Abstract
Objective
To examined the immediate and 24 hours post- irradiation germicidal effects of UV-C lamp on eggs and adults of house dust mites Dermatophagoides pteronyssinus (D. pteronyssinus) and Dermatophagoides farinae (D. farinae).
Methods
This study investigated the immediate and 24 hours post irradiation mortalities of adult mites exposed to UV-C at different exposure times (5 mins, 10 mins, 15 mins, 20 mins, 30 mins and 60 mins) and distances (10 cm, 25 cm, 35 cm, 45 cm and 55 cm). Fresh eggs of the 2 dust mites were also irradiated at 10, 35 and 55 cm for 0.5, 1, 2, 3, and 5 minutes, and observed daily post- irradiation for up to 7 days.
Results
Highest immediate mortality of 100% occurred with direct irradiation at 10 cm distance from UV-C lamp and for 60 mins, for both species of mites. The post 24 hours mean mortality rates were (58.4±17.4)% for D. pteronyssinus and (27.7±9.7)% for D. farinae when irradiated for 1 hour at 55 cm distance under UV-C lamp. When mites were irradiated in the presence of culture media, the highest mortality rates were lower compared to the direct irradiation; at 10 cm distance and 60 mins exposure, the mean mortality was (74.0±6.8)% for D. pteronyssinus and (70.3±6.7)% for D. farinae. Egg hatchability for both species of mites was also notably reduced by greater than 50% following irradiation.
Conclusions
Ultraviolet C irradiation is lethal to an array of organisms by damaging their nucleic acids (DNA and RNA). This study demonstrates the increasing mite mortalities with increasing exposure times and decreasing distances.
Keywords: Physical control, UV-irradiation, Dermatophagoides farina, Dermatophagoides pteronyssinus, Mortality
1. Introduction
House dust mites (HDM) are found in most homes. They are microscopic, eight-legged creatures closely associated with us, but they are not parasitic and do not bite. The concern about HDM is that some species produce allergens affecting humans. The HDM allergens cause allergic symptoms such as asthma and atopic dermatitis in atopic humans. A number of the allergen producing HDM belongs to the family Pyroglyphidae. Pyroglyphid mites usually account for >90% of the mite population in houses[1]. Dermatophagoides farinae and Dermatophagoides pteronyssinus are considered among the most important pyroglyphid mites because of their cosmopolitan occurrence and abundance in homes[2].
There are various approaches for the control of house dust mite and their allergen such as by reducing indoor relative humidity to below 50%, coupled with regular cleaning and use of encasement on mattress and pillows[3]. Several chemicals have been examined in laboratories but their effectiveness in the home is controversial or even if effective, they have not been commercialized for home use because have potential problems of toxicity to non targets such as humans and pets, produce unpleasant odor, damage household items, and unable to penetrate deeply into carpet and upholstery[4]. Physical strategies like irradiation has become an established technique for controlling aeroallergens because of residue free advantages over chemicals[5]. UV irradiation is widely used as a germicide and as an attractant for insects[6], in embryological physiological studies and for the surface disinfection of insect eggs[7]. Wharton[8] reported that UV irradiation (254 nm) killed nymphs of the American cockroach, Periplaneta americana. A number of other investigators also have considered the possibility of using UV rays to control, or at least to suppress development of various aeroallergens and insects[5],[9]. Ultraviolet light is known to damage or kill living organisms because it will destroy the DNA by forming covalent bonds between certain adjacent bases in the DNA, thereby preventing further replication and growth.
Ultraviolet C (UV-C) is a short wavelength (100-280 nm) radiation and is primarily used for the disinfection of air, surfaces and liquids from microbial contaminants[10]. To date the UV-C is the wavelength in germicidal applications and is also recommended by the Centre for Disease Control and Prevention. Ultraviolet light air purification has been used for years by the medical field to sanitize rooms and equipment in order to prevent the spread of illness and disease. High intensity UV light modifies proteins as well, so it is possible that UV light might render an allergen non-allergenic. The efficacy of UV-C had been previously demonstrated against some stored product beetle and mite pests[11]-[14] with sensitivity varying with species and doses. It is, however, difficult to make direct comparisons between studies as the level of UV dose achieved is not always stated and UV intensities vary with light sources. Long lists of bacteria, viruses and moulds also are often quoted to assert the killing power of UV-C. The implication that goes with those long lists is often made that UV-C will be just as effective on HDM.
