Abstract
Objective
To evalueate hepatoprotective effects Feronia elephantum (F. elephantum) correa against thioacetamide (TA) induced liver necrosis in diabetic rats.
Methods
Male wistar rats were made diabetic with alloxan (160 mg/kg) on day 0 of the study. They were intoxicated with hepatotoxicant (thioacetamide, 300 mg/kg, ip) on day 9 of study to produce liver necrosis. Effects of 7 day daily once administration (day 2 to day 9) of EF (400 and 800 mg/kg, po) were evaluated on necorosis of liver in terms of mortality, liver volume, liver weight, serum aspartate aminotransferase (AST) and serum alanine transaminase (ALT), and histopathology of liver sections (for signs of necorosis and inflammation) on day-9 of the study. Separate groups of rats with treated only with alloxan (DA control), thioacetamide (TA control) and both (TA+DA control) were maintained.
Results
FE significantly lowered the mortality rate and showed improvement in liver function parameters in TA-induced diabetic rats without change in liver weight, volume and serum glucose levels.
Conclusions
FE showed promising activity against TA-induced liver necorsis in diabetic rats and so might be useful for prevention of liver complications in DM.
Keywords: Feronia elephantum correa, Hepatic necrosis, Diabetes mellitus, Thioacetamide
1. Introduction
The incidence of obesity, diabetes mellitus (DM) and metabolic syndrome has increased in the world, reaching epidemic proportions. Recently, the association between prevalence of type 2 DM and liver diseases is established. Type 2 DM is a risk factor for progressive liver disease and liver-related deaths[1] Adults with newly diagnosed diabetes appeared to be at higher risk of advanced liver diseases. Such diabetes is known as hepatogenous diabetes (HD)[2] and said to be associated with diabetic hepatopathy. Non-alcoholic fatty liver disease (NAFLD), alcohol, hepatitis C virus (HCV) and haemochromatosis are often associated with DM[3]. On the other hand, about 96% of patients with cirrhosis may be glucose intolerant and 30% may be clinically diabetic[3]. Therefore, the aetiology of liver disease is becoming more important in the incidence and management of DM.
The liver has an important role in carbohydrate metabolism since it is responsible for the balance of blood glucose levels by glycogenogenesis and glycogenolysis. In the presence of hepatic disease, the metabolic homeostasis of glucose is impaired because of disorders such as insulin resistance, glucose intolerance and DM[4]. Therefore, treatment of diabetes in presence of the liver cirrhotic patient is complex. The presence metabolic changes due to liver damage and the hepatotoxicity of oral hypoglycemic drugs further complicate the matter. Therefore, pharmacological therapy must be closely monitored for the risk of hypoglycemia[5]. Therefore, management of hepatotoxicity in DM is of great interest from a clinical and mechanistic standpoint
HD is often less associated with microangiopathy, the natural history of DM patients with liver complications is much different from that without liver complications[6]. Patients with HD suffer more frequent and more severe liver complications of cirrhosis. Therefore, specific and effective drugs for HD are need of an hour.
Feronia elephantum (F. elephantum) correa (Synonym: Feronia limonia, Limonia acidissima or Schinus limonia) is plant form Rutaceae family. The fruit is traditionally used in India as a liver and cardiac tonic. Major claim for F. elephantum in traditional literature is and as hepatoprotectant for the treatment for many liver disorders like jaundice. In scientific studies, aqueous extract of F. elephantum (FE) was reported to posses hepatoprotective activity against allyl alcohol, carbon tetrachloride (CCl4)[7], and thioacetamide (TA)[8] induced liver damage with antioxidant potential[9]. Recently, anti-diabetic potential of F. elephantum fruit is also reported in scientific literature[10],[11]. Therefore, we undertook the present study to with the aim to evaluate potential of FE against HD. We evaluated efficacy of FE in rats with alloxan-induced DM and TH-induced severe liver toxicity.
