 0.17±0.03 | 151.1±6.7 | 0.39±0.02 |
| Group 2 (DC) | 6.3±1.9* | 4.7±1.6* | 12.7±1.3* | 76.7±3.9* | 0.77±0.06* | 0.25±0.02* | 109.2±5.1* | 0.59±0.03* |
| Group 3 (DAF1) | 9.2±1.1** | 5.1±0.9** | 10.4±1.1** | 54.3±2.4** | 0.65±0.03** | 0.21±0.03** | 122.4±2.2** | 0.48±0.02** |
| Group 4 (DAF2) | 11.8±3.6*** | 6.3±1.4*** | 7.2±1.2*** | 37.8±5.6*** | 0.45±0.04*** | 0.18±0.02*** | 142.3±5.5*** | 0.31±0.02*** |
| Group 5 (DG) | 10.1±2.8*** | 6.9±1.5*** | 8.6±1.6*** | 39.1±4.8** | 0.48±0.07** | 0.19±0.03** | 148.6±7.6** | 0.35±0.05** |
Values are given as mean±S.D. for six rats in each group.
aThe results are given as glucose equivalents (mg)/wet tissue (g).
*P<0.01 compared with the normal control (NC).
**P<0.001 and ***P<0.000 1 compared with diabetic control (DC).
3.4. Antihyperlipidemic activity
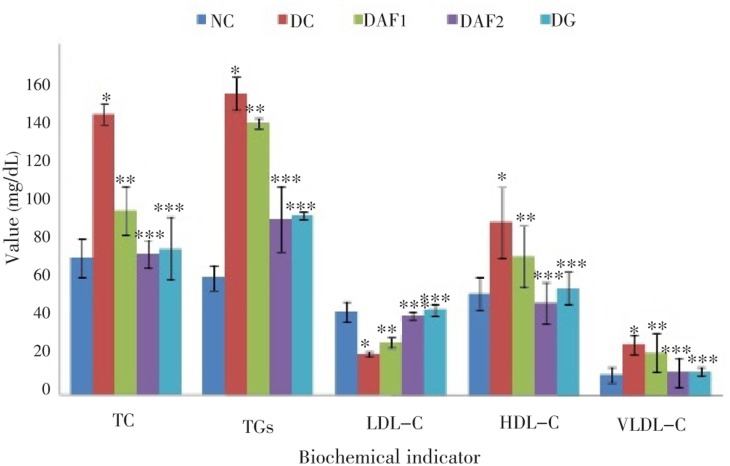
Figure 2 shows the dose dependent effect of the active fraction on the levels of serum total cholesterol, lipoproteins and TGs in the normal and experimental diabetic rats. The levels of total cholesterol, LDL cholesterol, VLDL cholesterol and TGs were significantly (P<0.01) increased, whereas the level of HDL cholesterol was significantly (P<0.01) decreased in the diabetic rats compared to those in the normals. Administration of the active fraction at a dose of 400 mg/kg to the diabetic rats for 30 d significantly (P<0.000 1) reduced total cholesterol to (64.5±6.5) mg/dL, LDL cholesterol to (42.3±9.4) mg/dL and VLDL cholesterols to (10.3±6.9) mg/dL compared with the diabetic untreated rats. The same dose level in the diabetic rats significantly (P<0.000 1) increased the HDL cholesterol to (36.3±1.9) mg/dL compared with the diabetic untreated rats. The levels of TGs were significantly (P<0.01) higher in the diabetic rats [(137.8±2.3) mg/dL] than in the normal rats [(53.7±5.6) mg/dL]. Treatment with the active fraction in the diabetic rats at a dose of 400 mg/kg resulted in a significant (P<0.000 1) decrease in the TGs levels compared with the diabetic control rats. The normal rats treated with the active fraction at a dose of 200 and 400 mg/kg showed no significant change in serum levels of total cholesterol, lipoproteins and TGs.