The aim of this study is to investigate the mortalities induced by UV-C irradiation on eggs and adults of 2 species of HDM, Dermatophagoides pteronyssinus (D. pteronyssinus) and Dermatophagoides farinae (D. farinae).
2. Materials and methods
2.1. Sources of mites
Adult males and females D. pteronyssinus and D. farinae, and their eggs, were obtained from colonies established since 1960 in the Acarology Unit, Institute for Medical Research (IMR), Malaysia. The colonies are reared in small glass bottles and sterile ground rat chow mixed with fish flake is used as culture medium. All bottles are kept in desiccators at (75±3) % relative humidity (RH) and at an average room temperature of (25±2) °C.
2.2. UV-C radiation source
The radiation source was a 30 watts UV germicidal lamp (G30T8, Sankyo Denki, Japan) measuring 88 cm x 2.5 cm, and emitting radiation at a wavelength of 254 nm. The lamp was fixed to the ceiling of a Laminar Flow cabinet (120 cm x 63 cm x 50 cm) that served as a test chamber; another similar cabinet without the lamp was used for controls. Bioassays were conducted at a room temperature of (25±2) °C.
2.3. Bioassay with adult mites for direct exposure
Sets of 30, 15 - 25 days old adult mites of mixed male and female were placed in Petri dishes of 14 cm diameter and 1.5 cm high. The dishes and mites were next placed inside the UV-C chamber and irradiated for different times (5, 10, 15, 20, 30 and 60 mins) and at different distances (10, 25, 35, 45 and 55 cm) from the UV lamp. Controls were similarly treated in the control chamber. Three replicates were tested and the procedure was repeated 3 times for each irradiation time and distance. The exposed mites were examined immediately after irradiation under 400x magnifications and the number of dead mites was recorded. Mites that do not move when gently prodded were considered dead. Irradiation mites were maintained at (75 ± 3) % RH and (25 ± 2) °C and mortalities were determined again after 24 hours.
2.4. Bioassay with adult mites in presence of culture medium
Thirty 15 - 25 days old adult mites of mixed male and female were placed in clean glass Petri dishes, 14.0 cm diameter and 1.5 cm high along with 0.25 g of sterile culture medium. The Petri dishes were then placed inside the UV-C chamber and irradiated at different exposure times and distances as above for direct exposure. Controls were similarly prepared but placed in the control chamber. There were 3 replicates for each treatment and the test was repeated 3 times. The number of dead mites after irradiation was examined under 400x magnification and the immediately mortalities were recorded. Irradiated mites were maintained at (75±3)% RH and (25±2) °C and mortalities were determined again after 24 hours.
2.5. Bioassay with eggs
Ten freshly oviposited eggs were collected using fine applicator sticks and placed in glass Petri dishes, 9.0 cm diameter and 1.2 cm high. The Petri dishes with eggs were placed inside the test chamber and irradiated for 0.5, 1, 2, 3 and 5 mins at distances of 10, 35, and 55 cm, from the UV lamp. Control eggs were similarly irradiated in the control chamber. After treatment, eggs were placed individually in clear glass vials measuring 3.5 cm high and 2.0 cm diameter that were secured with snap caps. The eggs were maintained at (75±3)% RH and (25±2) °C; hatchability was monitored daily for a week. All treatments were replicated 3 times, and the experiment repeated once.
2.6. Data and statistical analysis
Mean mortalities were compared and analyzed by independent sample t-test and one-way ANOVA at 95% confidence level using SPSS ver 11.0[15].
