Abstract
Objective
To investigate the inhibitory effect of Telfairia occidentalis Hook f. (Curcubitaceae) (T. occidentalis) leaf on key enzyme linked to type-2 diabetes (α - amylase and α - glucosidase) as well as assess the effect of blanching (a commonly practiced food processing technique) of the vegetable on these key enzymes.
Methods
Fresh leaves of T. occidentalis were blanched in hot water for 10 minutes, and the extracts of both the fresh and blanched vegetables were prepared and used for subsequent analysis. The inhibitory effect of the extract on α - amylase and α - glucosidase activities as well as some antioxidant parameter was determined in vitro.
Results
The result revealed that unprocessed T. occidentalis leaf reduce Fe3+ to Fe2+ and also inhibited α - amylase and α - glucosidase activities in a dose dependent manner. However, blanching of the leafy vegetables caused a significant (P<0.05) increase in the antioxidant properties but decrease their ability to inhibit α - amylase and α - glucosidase activities.
Conclusions
This antioxidant properties and enzyme inhibition could be part of the mechanism by which they are used in the treatment/prevention of type-2 diabetes. However, the blanched vegetable reduces their ability to inhibit both α - amylase and α - glucosidase activity in vitro.
Keywords: Blanching, Antioxidants, Vegetables, Telfairia occidentalis, α – amylase, α – glucosidase
1. Introduction
During onset and development of type 2 diabetes, cellular balance of carbohydrate and lipid metabolism is affected by improper glucose metabolism[1]. This improper regulation leads to elevated postprandial blood glucose levels. Prolonged imbalanced homeostasis, for an extended time, results in hyperglycemia leading to onset of noninsulin- dependent type 2 diabetes[2]. Type 2 diabetes is complicated by several factors inherent to the disease process, such as insulin resistance, hyperinsulinemia, impaired insulin secretion, reduced insulin mediated glucose uptake, and utilization[2]. A sudden rise in blood glucose levels, causing hyperglycemia in type 2 diabetes patients happens due to hydrolysis of starch by pancreatic α-amylase and uptake of glucose by intestinal α-glucosidases[3]. An effective strategy for type 2 diabetes management is the strong inhibition of intestinal α-glucosidases and mild inhibition of pancreatic α-amylase[3].
Amylase inhibitors are also known as starch blockers because they contain substances that prevent dietary starch from being absorbed by the body. Starch are complex carbohydrates that cannot be absorbed unless they are first broken down by the digestive enzyme amylase and other, secondary enzymes[4],[5]. Highly concentrated versions of amylase inhibitors did show potential for reducing carbohydrate absorption in humans[3]. Recently, it has been shown that phenolics play a role in mediating amylase inhibition and therefore have potential to contribute to the management of type 2 diabetes[6]. However, previous reports have also indicated that excessive inhibition of pancreatic α-amylase could result in the abnormal bacterial fermentation of undigested carbohydrates in the colon and therefore mild α- amylase inhibition activity is useful[7].
Vegetables contain compounds that are valuable antioxidants and protectants; the main protective action of vegetables has been attributed to the presence of antioxidants, especially antioxidant vitamins including ascorbic acid, α-tocopherol, β-carotene and phenolics[8]. However, numerous studies have conclusively shown that the majority of the antioxidant activity may be from compound such as flavonoids, isoflavone, flavones, anthocyanin, catechin and isocatechin, rather than vitamins C, E and β-carotene[8]. Several green leafy vegetables with high phenolic contents abound in tropical Africa, they are utilized either as condiments or spices in human diets[9]; these vegetables could be harvested at all stages in the process of growth, and could be fed upon in fresh, processed, or semiprocessed forms[10],[11]. They are very rich sources of β-carotene, ascorbic acid, minerals and dietary fiber[12]. Epidemiological analyses in a large Chinese population have revealed that consumption of vegetables is inversely associated with the risk of type 2 diabetes[13].
