Abstract
Objective
To evaluate the antioxidant and antiglycation potential of polyphenols from three spices; alligator pepper, ginger and nutmeg.
Methods
Polyphenol extracts of these spices were subjected to brine-shrimp lethality assay, phytotoxicity test, DPPH and superoxide anion radical scavenging as well as BSA-glucose antiglycation assay.
Results
Results obtained showed that polyphenol extract of ginger has the highest antioxidant potential with IC50 0.075 and 0.070 mg/mL for DPPH and superoxide anion radical scavenging assay while alligator pepper displayed highest antiglycation activity with IC50 0.125 mg/mL. However, nutmeg extract exhibited weakest cytotoxic and phytotoxic potential with LD50 4359.70 and 1490 µg/mL respectively.
Conclusions
It can be concluded that the polyphenol extracts of alligator pepper, ginger and nutmeg displayed good antioxidant as well as antiglycation potential and are safe for consumption.
Keywords: Glycation, Hyperglycemia, Polyphenols, Spices, Free radicals, Antioxidants
1. Introduction
Glycation is a non enzymatic condensation reaction between reducing sugars and amino groups of proteins that undergo rearrangements to stable ketoamines, leading to the formation of advanced glycation end products (AGEs)[1]. It is a spontaneous reaction which is dependent on the degree and duration of hyperglycaemia, half-life of the protein and permeability of the tissue to free glucose[2]. Increased glycation and build-up of tissue AGEs have been implicated in diabetic complications because they can alter enzymic activity, decrease ligand binding, modify protein half-life and alter immunogenicity[3]. Hyperglycemia is considered as a clinical hallmark of diabetes, the phenomenon that results into the formation of AGEs. Therefore glycation is also important therapeutic target for the treatment of diabetic complication.
Polyphenolic compound on the other hand, is a diverse class of plant secondary metabolites[4],[5] characterised by a polyphenol structure, which means that they have several hydroxyl groups on two or more benzene rings[6]. Several plant species such as fruits, spices and vegetables are reported to possess polyphenols which confer on them the ability to remove free radicals formed from glycation and its endproducts from the system. In this regard, several spices are among the top dietary sources of polyphenolics[7]. Not only do culinary spices provide high concentrations of bioactive compounds, they also tend to provide few calories which is an advantage in type 2 diabetes, which is often associated with abdominal obesity[8]. For the purpose of this study, alligator pepper, ginger and nutmeg are the spices of interest.
Alligator pepper (A framomum melegueta Roscoe K. Schum), also known as Guinea pepper or grains of paradise, is a member of the Zingiberaceae family native to West Africa. The seeds are used as a spice for flavouring food and have a wide range of ethnobotanical uses. They are used as a remedy for treating stomachache, diarrhoea, and snakebite[9],[10]. Zingiber officinale Roscoe [family: Zingiberaceae], commonly known as ‘ginger’, is one of the frequently used spices in many parts of the world. It has been cultivated for thousands of years for medicinal purposes and as a spice. It is used extensively in traditional medicine to treat headaches, nausea, febrile conditions, colds, arthritis, rheumatic disorders and muscular discomfort[11],[12]. Myristica fragrans Houtt (Myristicaceae) with a common name, nutmeg, is an aromatic tree cultivated in many tropical countries. Its dried kernel has been claimed to possess medicinal properties (digestive, carminative and expectorant) in Indian medicine[13]. It also possesses hypolipidaemic, antithrombotic, antiplatelet aggregation, antifungal, aphrodisiac, and anti-inflammatory activities[14].
Due to the global widespread incidence of diabetes and its complications in the recent time, the aim of this study is to evaluate the antiglycation and antioxidant potential of the polyphenol-rich extract of these spices. Their ability or inability to constitute danger to their consumer is also assessed through the cytotoxicity and phytotoxicity assay.
2. Materials and methods
2.1. Plant materials
Ginger, nutmeg and alligator pepper were purchased from the Central Spices Market in Mile 12 area, Ketu, Lagos. They were dried under room temperature, grounded into powder and kept in plastic containers in the refridgerator before the commencement of the study.
2.2. Chemicals
BSA (Bovine serum albumin) was obtained from the Research Organics Cleveland USA. 1, 1-diphenyl, 2-picryl hydrazyl (DPPH), ferric chloride and trichloroacetic acid were obtained from Sigma Chemical Co. (St. Louis, Mo., USA). All the chemicals were of analytical grade and the water was glass distilled.