3. Results
3.1. Immediate mortalities of D. pteronyssinus and D. farinae for direct irradiation
Mortality rates for direct irradiation of D. pteronyssinus and D. farinae at difference exposure times and distances are shown in Table 1. No control mites died. Generally, mortality rates for both species, increased with increasing exposure times and decreasing distances. At 10 cm distance from lamp and 60 minutes exposure, 100% mortality resulted in both species of mites. For similar exposure times at 55 cm distance from lamp, there was significant difference among species (P<0.05); the mean mortality rates were (32.5±8.9)% for D. pteronyssinus and (11.0 ± 9.8)% for D. farinae. Increasing the exposure period at each distance significantly increased mortalities (P<0.01) of both species. There was significant increase in D. farinae mortalities at each exposure period with decreasing distances (P<0.01); similar with D. pteronyssinus (P<0.03) except at 15 minutes exposure where the differences were not significant (P=0.16).
Table 1. Immediate mortalities and 24 hours post mortalities (%, mean ± SD) of D. pteronyssinus and D. farinae directly irradiated at difference distances and exposure times.
| Species | Distance (cm) | Immediate mortalities |
24 hours post mortalities |
||||||||||
| 5 min | 10 min | 15 min | 20 min | 30 min | 60 min | 5 min | 10 min | 15 min | 20 min | 30 min | 60 min | ||
| DP | 55 | 0.0±0.0 | 2.2±2.8 | 4.0±4.0 | 5.5±5.5 | 8.8±5.5 | 32.5±8.9 | 1.8±2.3 | 7.0±3.8 | 9.2±6.8 | 12.9±12.2 | 13.6±8.5 | 58.4±17.4 |
| 45 | 0.3±1.1 | 4.7±4.4 | 5.5±4.0 | 11.0±7.0 | 38.1±9.7 | 38.4±9.8 | 2.2±2.8 | 11.8±4.7 | 10.7±2.7 | 15.8±9.8 | 48.1±13.5 | 68.1±12.3 | |
| 35 | 2.2±1.6 | 6.2±4.2 | 7.7±5.9 | 12.9±4.2 | 55.8±9.6 | 74.7±9.7 | 4.7±2.3 | 9.2±6.8 | 12.2±8.9 | 18.1±5.0 | 69.9±7.9 | 98.8±2.3 | |
| 25 | 4.4±2.8 | 8.4±4.1 | 8.4±5.2 | 27.0±9.6 | 69.2±9.5 | 97.0±4.2 | 7.3±5.2 | 11.8±5.2 | 15.5±10.4 | 41.8±12.4 | 80.3±12.4 | 100.0±0.0 | |
| 10 | 4.4±3.3 | 8.8 ±7.6 | 11.0±9.7 | 35.9±9.3 | 88.8±9.6 | 100±0.0 | 8.8±5.2 | 15.5±6.6 | 16.6±11.7 | 54.0±17.2 | 96.6±4.4 | 100.0±0.0 | |
| DF | 55 | 0.0±0.0 | 1.1±2.3 | 1.8±2.3 | 1.1±1.6 | 5.9±4.6 | 11.0±9.8 | 2.2±2.3 | 6.2±3.5 | 8.4±5.0 | 8.5±4.4 | 8.6±6.4 | 27.2±9.7 |
| 45 | 0.3±1.1 | 1.4±3.3 | 2.2±3.3 | 2.9±3.5 | 6.2±3.5 | 16.6±9.9 | 2.5±3.2 | 6.9±5.1 | 9.6±2.6 | 13.6±6.9 | 9.9±6.6 | 26.2±12.3 | |
| 35 | 0.7±1.4 | 1.8±2.3 | 4.0±2.2 | 19.2±8.2 | 44.0±8.6 | 57.3±10.5 | 6.2±3.1 | 7.0±5.6 | 10.3±3.8 | 28.8±11.0 | 52.9±8.4 | 86.9±9.3 | |
| 25 | 3.3±1.6 | 4.7±3.3 | 6.6±2.3 | 19.6±7.8 | 49.6±10.9 | 90.3±10.1 | 8.4±2.9 | 10.3±5.3 | 14.0±4.9 | 29.2±15.0 | 79.2±14.1 | 99.6±1.1 | |
| 10 | 4.4±3.3 | 5.5±3.3 | 7.7±1.7 | 39.2±9.8 | 72.9±10.9 | 100.0±0.0 | 8.4±3.7 | 12.9±4.8 | 14.0±3.6 | 47.3±11.4 | 85.1±14.7 | 100.0±0.0 | |
DP-D. pteronyssinus, DF-D. farinae.