In Nigeria, green leafy vegetables are not usually consumed in their fresh form unlike fruits; however, they are usually blanched before consumption or in soup preparation[9]. Blanching stops the enzyme action, sets the colour, and shortens the drying and dehydration time[10]. Telfairia occidentalis Hook f. (Curcubitaceae) (T. occidentalis) is a fluted pumpkin and is one of the green leafy vegetables widely consumed in Nigeria[14]. Leaves from this plant constitute an important ingredient in soup making since they are good sources of proteins, vitamins (B-complex), minerals, fatty acids (linoleic and oleic acids), and fibers[10]. The vitamin C content of this plant is about 148.0 mg/100 g of dry matter[10]. T. occidentalis has been reported to protect against cancers of the esophagus, oral cavity, and stomach, to maintain blood vessel flexibility, and to improve circulation in the arteries of smokers[15],[16]. An extract from this plant has been shown to possess antidiabetic activity in both alloxan and streptozotocin diabetic animals[17]. Although a lot had been reported on the chemical characterization of phytoconstituents and antidiabetic properties of tropical green leafy vegetables, limited information is available on the possible mechanism by which T. occidentalis renders its antidiabetic properties. Hence, this study sought to investigate the inhibitory effect of T. occidentalis on key enzyme linked to type-2 diabetes (α - amylase and α - glucosidase) as well as assessing the effect of blanching (a commonly practiced food processing technique) on these key enzymes in order to provide some possible mechanism by which they are used in the management/prevention of type-2 diabetes.
2. Materials and methods
2.1. Sample collection
Fresh samples of T. occidentalis were sourced from the University garden of The Federal University of Technology, Akure. Authentication of the vegetables was carried in the Department of Biology, Federal University of Technology, Akure, Nigeria.
2.2. Chemicals
Chemicals and reagents used such as Hog pancreatic α-amylase, gallic acid, Folin-Ciocalteau's reagent, dinitrosalicylic acid, α-glucosidase, p-nitrophenyl-α-D-glucopyranoside were procured from Sigma-Aldrich, Inc., (St Louis, MO), trichloroacetic acid (TCA), quercetin, DPPH (1,1-diphenyl-2 picrylhydrazyl) were sourced from Sigma-Aldrich, Chemie GmbH (Steinheim, Germany), sodium carbonate, methanol, AlCl3, potassium acetate, potassium ferricyanide, ferric chloride and starch were of analytical grade while the water was glass distilled.
2.3. Preparation of 70% ethanol extract
The inedible parts of the vegetables were removed from the edible parts by hand picking. The edible parts were thoroughly washed in tap water to remove any dirt, chopped into small pieces by table knife. A portion of the chopped vegetables was then blanched for 10 minutes, while the other portion was not. The blanched portion was then drained of water. Both portions were then sun dried and milled to be obtained in a powder form. The powder was extracted with 70% ethanol then, the extract was filtered with Whatman filter paper and the filtrate was concentrated under reduced pressure to give a solid extract. The concentrated extract was further lyophilized. Then, the vegetable extract was reconstituted in distilled water and used for subsequent analysis.
2.4. α - Amylase inhibition assay
The α - amylase inhibitory activity was determined according to the method of Bernfield[18]. Appropriate dilutions of the vegetable extracts (500 µL) and 500 µL of 0.02 mol/L sodium phosphate buffer (pH 6.9 with 0.006 mol/L NaCl) containing Hog pancreatic α-amylase (EC 3.2.1.1) (0.5 mg/mL) were incubated at 25 °C for 10 minutes. Then, 500 µL of 1% starch solution in 0.02 mol/L sodium phosphate buffer (pH 6.9 with 0.006 mol/L NaCl) was added to the reacting mixture. Thereafter, the reaction mixture was incubated at 25 °C for 10 min and stopped with 1.0 mL of dinitrosalicylic acid (DNSA). The mixture was then incubated in a boiling water bath for 5 min, and cooled to room temperature. The reaction mixture was then diluted by adding 10 mL of distilled water, and absorbance measured at 540 nm in the JENWAY UV-Visible spectrophotometer. Then, the α-amylase inhibitory activity was calculated as percentage inhibition.