Figure 2. Effect of the active fraction of ethanol extract of R. arboreum on serum total cholesterol (TC), triglycerides (TGs), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) and very low density cholesterol (VLDL-C). NC, normal control; DC, diabetic control; DAF1, diabetic rats administered active fraction (200 mg/kg); DAF2, diabetic rats administered active fraction (400 mg/kg); DG, diabetic rats administered glibenclamide; “*” represents the significant (P<0.01) change as compared to NC and “**” represents the significant (P<0.001) and “***” represents the significant (P<0.000 1) change as compared to DC.
4. Discussion
In the present short-term study, the fraction 3 of R. arboreum flower ethanol extract showed maximum antihyperglycemic activity, bringing down the blood glucose levels to be almost normal in the treated diabetic rats[16]. The active fraction at a dose of 200 mg/kg b.w. produced a reduction of 73.6% in the blood glucose in the diabetic rats after 5 h of treatment. The efficacy of the active fraction in reducing the blood glucose levels was much higher than that of the standard oral hypoglycemic agent glybenclamide in the diabetic rats. The maximum antihyperglycemic effect shown by the active fraction at 5 h was followed by a increase in blood glucose level and this increase could be either due to cessation of the action of the antidiabetic active principle(s) or metabolic clearance of the active fraction in the animal system. No hypoglycemic condition was observed in the treated diabetic rats during the treatment period with the fraction 3. Drugs that normalize function, without causing hypoglycemia, would make attractive targets for diabetes[17].
In the present long-term study, streptozotocin was selected for induction of diabetes in rats rather than alloxan. Streptozotocin is well known for its selective pancreatic beta-cell cytotoxicity and has been extensively used to induce diabetes mellitus in animals[18], and it is less toxic than alloxan and allows a consistent maintenance of diabetes mellitus. The diabetic rats had lower body weights, decreased plasma insulin levels and increased levels of glycosylated haemoglobin as compared to the normal rats. The orally administered active fraction significantly (P<0.001 to P<0.000 1) increased the body weights and plasma insulin and decreased glycosylated haemoglobin levels in the diabetic rats in a dose-dependent manner. This is due to promotion in insulin secretion from the remnant pancreatic beta-cells in diabetic rats, consequently resulting in improvement in glycemic control. In our experiments, presence of low level insulin in the diabetic animals before treatment shows the presence of remnant beta-cells, indicating that streptozotocin at 60 mg/kg generated noninsulin-dependent diabetes mellitus by partial destruction of beta-cells. Further, treatment with glybenclamide, a known insulin secretogogue, confirmed the presence of remnant beta-cells in the diabetic animals as the glybenclamide treated group showed increased insulin levels along with reduced blood glucose levels after the treatment. After the treatment with the active fraction, there was a significant (P<0.001 to P<0.000 1) increase in the liver glycogen level in the diabetic rats. Hexokinase is insulin dependent key enzyme in the glycolytic pathway and crucial for the glucose homeostasis. Administration of the active fraction of R. arboreum flowers to diabetic rats enhanced the hepatic hexokinase activity and promoted glycolysis and glucose utilization for energy production. In the normal treated rats, the active fraction failed to bring any change in the activity of hexokinase, since there was no change in the insulin levels. The level of plasma insulin was found to increase significantly (P<0.001 to P<0.000 1) in the diabetic rats treated with the active fraction; the increased insulin levels may be due to the significant reduction in the activities of gluconeogenic enzymes. The reduction in the activities of gluconeogenic enzymes can decrease concentration of glucose in blood. The increased activities of gluconeogenic enzymes were shown to be reduced after treatment with other medicinal plants[19] in experimental diabetic animals and our results are comparable with these reports. The streptozotocin diabetic rats are associated with significant (P<0.01) increases in serum urea and creatinine levels and a decrease in plasma protein, indicating impaired renal function of diabetic rats. Streptozotocin induced diabetes in rats had been shown to be associated with functional and/or morphological changes in the kidney[20]. In our study, treatment of streptozotocin diabetic rats with active fraction showed promotion in urea and creatinine excretion[21]. The impairment of insulin secretion results in enhanced metabolism of lipids from the adipose tissue to the plasma[22]. It has been demonstrated that insulin deficiency in diabetes mellitus leads to accumulation of lipids such as total cholesterol and TGs in diabetic patients[23]. The abnormal high concentration of serum lipids in the diabetic subject is mainly due to increase in the mobilization of free fatty acids from the peripheral fat depots[24]. The effect of the active fraction on controlled mobilization of serum cholesterol and TGs are presumably mediated by controlling the hydrolysis of certain lipoproteins and their selective uptake and metabolism by different tissues. Significant (P<0.001 to P<0.000 1) lowering of total cholesterol and rise in HDL cholesterol is a very desirable biochemical state for prevention of atherosclerosis and ischemic conditions[25].