2.2. Preparation of crude polyphenol extracts
The powdered samples were extracted with 80% acetone (1:2 w/v) thrice each for 72 hours each time at room temperature. Pooled extracts were filtered with Whatman filter paper (type 2) under vacuum and the filtrate was concentrated under reduced pressure on rotatory evaporator (BÜCHI, R-3000, Switzerland) at 40 °C temperature. The concentrated extract was further lyophilized. The lyophilized extract was then used for the experiments[15].
2.3. Brine shrimp cytotoxicity assay
The test was conducted by taking half filled hatching tray (22 × 32 cm) with brine solution (sea salt 38 g/L), 500 mg eggs of brine shrimp (Artemia salina) were sprinkled and after covering with a lid, it was incubated at 27 °C for 2 days for hatching. The brine shrimp larvae were collected through a light source and Pasteur pipette. The extracts were tested by using initial concentrations of 10, 100 and 1000 µg/mL in vials containing 5 mL of brine and 10 shrimps in each of the three replicates. Survivors were counted after 24 h. The data was analyzed to determine LD50 values at 95% confidence intervals[16].
2.4. Phytotoxicity assay
This test was performed according to the modified protocol of McLaughlin et al [16]. The test fractions were incorporated with sterilized medium at different concentrations i.e. 10, 100, 1000 µg/mL in methanol. Sterilized conical flasks were inoculated with fractions of desired concentrations prepared from the stock solution and allowed to evaporate overnight. Each flask was inoculated with 20 mL of sterilized E-medium and then ten Lemna minor each containing a rosette of three fronds were placed on media. Other flasks were supplemented with methanol serving as negative control and reference inhibitor i.e. Paraquat served as positive control. Treatment was replicated three times and the flasks incubated at 30 °C in Fisons Fi-Totron 600 H growth cabinet for seven days, (56±10)% relative humidity and 12 h day length. Growth of Lemna minor (L. minor) in fraction containing flask was determined by counting the number of fronds per dose and growth inhibition was calculated with reference to negative control.
2.5. Diphenylpicrylhydrazine (DPPH) radical-scavenging activity
The radical scavenging property of the extract was evaluated by assessing the 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity[17]. The reaction mixture was prepared by mixing 5 µL extract (dissolved in DMSO) with 95 µL of DPPH (dissolved in ethanol). Different concentrations of extract were prepared, while the concentration of DPPH was kept as 300 µM in all reaction mixture. These reaction mixtures were dispensed in 96-well plate and incubated at 37 °C for 30 min and the absorbance was measured at 515 nm. The radical scavenging activity was calculated as a percentage of DPPH decolorization compared to control. Propyl gallate was used as a positive control.
% Inhibition = [1 - (Absorbance extract/ Absorbance control)] × 100
2.6. Superoxide anion scavenging (SAS) assay
The method of Ferda[18] was used in the determination of superoxide scavenging activity of the samples. Superoxide scavenging was assayed in phosphate buffer (0.1 M, pH 7.5). Xanthine oxidase (0.003 unit /well) and test samples in DMSO were mixed in 96 - well microliter plate and pre-incubated for 10 min at room temperature, WST-1 (15 µM) was added. The reaction was initiated by adding 0.1 mM of xanthine and uric acid formation was measured spectrophotometrically at 295 nm and the reduction of WST-1 was read at 450 nm by using molecular devices, spectrama × 340. The percentage inhibitory activity by the samples was determined against DMSO blank and calculated using the formula:
% Inhibition =[1 - (Absorbanceextract/ Absorbancecontrol)] × 100.
2.7. Antiglycation assay
In vitro antiglycation activity of the spices was examined by testing the ability of the extracts to inhibit the methyl glyoxal mediated development of fluorescence of bovine serum albumin (BSA)[19]. In 96-well plate assays, each well contained 60 µL reaction mixture of 20 µL BSA (10 mg/mL), 20 µL of glucose anhydrous (50 mg/mL), magnesium oxide (14 mM) and 20 µL test sample (extract). Glycated control contained 20 µL BSA, 20 µL glucose and 20 µL sodium phosphate buffer (0.1 M, pH 7.4) containing NaN3 (30 mM), while blank control contained 20 µL BSA and 40 µL sodium phosphate buffer. Reaction mixture was incubated at 37 °C for 9 days in the presence or absence of various concentrations of the extract. After 9 days of incubation, 60 µL TCA (100 %) was added in each well and centrifuged (15,000 rpm) for 4 minutes at 4 °C. After centrifugation, the pellet was washed with 60 µL 5 % TCA. The supernatant containing glucose, inhibitor and interfering substance, was removed and pellet containing advanced glycation endproducts (AGEs)-BSA was dissolved in 60 µL PBS. AGEs formation was measured by the fluorescence's intensity excitation (370 nm) to emission (440 nm) by using the spectrofluorometer RF-1500 (Shimadzu, Japan). Rutin was used as a positive control. The results are reported as follows:
% Inhibition = [1 - (Absorbance extract/Absorban cecontrol)] × 100.