% Inhibition =[(AbsControl - AbsSamples)/AbsControl] × 100
2.5. α - Glucosidase inhibition assay
The α - glucosidase inhibitory activity was determined according to the method of Apostolidis et al.[19]. Appropriate dilution of the vegetable extracts (50 µL) and 100 µL of α-glucosidase solution was incubated at 25 °Cfor 10 min. Thereafter, 50 µl of 5 mmol/l p-nitrophenyl-α-D-glucopyranoside solution in 0.1 mol/l phosphate buffer (pH 6.9) was added. The reacting mixture was then incubated at 25 °Cfor 5 min, before reading the absorbance at 405 nm in the JENWAY UV-Visible spectrophotometer. Then, the α - glucosidase inhibitory activity was expressed as percentage inhibition.
% Inhibition =[(AbsControl - AbsSamples)/AbsControl] × 100
2.6. Determination of total phenol content
The total phenol content was determined according to the method of Singleton et al.[20]. Briefly, appropriate dilution of the vegetable extracts were oxidized with 2.5 mL 10% Folin-Ciocalteau's reagent (v/v) and neutralized by 2.0 mL of 7.5% sodium carbonate. The reaction mixture was incubated for 40 minutes at 45 °C and the absorbance was measured at 765 nm in the UV-Visible spectrophotometer (Model 6305; Jenway, Bar lo world Scientific, Dunmow, United Kingdom). Then, the total phenol content was subsequently calculated as gallic acid equivalent.
2.7. Determination of total flavonoid content
The total flavonoid content was determined using a slightly modified method reported by Meda et al.[21]. Briefly 0.5 mL of appropriately diluted sample was mixed with 0.5 mL methanol, 50 µL 10% AlCl3, 50 µL 1 M Potassium acetate and 1.4 mL water, and allowed to incubate at room temperature for 30 minutes. The absorbance of the reaction mixture was subsequently measured at 415 nm in the UV-Visible spectrophotometer (Model 6305; Jenway, Barloworld Scientific, Dunmow, United Kingdom). Then, the total flavonoid content was subsequently calculated as quercetin equivalent.
2.8. Determination of reducing property
The reducing property of the vegetable extracts was determined by assessing the ability of the extract to reduce FeCl3 solution as described by Oyaizu[22]. 2.5 mL aliquot was mixed with 2.5 mL 200 mM sodium phosphate buffer (pH 6.6) and 2.5 mL 1% potassium ferricyanide. The mixture was incubated at 50 °C for 20 min. and then 2.5 mL 10 % trichloroacetic acid was added. This mixture was centrifuged at 650 rpm for 10 min. 5 mL of the supernatant was mixed with an equal volume of water and 1 ml 0.1% ferric chloride. The absorbance was measured at 700 nm in the JENWAY UV-Visible spectrophotometer. Then, the ferric reducing antioxidant property was subsequently calculated as ascorbic acid equivalent.
2.9. Data analysis
The result of three replicate experiments were pooled and expressed as mean±standard deviation[23]. A one-way analysis of variance (ANOVA) and Positive analysis was done using Duncan multiple test. Significance was accepted at P<0.05.