3.2. 24 hours post irradiation mortalities of D. pteronyssinus and D. farinae for direct exposure
D. pteronyssinus and D. farinae mortalities at all distances were significantly different between exposure times (P<0.05) (Table 2); 5 minutes exposure caused the lowest mortalities. At each exposure time, there were significant differences in D. pteronyssinus mortalities between various distance (P<0.01), however the difference were not significant for 15 minutes exposure times (P=0.34). For D. farinae mortalities, there were significant difference for all exposure time (P<0.03). At 60 minutes exposure time and 55 cm distance, the mean mortality rates by species were significantly different from each other (P=0.001).
3.3. Immediate mortalities of D. pteronyssinus and D. farinae in presence of culture media
Overall the mean mortality rates after exposure to UV-C in the presence of culture media for both mites were lower compared to the direct exposure. It is shown in Table 3 that mortalities increased with decreasing distance from the UV-C lamp. There was no death in the controls. At the highest exposure time of 60 min and 10 cm distance, there was no significant difference among species (P=0.97); the mean mortality rate were (74.0±6.8)% for D. pteronyssinus and (70.3±6.7)% for D. farinae. At the same exposure time but 55 cm distance, the mortalities between D. pteronyssinus and D. farinae were not significantly different (P< 0.05). Increasing the time exposed to UV-C at each distance significantly increased the mortalities of both species of mites (P<0.01). Decreasing distance from the UV lamp at each exposure times significantly increased the mortalities of D. pteronyssinus (P<0.01) but for D. farinae mortality significantly increased at each exposure (P<0.05) except at 5 minutes (P=0.07).
Table 3. Immediate mortality rates (mean ± SD) of D. pteronyssinus and D. farinae in the presence of culture media at different exposure period and distances from UV-C lamp.
| Species | Distance (cm) | Exposure time (minutes) |
|||||
| 5 | 10 | 15 | 20 | 30 | 60 | ||
| D. pteronyssinus | 55 | 0.0±0.0 | 0.3±1.1 | 1.1±1.6 | 1.8±2.3 | 2.9±1.9 | 12.1±6.4 |
| 45 | 0.0±0.0 | 0.7±1.4 | 1.1±1.6 | 5.5±4.6 | 5.5±2.3 | 26.6±8.9 | |
| 35 | 1.1±1.6 | 2.9±2.5 | 4.7±3.3 | 10.7±4.0 | 22.5±7.2 | 37.0±8.8 | |
| 25 | 2.5±2.7 | 3.6±3.5 | 7.0±4.5 | 17.7±4.4 | 40.3±6.3 | 69.9±10.0 | |
| 10 | 2.5±2.7 | 4.7±3.3 | 7.7±2.3 | 31.0±8.1 | 64.0±5.4 | 74.0±6.8 | |
| D. farinae | 55 | 0.0±0.0 | 0.0±0.0 | 1.1±1.6 | 0.7±1.4 | 4.0±3.2 | 6.9±3.8 |
| 45 | 0.0±0.0 | 0.3±1.1 | 1.1±1.6 | 2.5±2.2 | 4.7±2.9 | 19.5±7.1 | |
| 35 | 0.7±1.4 | 2.2±2.8 | 2.9±3.0 | 9.2±4.6 | 22.9±8.2 | 30.7±3.2 | |
| 25 | 1.8±2.9 | 1.4±1.7 | 4.4±3.7 | 17.3±4.3 | 36.6±6.2 | 69.9±10.0 | |
| 10 | 1.8±2.3 | 4.0±4.0 | 6.2±3.5 | 34.0±10.8 | 62.5±4.9 | 70.3±6.7 | |
3.4. 24 hours post irradiation mortalities of D. pteronyssinus and D. farinae in presence of culture media
It is shown in Table 4 that D. pteronyssinus and D. farinae mortalities were significantly difference at all exposure times for various distance (P<0.01). At each distance, there was significant differences in D. pteronyssinus and D. farinae mortalities between various exposure times (P<0.05). However, at the 10 cm distance with the longest exposure time the mean mortality rates by species were not significantly different from each other (P=0.40).