In the present study, active fraction of R. arboreum flower aqueous ethanol extract possesses the antihyperglycemic and antihyperlipidemic active principle(s) which acts by promoting insulin secretion and alterations in the carbohydrate and lipid metabolism. These results support the traditional use of R. arboreum as an antidiabetic plant. Identification of the active compound(s) found in R. arboreum may be the basis for the development of new antidiabetic drugs.
Footnotes
Foundation Project: Supported in part by grant Number GAP-274625 from Department of Science & Technology (DST), New Delhi, India.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Bavarva JH, Narasimhacharya AV. Leucas cephalotes regulates carbohydrate and lipid metabolism and improves antioxidant status in IDDM and NIDDM rats. J Ethnopharmacol. 2010;127(1):98–102. doi: 10.1016/j.jep.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in type 2 diabetes. Diabet Med. 2008;25(3):245–254. doi: 10.1111/j.1464-5491.2007.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhandary MR, Kawabata J. Antidiabetic activity of Laligurans (Rhododendron arboreum Sm.) flower. J Food Sci Technol. 2008;4:61–63. [Google Scholar]
- 4.Shyam SA, Kalpana S. Anti-inflammatory activity of flowers of Rhododendron arboreum (SMITH) in rat's hind paw edema induced by various phlogistic agents. Indian J Pharmacol. 1988;20:86–89. [Google Scholar]
- 5.Verma N, Singh AP, Amresh G, Sahu PK, Rao ChV. Anti-inflammatory and anti-nociceptive activity of Rhododendron arboreum. J Pharm Res. 2010;3:1376–1380. [Google Scholar]
- 6.Murty D, Rajesh E, Raghava D, Raghavan TV, Surulivel MK. Hypolipidemic effect of arboreum plus in experimentally induced hypercholestermic rabbits. Yakugaku Zasshi. 2010;130(6):841–846. doi: 10.1248/yakushi.130.841. [DOI] [PubMed] [Google Scholar]
- 7.Verma N, Singh AP, Amresh G, Sahu PK, Rao ChV. Protective effect of ethyl acetate fraction of Rhododendron arboreum flowers against carbon tetrachloride-induced hepatotoxicity in experimental models. Indian J Pharmacol. 2011;43(3):291–295. doi: 10.4103/0253-7613.81518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.OECD OECD Guidelines for the testing of chemicals (Acute Oral Toxicity–Up and Down-Procedure). [Online] Available from: http://www.oecd.org/dataoecd/17/51/1948378.pdf [Accessed on 2006 March 23]
- 9.Erejuwa OO, Sulaiman SA, Wahab MS, Salam SK, Salleh MS, Gurtu S. Antioxidant protective effect of glibenclamide and metformin in combination with honey in pancreas of streptozotocin-induced diabetic rats. Int J Mol Sci. 2010;11(5):2056–2066. doi: 10.3390/ijms11052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar PR, Bhansali A, Ravikiran M, Bhansali S, Dutta P, Thakur JS, et al. Utility of glycated hemoglobin in diagnosing type 2 diabetes mellitus: A community-based study. J Clin Endocrinol Metab. 2010;95(6):2832–2835. doi: 10.1210/jc.2009-2433. [DOI] [PubMed] [Google Scholar]
- 11.Eross J, Kreutzmann D, Jimenez M, Keen R, Rogers S, Cowell C, et al. Colorimetric measurement of glycosylated protein in whole blood cells plasma and dried blood. Ann Clin Biochem. 1984;21(Pt 6):519–522. doi: 10.1177/000456328402100606. [DOI] [PubMed] [Google Scholar]