3. Results
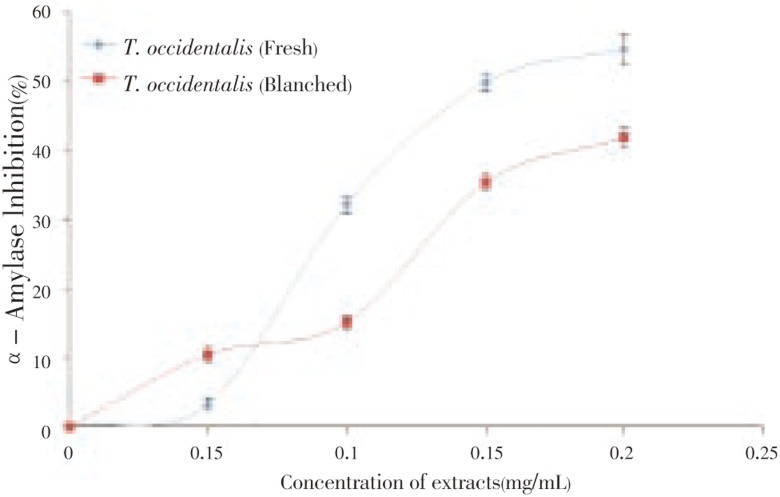
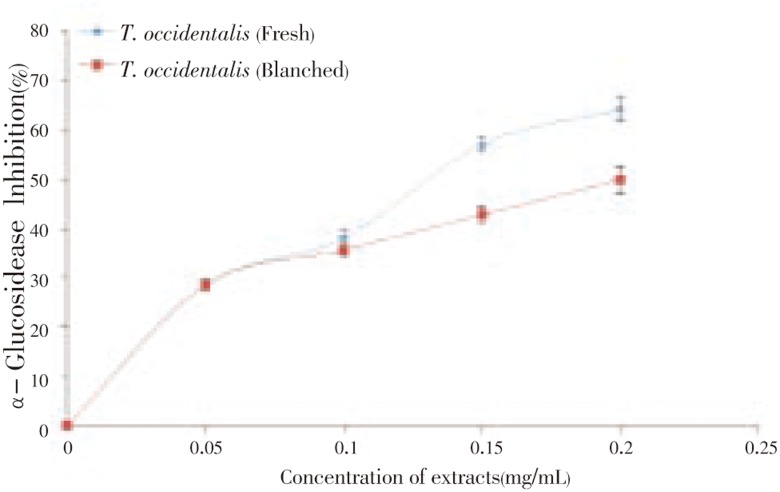
First, the ability of T. occidentalis leaf extract to inhibit α-amylase activity in vitro was investigated and the result is presented in Figure 1. The results revealed that T. occidentalis leaf extracts inhibited α-amylase in a dose-dependent manner (0-0.2 mg/mL). However, as revealed by the EC50 (extract concentration causing 50% enzyme inhibition) values (Table 1), unprocessed T. occidentalis (0.17 mg/mL) had a significantly (P<0.05) higher α-amylase inhibitory activity than blanched T. occidentalis (0.24 mg/mL). In the same vein, the ability of the vegetable extracts to inhibit α-glucosidase activity in vitro was investigated and the result is presented in Figure 2. The results revealed that Telfairia occidentalis leaf extracts inhibited α-glucosidase in a dose-dependent manner (0 - 0.2 mg/mL). However, as revealed by the EC50 values (Table 1), unprocessed T. occidentalis (0.14 mg/mL) had a significantly (P<0.05) higher α-glucosidase inhibitory activity than blanched T. occidentalis (0.18 mg/mL).
Figure 1. α-Amylase inhibitory activity of T. occidentalis leaf extract.
Values represent mean±standard deviation, n = 3
Table 1. EC50 values (mg/mL) of α- Amylase and α- Glucosidase inhibitory activity of T. occidentalis leaf as affected by blanching.
| Samples | α- Amylase (mg/mL) | α- Glucosidase (mg/mL) |
| Fresh | 0.17± 0.05b | 0.14± 0.01a |
| Blanched | 0.24± 0.09c | 0.18±0.08b |
Values represent mean±standard deviation of triplicate experiments.
Values with the same superscript letter along the same column are not significantly different (P<0.05)
Figure 2. α-Glucosidase inhibitory activity of T. occidentalis leaf extract.
Values represent mean±standard deviation, n = 3.
Furthermore, the result of the total phenol and flavonoid content of T. occidentalis leaf is presented in Table 2. The result revealed that unprocessed T. occidentalis leaf had a significantly (P<0.05) higher total phenol (13.0 mg/100g) and flavonoid (7.3 mg/100g) content than blanched T. occidentalis leaf[total phenol (5.8 mg/100g) and flavonoid (1.1 mg/100g) content].
Table 2. Total phenol and flavonoid content of T. occidentalis leaf (mg/100g) as affected by blanching.
| Samples | Total phenol | Total flavonoid |
| Fresh | 13.00±0.30b | 7.3± 0.30a |
| Blanched | 5.80± 0.30c | 1.10± 0.00b |
Values represent mean±standard deviation of triplicate experiments
Values with the same superscript letter along the same column are not significantly different (P<0.05)
Subsequently, the reducing power of T. occidentalis leaf is presented as ascorbic acid equivalent. The result revealed that T. occidentalis leaf was able to reduce Fe (III) to Fe (II). However, blanched T. occidentalis (67.4 mg AAE/100 g) had a significantly (P<0.05) higher reducing power than unprocessed T. occidentalis leaf (61.2 mg AAE/100 g).