3. Results
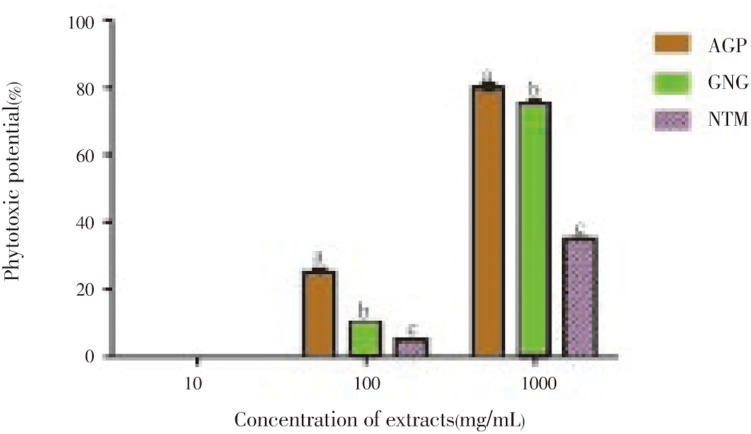
The result of phytotoxic activity of these extracts is displayed in Figure 1. All the extracts did not inhibit the growth of the plant at 10 µg/mL and showed low activities at 100 µg/mL. At a high concentration of 1 000 µg/mL, alligator pepper and ginger showed very high activity. The activities of the different extracts were significantly different (P<0.05) from one another at 100 and 1 000µg/mL. Nutmeg displayed the highest LD50 (1490µg/ml) as against alligator pepper (350) and ginger (600) as shown in Table 1.
Figure 1. Phytotoxic effect of the polyphenolic extracts of spices against L. minor.
The values are expressed as means±SEM of triplicate tests. Means not sharing a common letter at the same concentration were significantly different (P < 0.05) when analysed by ANOVA and Bonferroni post hoc test. AGP: Alligator pepper, GNG: Ginger, NTM: Nutmeg.
Table 1. LD50 values of polyphenolc extracts of alligator pepper, ginger and nutmeg in cytotoxity and phytotoxicity assays (µg/mL).
| Spices | LD50 |
|
| Cytotoxicity | Phytotoxicity | |
| Alligator pepper | 749.67±9.56 | 350.00±3.85 |
| Ginger | 187.85±3.25 | 600.00±5.29 |
| Nutmeg | 4359.70±12.33 | 1490.00±6.12 |
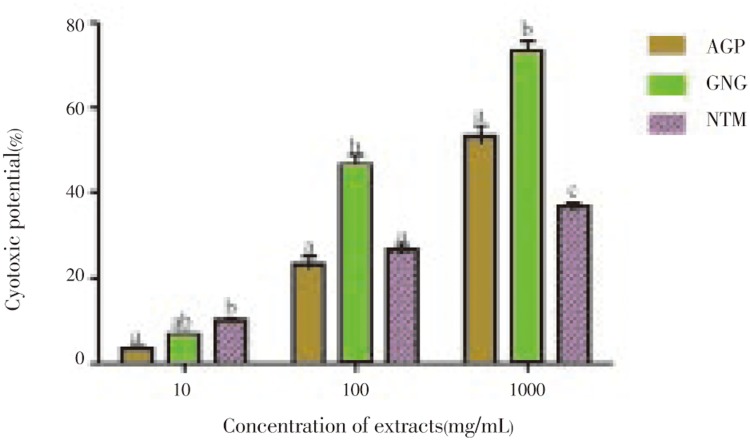
The effect of the extracts on the survival of brine shrimps (Artemia salina) is shown in Figure 2. At low concentrations (10 and 100µg/mL), the extracts showed low activity (less than 50%) against the organism but were active at high concentration (1 000µg/mL) except nutmeg which still possesses less than 50% mortality of the organism. However, ginger showed the highest activity against the brine shrimps (46.67 and 73.33 at 100 and 1 000µg/ml respectively). So the result of ginger was significantly different (P<0.05) from the other spices at these concentrations. Their LD50 (µg/mL) is in this order; ginger (187.85), alligator pepper (749.67), nutmeg (4359.70) (Table 1).