Table 4. Hatchability (%) of controls and eggs exposed to UV-C for 5 minutes.
| Species | Distance (cm) | Eggs | Days post irradiation |
||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| D. pteronyssinus | 55 | Control | 0 | 0 | 24 | 60 | 76 | 84 | 88 |
| Irradiated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 35 | Control | 0 | 0 | 0 | 30 | 40 | 54 | 82 | |
| Irradiated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 10 | Control | 0 | 4 | 14 | 32 | 52 | 68 | 74 | |
| Irradiated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| D. farinae | 55 | Control | 0 | 0 | 28 | 56 | 84 | 96 | 96 |
| Irradiated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 35 | Control | 0 | 4 | 4 | 24 | 42 | 52 | 58 | |
| Irradiated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 10 | Control | 0 | 10 | 32 | 46 | 52 | 52 | 60 | |
| Irradiated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
3.5. Egg hatchability
No eggs hatched when exposed to UV-C radiation at any of the exposure times and distances; in comparison, >70% of the control eggs hatched. Most (88%) of the treated eggs were dry on day 3 post irradiation. Microscopic examination immediately after irradiation showed no difference with controls and irradiated eggs that fail to hatch; however day 3 post irradiation, treated eggs were wrinkled and dry (Figure 1). This is probably due to leakage of the inner contents when the chorions of treated egg wrinkled. For D. farinae, 122 out of 150 (71.3%) control eggs were hatched while 81.3% of D. pteronyssinus control eggs were hatched into viable larvae (Table 4).
Figure 1. Microscopic examination of UV-C irradiated eggs.
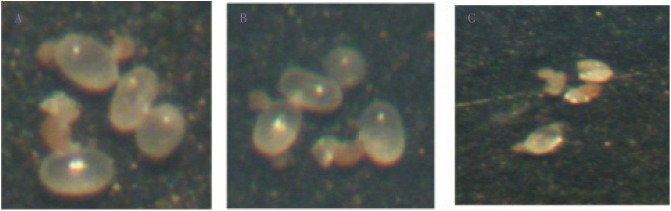
A: before irradiation; B: immediately after 5 minutes irradiation; C: on the third day post irradiation.
4. Discussion
The findings demonstrated UV-C radiation affected the egg stages more than the adult mites. It is possible for ultra-violet radiation to stop the development of house dust mites at early stage of its life cycle because none of the treated eggs hatched. This effect probably was due to the thinness of the chorion and the delicateness of the dust mite eggs in general. Needham et al[16] came out with similar results and concluded that UV-C has potential to break the life cycle of house dust mites by killing the embryonic stage thus stopping the production of allergens. The nature of the UV-C effect on early developmental stages of mites is attributed to transmission of UV energy into the tissues. Provided it reaches the cells, UV-C radiation is able to impair cellular functions directly by damaging DNA, or indirectly by inducing increased formation of reactive free radicals leading to oxidative stress[17].
In the present study, the mortality of irradiated mites was directly proportional to the exposure times but indirectly with distances. Similar increase in adult mortality was reported by Faruki et al[5] working with UV-irradiated of A. diaperinus. This finding agreed also with the results of Faruki et al[14] working with the Almond Moth, Cadra cautella using UV rays mentioned that larval mortality was positively correlated with radiation exposure periods. Begum et al[18] also conducting a similar study with darkling beetle and reported that 100% reduction in the population which was exposed to UV-irradiation at certain times. Germicidal effect of UV-C also would be lethal to mites because of their small size; i.e., their body surface area per weight is large thus accelerate rate of UV absorption and dispersion of damage[19].