4. Discussion
Management of the blood glucose level is a critical strategy in the control of diabetes complications. Inhibitors of saccharide hydrolysing enzymes (α - amylase and α - glucosidase) have been useful as oral hypoglycemic drugs for the control of hyperglycemia especially in patients with type-2 diabetes mellitus. Inhibition of these enzymes delay carbohydrate digestion and prolong overall carbohydrate digestion time, causing a reduction in the rate of glucose absorption and consequently reducing the postprandial plasma glucose rise[3]. First, the ability of T. occidentalis leaf extract to inhibit α-amylase activity in vitro was investigated and the result is presented in Figure 1. The results revealed that T. occidentalis leaf extracts inhibited α-amylase in a dose-dependent manner (0-0.2 mg/mL). However, as revealed by the EC50 (extract concentration causing 50% enzyme inhibition) values (Table 1), unprocessed T. occidentalis (0.17 mg/mL) had a significantly (P<0.05) higher α-amylase inhibitory activity than blanched T. occidentalis (0.24 mg/mL). This significant (P<0.05) decrease in the inhibition of α-amylase activity as a result of blanching of the vegetable could be attributed to the damage/loss of physiologically active phytochemicals having α-amylase inhibitory activities during the heat processes involved in blanching such as observed in phenol content (Table 2). Nevertheless, the determined α-amylase inhibitory activity of the vegetable agreed with some earlier reports where plant phytochemicals from pepper inhibited saliva α-amylase activity[24],[25] and inhibitory effects of Allium spp. on α-amylase activity[26]. This also agreed with a recent worked where red and white ginger inhibited α-amylase activity in vitro[27].
Furthermore, the ability of the vegetable extracts to inhibit α-glucosidase activity in vitro was investigated and the result is presented in Figure 2. The result revealed that T. occidentalis leaf extracts inhibited α-glucosidase in a dose-dependent manner (0 - 0.2 mg/mL). However, as revealed by the EC50 (extract concentration causing 50% enzyme inhibition) values (Table 1), unprocessed T. occidentalis (0.14 mg/mL) had a significantly (P<0.05) higher α-glucosidase inhibitory activity than blanched T. occidentalis (0.18 mg/mL). This significant (P<0.05) decrease in the inhibition of α-amylase activity as a result of blanching of the vegetable could not be categorically stated, however, it could be attributed to the excessive loss of physiologically active phytochemicals as a result of blanching such as observed in Table 2. The determined α-glucosidase inhibitory activity follows the same pattern as observed in Figure 1. This result is in agreement with a recent worked reported by Oboh et al.[27] where red and white ginger inhibited α-glucosidase activity in vitro.
The results of the enzyme (α-amylase and α-glucosidase) inhibitory assays showed that the extracts of the unprocessed and blanched T. occidentalis were strong inhibitors of α-glucosidase, but mild inhibitors of α-amylase as shown in Figures 1 and 2. This however, is in agreement with earlier reports that showed that plant phytochemicals are mild inhibitors of α-amylase and strong inhibitors of α-glucosidase activity[3]. A property that confers advantage over synthetic drugs such as Acarbose; use by diabetics in the management of postprandial blood glucose, which strongly inhibit α-amylase. Stronger inhibition of α-glucosidase activity and mild inhibition of α-amylase activity of the vegetable extracts could address the major drawback of currently used α-glucosidase and α-amylase inhibitor drugs with side effects such as abdominal distention, flatulence, meteorism and possibly diarrhea[28]. It has been suggested that such adverse effects might be caused by the excessive pancreatic α-amylase inhibition resulting in the abnormal bacterial fermentation of undigested carbohydrates in the colon[3]. Therefore, this study buttress the claim that natural inhibitors from dietary plants have mild inhibitory effect on α-amylase activity but strong α-glucosidase inhibitory activity and could be used as effective therapy for the management of postprandial hyperglycemia with minimal side effects[3] this agrees with the finding on eggplant phenolics, which have been recommended as a choice diet for the management of type 2 diabetes[28]. Also agrees with Oboh et al.[27] for ginger varieties and Saliu et al.[29] for bitter leaf extract
The result of the total phenol and flavonoid content of T. occidentalis leaf was observed as reported by Oboh et al.[30]. The result revealed that unprocessed T. occidentalis leaf had a significantly (P<0.05) higher total phenol (13.0 mg/100g) and flavonoid (7.3 mg/100g) content than blanched T. occidentalis leaf[total phenol (5.8 mg/100g) and flavonoid (1.1 mg/100g) content]. The values were lower than what Oboh[10] reported for some tropical green leafy vegetables (1 - 3 mg/g). The difference in phenolic value is as a result of the extraction medium used in the study. However, there was a decrease in the phenolic content due to blanching. The basis of the decrease could not be categorically stated, however, it could be that during blanching some of the phenols would have been leached into the water. However, the result was in agreement with Chen & Lin[31] that phenolics content in cooked yams prepared at different temperatures (50 - 100 °C) was lower compared to the raw ones. Also, this result was in line with Chung et al.[32] that more than 40% of phenolic content in yam peels were lost after blanching at 85 °Cfor 30 seconds.