Figure 2. Cytotoxic effect of polyphenolic extracts of selected spices on Artemia salina.
The values are expressed as means±SEM of triplicate tests. Means not sharing a common letter at the same concentration were significantly different (P< 0.05) when analysed by ANOVA and Bonferroni post hoc test. AGP: Alligator pepper, GNG: Ginger, NTM: Nutmeg.
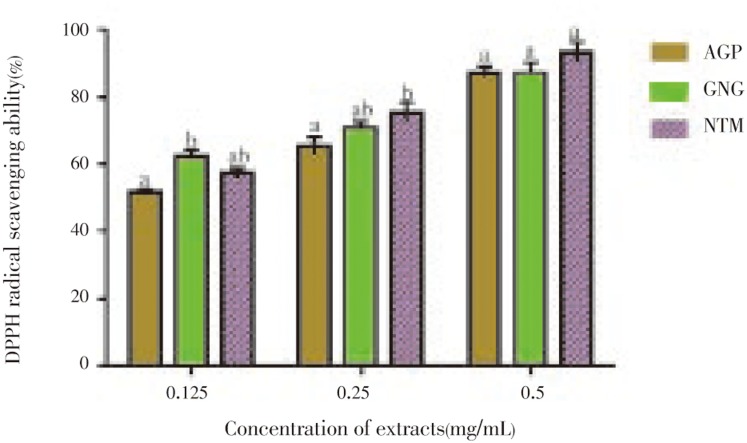
The DPPH radical scavenging activity of the spices is shown in Figure 3. All the extracts showed dose-dependent inhibition. At all the concentrations tested, the extracts exhibited more than 50% inhibition of the radical. Though, the extracts had different inhibitions at lower concentrations, their percentage inhibition at 0.5 mg/mL were not significantly different (P<0.05) from one another. However, the IC50 shown in table 2 depicted that ginger had the lowest IC50 value (0.075) when compared to nutmeg (0.1) and alligator pepper (0.11).
Figure 3. Inhibitory capacities of the polyphenolic extracts of alligator peppper on the formation of DPPH radicals.
The values are expressed as means±SEM of triplicate tests. Means not sharing a common letter at the same concentration were significantly different (P< 0.05) when analysed by ANOVA and Bonferroni post hoc test. AGP: Alligator pepper, GNG: Ginger, NTM: Nutmeg.
Table 2. IC50 values of the extracts of alligator pepper, ginger and nutmeg in antiglycation and antioxidant assays (mg/mL).
| Spices | IC50 |
||
| Antiglycation | 1DPPH Assay | 2SAS Assay | |
| Alligator pepper | 0.125±0.020 | 0.110±0.010 | 0.105±0.020 |
| Ginger | 0.285±0.010 | 0.075±0.000 | 0.070±0.010 |
| Nutmeg | 0.200±0.030 | 0.100±0.010 | 0.135±0.010 |
1DPPH; Diphenylpicrylhydrazine, 2SAS; Superoxide anion scavenging.
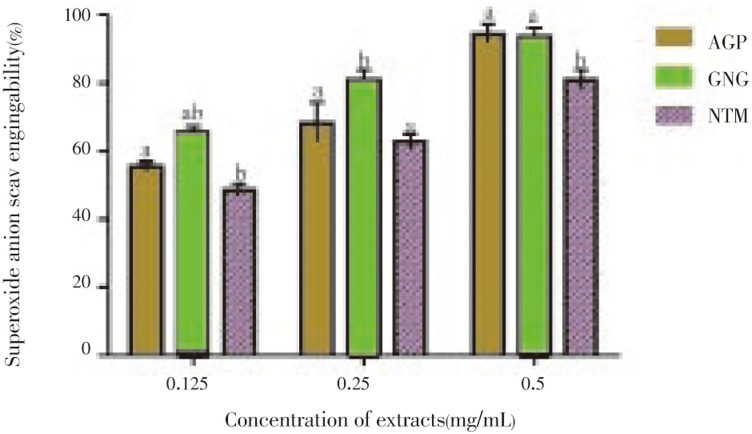
Figure 4 showed that ginger offered the highest inhibition to the superoxide anion radicals; 65.52%, 80.66% and 93.18% at 0.125, 0.25 and 0.5mg/ml concentration respectively. However, both alligator pepper and ginger were not significantly different (P<0.05) at the highest concentration tested. This trend is corroborated by the lowest IC50 displayed by ginger (0.07), followed by alligator pepper (0.105) and nutmeg (0.135)
Figure 4. Inhibitory capacities of the polyphenolic extracts of selected spices on the formation of superoxide anion radicals.