- 12.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin-phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 13.Guruvayoorappan C, Sudha G. Phytopharmacological evaluation of Byesukar for hypoglycaemic activity and its effect on lipid profile and hepatic enzymes of glucose metabolism in diabetic rats. Ann Hepatol. 2008;7(4):358–363. [PubMed] [Google Scholar]
- 14.Jayaraman J. New Delhi: New Age International; 1981. Laboratory manual in biochemistry; pp. 154–155. [Google Scholar]
- 15.Antia BS, Okokon JE, Umoh EE, Udobang JA. Antidiabetic activity of ethanolic leaf extract of Panicum maximum. Int J Drug Dev Res. 2010;2(3):488–492. [Google Scholar]
- 16.Ottah Anaga Aruh, Augustine Onuoha, Obiora Igboeli Okechukwu, Maxwell Ezeja. Antihyperglycemic effects of the methanol leaf extract of Diaphananthe bidens in normoglycemic and streptozotocin-induced hyperglycemic rats. Asian Pac J Med. 2012;5(3):192–196. doi: 10.1016/S1995-7645(12)60023-6. [DOI] [PubMed] [Google Scholar]
- 17.Rajasekhar MD, Ramesh Babu K, Vinay K, Sampath MR, Sameena SK, Apparao Ch. Antihyperglycemic and antioxidant activities of act/ive fraction from the aqueous extract of Momordica cymbalaria fruits in Streptozotocin induced diabetic rats. Pharmacogn Res. 2009;1(6):352–358. [Google Scholar]
- 18.Raju K, Balaraman R. Antidiabetic mechanisms of saponins of Momordica cymbalaria. Pharmacogn Mag. 2008;4:197–206. [Google Scholar]
- 19.Pushparaj PN, Low HK, Manikandan J, Tan BKH, Tan CH. Antidiabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2007;111:430–434. doi: 10.1016/j.jep.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Zafar M, Naqvi SN, Ahmed M, Kaimkhani ZA. Altered kidney morphology and enzymes in streptozotocin-induced diabetic rats. Int J Morphol. 2009;27(3):783–790. [Google Scholar]
- 21.Mishra Shanti Bhushan, Verma Amita, Mukerjee Alok, Vijayakumar M. Anti-hyperglycemic activity of leaves extract of Hyptis suaveolens L. Poit in streptozotocin induced diabetic rats. Asian Pac J Med. 2011;4(9):689–693. doi: 10.1016/S1995-7645(11)60175-2. [DOI] [PubMed] [Google Scholar]
- 22.Pattabiraman K, Muthukumaran P. Antidiabetic and antioxidant activity of Morinda tinctoria roxb fruits extract in streptozotocin-induced diabetic rats. Asian J Pharm Tech. 2011;1(2):34–39. [Google Scholar]
- 23.Sharma SB, Tanwar RS, Nasir A, Prabhu KM. Antihyperlipidemic effect of active principle isolated from seed of Eugenia jambolana on alloxan-induced diabetic rabbits. J Med Food. 2011;14(4):353–359. doi: 10.1089/jmf.2010.1227. [DOI] [PubMed] [Google Scholar]
- 24.Gayathri M, Kannabiran K. Antidiabetic activity of 2-hydroxy 4-methoxy benzoic acid isolated from the roots of Hemidesmus indicus on streptozotocin-induced diabetic rats. Int J Diabetes Metabolism. 2009;17:53–57. [Google Scholar]
- 25.Wani VK, Dubey RD, Verma S, Sengottuvelu S, Sivakumar T. Antidiabetic activity of methanolic root extract of Mukia maderaspatana in alloxan-induced diabetic rats. Int J Pharm Tech Res. 2011;3(1):214–220. [Google Scholar]