Phenolic compounds can protect the human body from free radicals, whose formation is associated with the normal metabolism of aerobic cells. They are strong antioxidants capable of removing free radicals, chelate metal catalysts, activate antioxidant enzymes, reduce α-tocophenol radicals and inhibit oxidases[8]. The presence of derivatives of flavonoids has been found in many fruits and vegetables; moreover, numerous studies have conclusively shown that the majority of the antioxidant activity maybe from compounds such as flavonoids, isoflavones, flavones, anthocyanins, catechin and isocatechin rather than from vitamins C, E and β-carotene[33]. Flavonoids have antioxidant activity and could therefore lower cellular oxidative stress[33],[34]. Polyphenols are considered to be strong antioxidants due to the redox properties of their hydroxyl groups[33].
Reducing power is a novel antioxidation defence mechanism; the mechanisms available to affect this property are by electron transfer and hydrogen atom transfer[35]. This is because the ferric-to-ferrous ion reduction occurs rapidly with all reductants with half reaction reduction potentials above that of Fe3+/Fe2+, the values in the Ferric reducing antioxidant property (FRAP) assay will express the corresponding concentration of electron-donating antioxidants[34],[36]. The reducing power of T. occidentalis leaf is presented as ascorbic acid equivalent in Figure 3. The result revealed that T. occidentalis leaf was able to reduce Fe (III) to Fe (II). However, blanched T. occidentalis (67.4 mg AAE/100 g) had a significantly (P<0.05) higher reducing power than unprocessed T. occidentalis leaf (61.2 mg AAE/100 g). The basis for the significant increase in the reducing power could not be categorically stated, however, it could be reasoned out that the temperature at which blanching is carried out would have enhance the activity of the phenolic compound or other Fe3+ reducing agents in the blanched vegetable to the extent that the high phenol content observed in the unprocessed vegetable could not shield their effect.
In conclusion, T. occidentalis leaf exhibited antioxidant properties and inhibited α - amylase and α - glucosidase (key enzyme linked to type-2 diabetes) activities. This antioxidant properties and enzyme inhibition could be part of the possible mechanism by which T. occidentalis leaf is used in the management/prevention of type-2 diabetes. However, blanching of the vegetable could reduce their ability to inhibit both α - amylase and α - glucosidase activity, but could enhance their antioxidant properties in vitro.
Acknowledgments
The authors graciously acknowledge the financial backing of International Foundation for Science (IFS) for granting Dr. G. Oboh the research grant for the execution of this work (IFS Grant Agreement No. E/4625-1).
Footnotes
Foundation Project: The authors graciously acknowledge the financial backing of International Foundation for Science (IFS) for granting Dr. G. Oboh the research grant for the execution of this work (IFS Grant Agreement No. E/4625-1).
Conflict of interest statement: The authors declare no conflict of interest.