The values are expressed as means±SEM of triplicate tests. Means not sharing a common letter at the same concentration were significantly different (P < 0.05) when analysed by ANOVA and Bonferroni post hoc test. AGP: Alligator pepper, GNG: Ginger, NTM: Nutmeg.
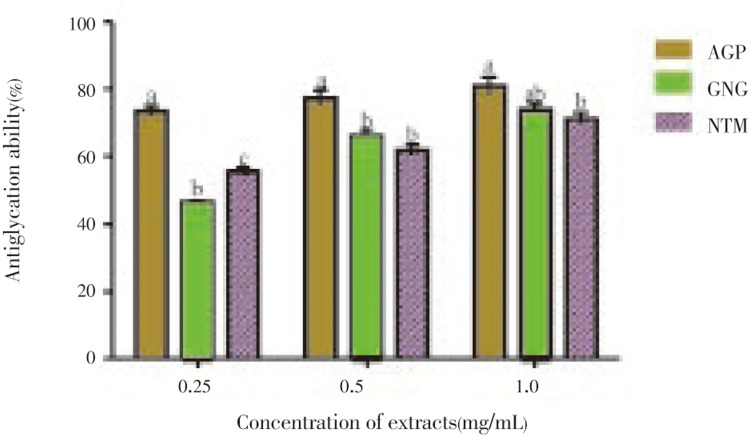
Figure 5 presents the antiglycation capacities of the polyphenolic extracts, as evaluated by their inhibition of the formation of AGEs in the BSA/glucose system. All the extracts inhibited the glucose-mediated formation of fluorescent AGEs in a dose-dependent manner. At 1.0 mg/mL, all the extracts exhibited high inhibition towards the formation of AGEs which was retained by alligator pepper at all concentrations. Its inhibitory capacity was significantly different (P<0.05) compared to ginger and nutmeg at all concentrations tested. At 0.25mg/ml, the antiglycation capacity of the extracts followed this order; alligator pepper (73.4%), nutmeg (55.5%), ginger (46.4%). Table 2 also showed that alligator pepper had the lowest IC50 (0.125) compared to that of ginger (0.285) and curry (0.200).
Figure 5. Inhibitory capacities of the polyphenolic extracts of alligator pepper, ginger and nutmeg on the formation of advanced glycation endproducts (AGEs).
The values are expressed as means±SEM of triplicate tests. Means not sharing a common letter at the same concentration were significantly different (P < 0.05) when analysed by ANOVA and Bonferroni post hoc test. AGP: Alligator pepper, GNG: Ginger, NTM: Nutmeg.
4. Discussion
Brine shrimp lethality is a general bioassay, which is indicative of cytotoxicity of an extract or compound[16]. The result indicates that crude polyphenolic extract of ginger is the most cytotoxic of the three. This is attested to by the large difference in their LD50 values (187, 749 and 4359µg/mL for ginger, alligator pepper and nutmeg respectively). However, all the extracts can still be considered safe due to their relatively high LD50. Anderson et al [20]. stated that extracts which showed LD50 higher than 100µg/ml in the brine shrimp lethality test can be considered inactive and so safe for consumption.
To assess the possible phytotoxic potential of these extracts, their effect on the growth of plant Lemna minor was determined. From the LD50 values generated from their phytotoxic study, it showed that all the extracts had high LD50, the implication of which is that they are non-toxic to the plant except at very high concentrations. According to Khan et al[21], a number of plants, their extracts or their purified active constituents can act as allelochemicals to other plants. These spices are not suitable for such purpose due to their inactivity.
The DPPH free radical scavenging ability of the extracts showed that all the spices inhibited the free radicals in concentration-dependent manner. These high inhibition possessed by the spices at all concentrations may not be unconnected with the large quantities of phenolics[15] in them. This is also shown by their low IC50 signifying that ginger seems to be the most active against DPPH radicals due to its least IC50 of 0.075 mg/mL.