Our results also show there was significant difference in immediate and 24 hours post irradiation mortalities between D. pteronyssinus and D. farinae when directly irradiated at longest distance and times. This would suggest that UV-C treatment may have affected the mites quickly and thus killed them immediately. The recovery of mites from irradiation effect was very slow in most cases, which agrees well with other studies[20]. In case of post 24 hours mortality for the same treatment, the mean mortality rates of D. pteronyssinus were significantly higher than D. farinae; it could be due to D. farinae mites more resistance to UV radiation or D. pteronyssinus has been in a poorer condition to begin with.
Although UV-C radiation kill dust mites, its application to control of such house dust mites population in their natural habitat such as mattresses or carpet, is not yet practical because it requires a prolonged exposure and has inherent operational drawbacks which are likely to influence its accuracy[21]. One possible application is combating pest infestations associated with the structure of a building and may serve as a potential new hygiene measure[10],[22]. The limited penetration however precludes its use as a treatment on bulk commodities. It may also be able to offer potential as a surface hygiene in empty stores. The lack of irradiation effect on lower mortality when food was present in this finding demonstrates the limited penetration of UV-C through substrates. Adult mites and eggs concealed in carpeting and dense fabrics may escape exposure to the UV-C light radiation altogether. House dust mites are found most of the time inside mattresses or carpet since the microclimate and dampness are often highly favorable to mites. In order for UV-C treatment to be fully effective, the mites must be directly exposed to the UV-C for the required duration; a shorter distance between the mites and the UV-C source is advantageous. Anything that can shield the mites from exposure, e.g., food particles, dust, debris will affect efficacy. Since mortality rates increase with increasing exposure period, irradiation for more than 1 hour may result in higher mortality rates. When radiation kills or suppress, dust mite colony will be minimal and allergen levels may kept below threshold. However, there is need a balance the effect on the mites with the effect on the store products or house materials since prolonged exposure to UV radiation may reduce the quality of the stored products.
The reduced egg hatching and adult mortality of HDM caused by UV-irradiation is promising from control point of view. It may be concluded that irradiation is a clean method to eliminate mite population and consequently allergen reduction. Practical applications of UV-C within the house environment may, therefore, lie in the treatment of structural and equipment surfaces such as conveyor systems[10]. However, cleaning and weekly vacuuming of carpets and sofas in homes is an important consideration as the presence of particles may affect UV-C efficacy. Repeated UV-C irradiations are probably required because the shorter wavelength rays of UV-C can penetrate only surface of carpets and mattresses -a single UV-C exposure would not eliminate dust mites and its allergen located in deeper layers of mattresses. The cost and safety implications of UV-C irradiation should also be considered and more comprehensive research is needed.
Acknowledgments
The authors wish to thank the Director-General of Health, Ministry of Health Malaysia, for permission to publish this paper.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Kim HK, Yun YK, Ahn YJ. Fumigant toxicity of cassia bark and cassia and cinnamon oil compounds to Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae) Exp Appl Acarol. 2008;44:1–9. doi: 10.1007/s10493-008-9129-y. [DOI] [PubMed] [Google Scholar]
- 2.Platt-Mills TAE, Thomas WR, Aalberse RC, Vervvloet D, Chapman MD. Dust mite allergens and asthma; report of a second international workshop. J Allergy Clin Immunol. 1992;89:1046–1062. doi: 10.1016/0091-6749(92)90228-t. [DOI] [PubMed] [Google Scholar]