References
- 1.Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2010;51(5):993–999. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruenwald J, Freder J, Armbruester N. Cinnamon and health. Crit Rev Food Sci Nutr. 2010;50(9):822–834. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- 3.Kwon YI, Apostolidis E, Kim YC, Shetty K. Health benefits of traditional corn, beans and pumpkin: In vitro studies for hyperglycemia and hypertension management. J Med Food. 2007;10:266–275. doi: 10.1089/jmf.2006.234. [DOI] [PubMed] [Google Scholar]
- 4.Ranilla LG, Kwon YI, Apostolidis E, Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour Technol. 2010;101(12):4676–4689. doi: 10.1016/j.biortech.2010.01.093. [DOI] [PubMed] [Google Scholar]
- 5.El-kaissi S, Sherbeeni S. Pharmacological management of type 2 fiabetes mellitus: An Update. Curr Diabetes Rev. 2011;7(6):392–405. doi: 10.2174/157339911797579160. [DOI] [PubMed] [Google Scholar]
- 6.Cheplick S, Kwon Y, Bhowmik P, Shetty K. Phenolic-linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertension. Bioresource Technol. 2010;101:404–413. doi: 10.1016/j.biortech.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Narwal S, Kumar V, Prakash O. α-glucosidase inhibitor from plants. A natural approach to treat diabetes. Phcog Rev. 2011;5:19–29. doi: 10.4103/0973-7847.79096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oboh G, Rocha JBT. Antioxidant in foods: A new challenge for food processors: Leading edge antioxidants research. New York: Nova Science Publishers Inc; 2007. pp. 35–64. [Google Scholar]
- 9.Akindahunsi AA, Oboh G. Effect of some post-harvest treatments on the bio-vailability of zinc from some selected tropical vegetables. La Riv Ital Delle Sost Grasse. 1999;76:285–287. [Google Scholar]
- 10.Oboh G, Akindahunsi AA. Change in the ascorbic acid, total phenol and antioxidant activity of sun-dried commonly consumed green leafy vegetables in Nigeria. Nutri Health. 2004;18:29–36. doi: 10.1177/026010600401800103. [DOI] [PubMed] [Google Scholar]
- 11.Kasim LS, Ferro VA, Odukoya OA, Drummond A, Ukpo GE, Seidel V, et al. Antimicrobial agents from the leaf of Struchium sparganophora (Linn) Ktze, Asteraceae. J Microbiol Antimicrob. 2011;3(1):13–17. [Google Scholar]
- 12.Makobo ND, Shoko MD, Mtaita TA. Nutrient content of Amaranth (Amaranthus cruentus L.) under different processing and preservation methods. World J Agric Sci. 2010;6:639–643. [Google Scholar]
- 13.Tang GY, Li XJ, Zhang HY. Antidiabetic components contained in vegetables and legumes. Molecules. 2008;13:1189–1194. doi: 10.3390/molecules13051189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adaramoye OA, Achem J, Akintayo OO, Fafunso MA. Hypolipidemic effect of Telfairia occidentalis (Fluted Pumpkin) in rats fed a cholesterol-rich diet. J Med Food. 2007;10(2):330–336. doi: 10.1089/jmf.2006.213. [DOI] [PubMed] [Google Scholar]
- 15.Pinto MDS, Kwon YI, Apostolidis E, Lajolo FM, Genovese MI, Shetty K. Potential of Ginkgo biloba L. leaves in the management of hyperglycemia and hypertension using in vitro models. Bioresour Technol. 2009;100:6599–6609. doi: 10.1016/j.biortech.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Eko ME, Eteng MU, Eyong EU. Phytochemical composition and effect of aqueous extract of Struchium sparganophora (L) on cockroach crude extract induced -airway inflammatory responses in Wistar Rats. Global J Pure Appl Sci. 2008;14(4):29. [Google Scholar]
- 17.Nwozo SO, Adaramoye OA, Ajaiyeoba EO. Anti-diabetic and hypolipidemic studies of Telifairia occidentalis on alloxan-induced diabetic rabbits. Nigerian J Natl Prod Med. 2004;8:37–39. [Google Scholar]