In order to further confirm the antioxidant activity of these extracts, their ability to scavenge superoxide anion radical was evaluated. Extract of ginger also displayed the highest inhibition at all concentrations. This is testified to by its IC50 0.07 mg/mL as against 0.105 mg/mL and 0.135 mg/mL for alligator pepper and nutmeg. The antiradical activity of polyphenols is principally based on the redox properties of their hydroxyl groups and the structural relationships between different parts of their chemical structure[22],[23]. In accordance, several reports had established a correlation between the total phenol content of plant food and their antioxidant properties[15],[24]
Free radicals especially reactive oxygen species (ROS) had been implicated in a lot of degenerative diseases such as diabetes and cardiovascular diseases. Overproduction of ROS can directly attack the polyunsaturated fatty acids of the cell membranes and induce lipid peroxidation[25]. Antioxidants carry out their protective properties on cells either by preventing the production of free radicals or by neutralizing/scavenging free radicals produced in the body[26]. The free radical scavenging ability of all these extracts is an indication that these spices promise to be excellent dietary source of antioxidants.
Hyperglycemia is regarded as a key factor in the development of diabetic complications. Chronic exposure of tissues to high levels of blood glucose can lead to adverse intracellular effects, leading to what is known as glucose toxicity. Several major mechanisms have been proposed for hyperglycemia induced-tissue damages, including increased polyol pathway flux, increased de-novo diacylglycerol synthesis with resultant activation of protein kinase C isoforms, hexoamine pathway and AGEs formation [27]-[29]. A large body of evidences point to glycation as a key molecular basis of diabetic complications. The fact that glycoxidative reactions cause protein dysfunction strongly suggests a role for such reactions as pathogenic factors in diabetes[30]-[39]. AGEs contribute to diabetic complications through a series of pathological changes such as increased atherogenicity of LDL, increased basement membrane permeability and decreased insulin binding to its receptors. AGEs play, as well, an important role in diabetic micro- and macroangiopathy where it deposits under endothelial cells[40],[41].
The extracts of all the spices tested displayed good antiglycation ability which increases with the increase in concentration, though alligator pepper extract showed highest potential at all concentrations tested. Glycation and AGE-induced toxicity are known to be associated with increased free radical production. Therefore, agents that possess good antioxidant activity by mopping up free radicals can simultaneously inhibit the formation of advanced glycation endproducts[42],[43]. As such, polyphenols from these spices can effectively serve as an antioxidant and antiglycation agent in the diets of diabetics.
Acknowledgments
The study is conducted through ICCBS-TWAS postgraduate fellowship (FR number: 3240223509) granted M. I. Kazeem tenable at the Dr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences (ICCBS), University of Karachi, Karachi, Pakistan. Academy of Science for the Developing World (TWAS), Trieste, Italy and International Center for Chemical and Biological Sciences (ICCBS), University of Karachi, Karachi, Pakistan is gratefully acknowledged for the fellowship.
Footnotes
Foundation Project: The study is financially supported by ICCBS-TWAS postgraduate fellowship (FR number: 3240223509).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Ahmed N. Advanced glycation endproducts - role in pathology of diabetic complications. Diabetes Res Clin Pr. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Berrou J, Tostivint I, Verrecchia F, Berthier C, Boulanger E, Mauviel A, et al. Advanced glycation end-products regulate extracellular matrix protein and protease expression by human glomerular mesangial cells. Int J Mol Med. 2009;23:513–520. doi: 10.3892/ijmm_00000159. [DOI] [PubMed] [Google Scholar]
- 3.Kostolanska J, Jakus V, Barak L. Monitoring of early and advanced glycation in relation to the occurrence of microvascular complications in children and adolescents with type 1 diabetes mellitus. Physiol Res. 2009;58:553–561. doi: 10.33549/physiolres.931612. [DOI] [PubMed] [Google Scholar]