- 3.Arlian LG, Neal JS, Morgan MS, Vyszenski-Moher DL, Rapp CM, Alexander AK. Reducing relative humidity is a practical way to control dust mites and their allergens in homes in temperate climate. J Allergy Clin Immunol. 2001;107:99–104. doi: 10.1067/mai.2001.112119. [DOI] [PubMed] [Google Scholar]
- 4.Codina R, Lockey RF, Diwadkar R, Mobly LL, Godfrey S. Disodium octaborate tetrahydrate (DOT) application and vacuum cleaning, a combined strategy to control house dust mites. Allergy. 2003;58:318–324. doi: 10.1034/j.1398-9995.2003.00100.x. [DOI] [PubMed] [Google Scholar]
- 5.Faruki SI, Das DR, Khatun S. Effects of UV-radiation on the larvae of the Lesser Mealworm, Alphitobius diaperinus (panzer) (Coleoptera:Tenebrionidae) and their progeny. J Biol Sci. 2005;5(4):444–448. [Google Scholar]
- 6.Bruce WA. Effect of UV radiation on egg hatch of Plodia interpunctella (Lepidoptera: Pyralidae) J Stored Prod Res. 1975;11:243–244. [Google Scholar]
- 7.Guerra AA, Ouye MT, Bullock HR. Effect of ultraviolet irradiation on egg hatch, subsequent larval development and adult longevity of the tobacco budworm and the bollworm. J Econ Entomol. 1968;61:541–542. [Google Scholar]
- 8.Wharton DRA. Ultraviolet repellent and lethal action on the American cockroach. J Econ Entomol. 1971;64:252–255. [Google Scholar]
- 9.Sharma MK, Dwivedi SC. Investigation on the effects of ultraviolet and infra-red light on the life cycle of Callosobruchus chinensis Linn. J Adv Zool. 1997;18:27–31. [Google Scholar]
- 10.Collins DA, Kitchingman L. The effect of ultraviolet C radiation on stored product pests. 10th International Working Conference of Stored Product Protection. Julius Kuhn-Archiv. 2010;425:632–636. [Google Scholar]
- 11.Calderon M, Navvaro S. Effects of Ultra-violet irradiation on the eggs of Ephestia cautellaa (Wlk.) (Lepidoptera: Phycitidae) J Stored Prod Res. 1971;7:309–311. [Google Scholar]
- 12.Bruce WA, Lum PTM. The effects of UV radiation on stored-product insects. . In: Caswell GH, Boshoff WH, Daramola AM, editors. Ibadan, Nigeria, Savannah, Georgia: Proceedings of the Second International Working Conference on Stored-Product Entomology; 1978. Sep 10-16, pp. 10–16. [Google Scholar]
- 13.Calderon M, Bruce WA, Leecsh LG. Effect of UV radiation on eggs of Tribolium castaneum. Phytoparasitica. 1985;13:179–244. [Google Scholar]
- 14.Faruki SI, Das DR, Khan AR, Khatun M. Effects of ultraviolet (254nm) irradiation on egg hatching, and adult emergence of the flour beetles, Tribolium castaneum, T. confusum and the almond moth, Cadra cautella. J Insects Sci. 2007;7(36):1–6. doi: 10.1673/031.007.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheridan JC, Lyndall GS. Chicago: SPSS Inc; 2002. SPSS: Analysis without anguish version 11.0 for windows. [Google Scholar]
- 16.Needham G, Begg C, Buchanan S. Ultraviolet C exposure in fatal to American house dust mite eggs. J Allergy Cli Immu. 2006;117(2):S28. [Google Scholar]
- 17.Ahmed RG. Damage pattern as function of various types of radiations. Med J Islamic World Academy Sci. 2005;15:135–147. [Google Scholar]
- 18.Begum M, Parween S, Faruki SI. Combined effect of UV-radiation and triflumuron on the progeny of Alphitobius diaperinus (Panzer) (Coleoptera:Tenebrionidae) at different storage period. University Journal of Zoology Rajshahi Univ. 2007;26:45–48. [Google Scholar]
- 19.Suzuki T, Watanabe M, Takeda M. UV tolerance in the two spotted spider mite, Tetranychus urticae. J Insect Physiol. 2009;55:649–654. doi: 10.1016/j.jinsphys.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Wang Y. Photocatalytic effect on plasmid DNA damage under different UV irradiation time. Building & Enviro. 2008;43(3):253–257. [Google Scholar]
- 21.Sinha RP, Hader DP. UV-induced DNA damage and repair: a review. Photochem Photoobiol Sci. 2002;1:225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- 22.Ghanem I, Shamma M. Effect of non-ionizing radiation (UVC) on the development of Trogoderma granarium Everts. J Stored Prod Res. 2007;43:362–366. [Google Scholar]