- 18.Bernfield P. Enzymes of starch degradation and synthesis. Adv Enzymol. 1951;12:379–380. doi: 10.1002/9780470122570.ch7. [DOI] [PubMed] [Google Scholar]
- 19.Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit, and funga-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Inn Food Sci Emerg Technol. 2007;8:46–54. [Google Scholar]
- 20.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Cioalteau reagent. Meth Enzymol. 1999;299:152–178. [Google Scholar]
- 21.Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. [Google Scholar]
- 22.Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jap J Nutri. 1996;44:307–315. [Google Scholar]
- 23.Zar JH. Biostatistical analysis. USA: Prentice-Hall Inc; 1984. p. 620. [Google Scholar]
- 24.Oboh G, Ademiluyi AO, Faloye YM. Effect of combination on the antioxidant and inhibitory properties of tropical pepper varieties against α-Amylase and α-Glucosidase activities in vitro. J Med Food. 2011;14(10):1152–1158. doi: 10.1089/jmf.2010.0194. [DOI] [PubMed] [Google Scholar]
- 25.Aguilar-Santamaria L, Ramirez G, Nicasio P, Alegria-Reyes C, Herrera-Arellano A. Antidiabetic activities of Tecoma stans (L.) Juss, ex Kunth. Journal of Ethnopharmacol. 2009;124:284–288. doi: 10.1016/j.jep.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Nickavar B, Yousefian N. Inhibitory effects of six Allium species on α-amylase enzyme activity. Iran J Pharm Res. 2009;8(1):53–57. [Google Scholar]
- 27.Oboh G, Akinyemi AJ, Ademiluyi AO, Adefegha SA. Inhibitory effects of aqueous extract of two varieties of ginger on some key enzymes linked to type-2 diabetes in vitro. J Food Nutri Res. 2010;49(1):14–20. [Google Scholar]
- 28.Pinto MDS, Ranilla LG, Apostolidis E, Lajolo FM, Genovese MI, Shetty K. Evaluation of anti-hyperglycemia and anti-hypertension potential of native Peruvian fruits using in vitro models. J Med Food. 2009;12:278–291. doi: 10.1089/jmf.2008.0113. [DOI] [PubMed] [Google Scholar]
- 29.Saliu JA, Ademiluyi AO, Akinyemi AJ, Oboh G. In vitro antidiabetes and antihypertension properties of phenolic extracts from bitter leaf (Vernonia amygdalina Del.) J Food Biochem. 2011 doi: 10.1111/j.1745-4514.2011.00576. [DOI] [Google Scholar]
- 30.Oboh G, Akinyemi AJ, Ademiluyi AO, Bello FO. Inhibitory effect of some tropical green leafy vegetables on key enzymes linked to Alzheimer's disease and some pro-oxidant induced lipid peroxidation in rats' brain. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YT, Lin KW. Effects of heating temperature on the total phenolic compound, antioxidative ability and the stability of dioscorin of various yam cultivars. Food Chem. 2007;101(3):955–963. [Google Scholar]
- 32.Chung YC, Chiang BH, Wei JH, Wang CK, Chen PC, Hsu CK. Effects of blanching, drying and extraction processes on the antioxidant activity of yam (Dioscorea alata) Int J Food Sci Technol. 2008;43(5):859–864. [Google Scholar]
- 33.Rong T. Chemistry and biochemistry of dietary polyphenol; A Review. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oboh G, Puntel RL, Rocha JBT. Hot pepper (Capsicum annuum, Tepin & Capsicum chinese, Habanero) prevents Fe2+ - induced lipid peroxidation in brain - In vitro. Food Chem. 2007;102(1):178–185. [Google Scholar]
- 35.Dastmalchi K, Dorman HJD, Kosar M, Hiltunen R. Chemical composition and in vitro antioxidant evaluation of a water soluble Moldavian balm (Dracocephalum moldavica L.) extract. Lebensm Wissen Und Technol. 2007;40:239–248. [Google Scholar]
- 36.Oboh G, Akomolafe TL, Adefegha SA, Adetuyi AO. Inhibition of cyclophosphamide induced oxidative stress in brain by polar and non-polar extracts of Annatto (Bixa orellana) seeds. Exp Toxicol Pathol. 2011;63:257–262. doi: 10.1016/j.etp.2010.01.003. [DOI] [PubMed] [Google Scholar]