- 4.Jaejoon H, Michel B, Daniel S, Claude PC, Patrick F, Stéphane S, et al. Polyphenolic compounds as functional ingredients in cheese. Food Chemistry. 2011;124:1589–1594. [Google Scholar]
- 5.Hasna E. Polyphenols: food sources, properties and applications - a review. Int J Food Sci Tech. 2009;44:2512–2518. [Google Scholar]
- 6.D'Archivio M, Filesi C, Di Benedetto R. Polyphenols, dietary sources and bioavailability. Annali dell'Istituto Superiore Di Sanita. 2007;43:348–361. [PubMed] [Google Scholar]
- 7.Cinta B, Lluis A, Salvado M. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol Nutr Food Res. 2010;54:37–59. doi: 10.1002/mnfr.200900476. [DOI] [PubMed] [Google Scholar]
- 8.Schroder H. Protective mechanisms of the Mediterranean diet in obesity and type 2 diabetes. J Nutr Biochem. 2007;18:149–160. doi: 10.1016/j.jnutbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Akendengue B, Loius A. Medicinal plants used by Masongo people of Gabon. J Ethnopharmacol. 1994;41:193–200. doi: 10.1016/0378-8741(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 10.Umukoro S, Ashorobi RB. Further studies on the antinociceptive action of aqueous seed extract of Aframomum melegueta. J Ethnopharmacol. 2007;109:501–504. doi: 10.1016/j.jep.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Khaki A, Fatemek F, Mohammad N, Amir AK, Chelar CO, Marefat N, et al. The effects of ginger on spermatogenesis and sperm parameters. Iran J Reprod Med. 2009;7(1):7–12. [Google Scholar]
- 12.Shalaby MA, Hamowieh AR. Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats. Food Chem Toxicol. 2010;48:2920–2924. doi: 10.1016/j.fct.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Nadkarni KM. Indian materia medica. 3rd ed. Mumbai: Bombay Popular Prakashan; 1998. pp. 830–834. [Google Scholar]
- 14.Evans WC. Trease and Evans' pharmacognosy. 14th ed. Singapore: Harcourt Brace & Co. Asia; 1996. pp. 273–275. [Google Scholar]
- 15.Chu Y, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activity of common vegetables. Journal of Agricultural and Food Chemistry. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin JL, Chang CJ, Smith DL. “Bench-Top” bioassays for the discovery of bioactive natural products; an update. In: Atta-ur-Rahman, editor. Amsterdam: Studies in Natural Products Chemistry, Elsevier Science Publishers; 1991. [Google Scholar]
- 17.Gyamfi MA, Yonamine M, Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. General Pharmacology. 1999;32:661–667. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- 18.Ferda C, editor. Effect of Rhus coriarial L (Anacardiaceae) on Superoxide radical scavenging and xanthine oxidase activity. J Enzyme Inhibition Med Chem. 2003;18:59–62. doi: 10.1080/1475636031000069273. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda H., Wang T., Managi H., Yoshikawa M. Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg Med Chem. 2003;11:5317–5323. doi: 10.1016/j.bmc.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JE, Goetz CM, McLaughlin JL, Suffness MA. A blind comparison of simple bench-top bioassays and human tumour cell cytotoxicities as antitumor prescreens. Phytochemical Analysis. 1991;2:107. [Google Scholar]
- 21.Khan T, Zahid M, Asim M, Shahzad-ul-Hussan IZ, Choudhary MI, Ahmad VU. Pharmacological activities of crude acetone extract and purified constituents of Salvia moorcraftiana Wall. Phytomedicine. 2002;9:749–752. doi: 10.1078/094471102321621386. [DOI] [PubMed] [Google Scholar]
- 22.Oboh G, Henle T. Antioxidant and Inhibitory Effects of Aqueous Extracts of Salvia officinalis Leaves on Pro-Oxidant-Induced Lipid Peroxidation in Brain and Liver In Vitro. J Med Food. 2009;12(1):000–000. doi: 10.1089/jmf.2008.0007. [DOI] [PubMed] [Google Scholar]
- 23.Oboh G. Neuroprotective potentials of Sour (Hibiscus sabdarifa, calyx) and Green (Camellia sinensis) teas on some pro-oxidants induced oxidative stress in brain. Asian J Clinical Nutrition. 2009;1(1):40–49. [Google Scholar]
- 24.Oboh G, Puntel RL, Rocha JBT. Hot pepper (Capsicum annuum, Tepin and Capsicum chinese, Habanero) prevents Fe2+-induced lipid peroxidation in brain - in vitro. Food Chemistry. 2007;102:178–185. [Google Scholar]
- 25.Jeffrey WS, Manish PK, Stephen CB. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202:321–329. doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Jorge L, María EG. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutation Research. 2009;674:137–147. doi: 10.1016/j.mrgentox.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Jagtap AG, Patil PB. Antihyperglycemic activity and inhibition of advanced glycation end product formation by Cuminum cyminum in streptozotocin induced diabetic rats. Food and Chemical Toxicology. 2010;48:2030–2036. doi: 10.1016/j.fct.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 28.Jarinyaporn N, Patchareewan P, Veerapol K, Bunkerd K, Upa K. Antihyperglycemic, Antioxidant and Antiglycation Activities of Mulberry Leaf Extract in Streptozotocin-Induced Chronic Diabetic Rats. Plant Foods Hum Nutr. 2009;64:116–121. doi: 10.1007/s11130-009-0112-5. [DOI] [PubMed] [Google Scholar]
- 29.Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radical Biol Med. 2006;41:177–184. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 30.Singh R, Barden A, Mori T, Beilin L. Advanced glycation endproducts: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 31.Kannan RRR, Arumugam R, Anantharaman P. In vitro antioxidant activities of ethanol extract from Enhalus acoroides (L.F.) Royle. Asian Pac J Med. 2010;3(11):898–901. [Google Scholar]
- 32.Kumar BSA, Lakshman K, Jayaveera KN, Shekar DS, Kumar AA, Manoj B. Antioxidant and antipyretic properties of methanolic extract of Amaranthus spinosus leaves. Asian Pac J Med. 2010;3(9):702–706. [Google Scholar]
- 33.Ganga RB, Madhu KP, Vijaya RAD. Investigation of antioxidant and anti-inflammatory activity of leaves of Dalbergia paniculata (Roxb) Asian Pac J Med. 2012;5(6):455–458. doi: 10.1016/S1995-7645(12)60077-7. [DOI] [PubMed] [Google Scholar]
- 34.Kumar DJ, Santhi RJ. Antioxidant and cytotoxic effects of hexane extract of Morinda pubescens leaves in human liver cancer cell line. Asian Pac J Med. 2012;5(5):362–366. doi: 10.1016/S1995-7645(12)60060-1. [DOI] [PubMed] [Google Scholar]
- 35.Meriga B, Mopuri R, Krishna TM. Insecticidal, antimicrobial and antioxidant activities of bulb extracts of Allium sativum. Asian Pac J Med. 2012;5(5):391–395. doi: 10.1016/S1995-7645(12)60065-0. [DOI] [PubMed] [Google Scholar]
- 36.Osadebe PO, Okoye FBC, Uzor PF, Nnamani NR, Adiele IE, Obiano NC. Phytochemical analysis, hepatoprotective and antioxidant activity of Alchornea cordifolia methanol leaf extract on carbon tetrachloride-induced hepatic damage in rats. Asian Pac J Med. 2012;5(4):289–293. doi: 10.1016/S1995-7645(12)60041-8. [DOI] [PubMed] [Google Scholar]
- 37.Piaru SP, Mahmud R, Majid AMSA, Nassar ZDM. Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia. Asian Pac J Med. 2012;5(2):294–298. doi: 10.1016/S1995-7645(12)60042-X. [DOI] [PubMed] [Google Scholar]
- 38.Taher M, Susanti D, Rezali MF, Zohri FSA, Ichwan SJA, Alkhamaiseh SI, Ahmad F. Apoptosis, antimicrobial and antioxidant activities of phytochemicals from Garcinia malaccensis Hk.f. Asian Pac J Med. 2012;5(2):136–141. doi: 10.1016/S1995-7645(12)60012-1. [DOI] [PubMed] [Google Scholar]
- 39.Poongothai K, Ponmurugan P, Ahmed K Syed Zameer, Kumar B Senthil, Sheriff SA. Antihyperglycemic and antioxidant effects of Solanum xanthocarpum leaves (field grown & in vitro raised) extracts on alloxan induced diabetic rats. Asian Pac J Med. 2011;4(10):778–785. doi: 10.1016/S1995-7645(11)60193-4. [DOI] [PubMed] [Google Scholar]
- 40.Daroux M, Prévost G, Maillard-Lefebvre H, Gaxatte C, D'Agati VD, Schmidt AM, Boulanger E. Advanced glycation end-products: Implications for diabetic and non-diabetic nephropathies. Diabetes & Metabolism. 2010;36:1–10. doi: 10.1016/j.diabet.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Park CH, Noh JS, Yamabe N, Kang KS, Tanaka T, Yokozawa T. Beneficial effect of 7-O-galloyl-D-sedoheptulose on oxidative stress and hepatic and renal changes in type 2 diabetic db/db mice. European Journal of Pharmacology. 2010;640:233–242. doi: 10.1016/j.ejphar.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad MS, Ahmed N. Antiglycation properties of aged garlic extract: possible role in prevention of diabetic complications. Journal Nutrition. 2006;136(Suppl. 3):796S–799S. doi: 10.1093/jn/136.3.796S. [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa T, Yokozawa T, Terasawa K. Protective activity of green tea against free radical- and glucose-mediated protein damage. J Agric Food Chem. 2002;50:2418–2422. doi: 10.1021/jf011339n. [DOI] [PubMed] [Google Scholar]