2. Material and methods
2.1. Animals
Wistar male rats (120-200 g) were bought from National Toxicology Centre, Pune, India and used for the study. The rats were divided into groups of six and housed in polypropylene cages with 12 h-12 h light-dark cycle, with free access to standard pellet feed (Chakan Oil Mill, India) and clean drinking water. All experiments were carried out between 08:00 h and 17:00 h in a quiet laboratory with an ambient temperature of (24 ± 1) °C. The research protocol was approved by Institutional Animal Ethics Committee (IAEC) constituted under the norms of Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA), New Delhi, India.
2.2. Drugs and chemicals
Alloxan Monohydrate (Sigma Aldrich, USA), Chloroform (Merck, India), Allyl Alcohol (Hi-Media Lab Private Ltd., Mumbai, India), Thioacetamide (Hi-Media Lab Private Ltd., Mumbai, India), Anaesthetic ether (TKM pharma, Hyderabad, India), and Formaldehyde (Qualigen Fine chemicals, Mumbai, India) were purchased form respective vendors. Diagnostic kit for Glucose estimation was purchased from Accurex Biomedical Pvt. Ltd., Mumbai, India.
2.3. Authentication of plant
F. elephantum leaves were collected from Pune district of Maharashtra, India and authenticated at Agharkar Research Institute, Pune by Dr. A.M. Mujumdar, Head, Botany Department and voucher specimen was deposited at that Institute.
2.4. Preparation of extract
Leaves were dried in shade, powdered and passed through sieve no-22. The obtained fine powder was macerated with sufficient amount of 2.5% chloroform water in a closed percolator for 72 h, shaking often for 6 h and then allowed to stand for sixty-six hours. Sufficient overhead volume of water was kept initially. Then it was allowed to elute and filter through a fine muslin cloth. The filtrate was placed in a round bottom flask and was evaporated below 60 °C under reduced pressure. The obtained residue was collected (yield- 10%) and stored as extract in amber coloured bottle in a cool and dark place. The obtained extract (code name - FE) was kept in airtight amber coloured bottles, stored at room temperature until use. Fresh suspension of FE with the help of tragacanth (4% w/v) was prepared each day of study and used for pharmacological evaluation.
2.5. Treatment schedule
The animals were divided six groups of 6 rats each. Group I was treated as normal control, which did not receive any treatment and was non-diabetic. Group II (called as TA) was treated as hepatotoxin (thioacetamide), and treated only with thioacetamide on day 6 of study and were non-diabetic.
2.6. Induction of diabetes in rats by alloxan[12]
On start of study (day 0), the remaining 4 group of rats were made diabetic with administration of alloxan (160 mg/kg, i.p.). Forty-eight hours later, blood samples were removed by retro-orbital plexus, serum was separated. Rats were considered diabetic if plasma glucose was >200 mg/dL, as measured using glucose oxidase peroxidase (GOD/POD) method. These rats were divided into group III, IV, V and VI, each containing 6 rats.
2.7. Thioacetamide induced liver damage[13]
On day 7 of study, the rats of group III, IV, V and VI were intoxicated with single dose of hepatotoxin (thioacetamide, 300 mg/kg, p.o.) after 1 h of FE administration. Group III (called as DA) was treated as diabetic control and did not receive thioacetamide. Group IV (called as TA+DA) received both thioacetamide and alloxan treatment. Group V and VI received oral treatment of FE in doses of 400 mg/kg and 800 mg/kg, p.o. respectively from day 2 to day 9 of study and had both thioacetamide and alloxan treatment. Mortality of rats was observed. Number of Dead animals in each group were noted and presented as Table 1.
Table 1. Percent (%) survival of normal and diabetic rats during the study period.
| Treatment group | % survival |
||||
| Day 0 | Day 2 | Day 6 | Day 7 | Day 9 | |
| Normal | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| TA control | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| DA control | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| DA+TA | 100.0 | 75.0 | 37.5 | 25.0 | 0.0 |
| DA+TA+FE (400 mg/kg, p.o) | 100.0 | 100.0 | 87.5 | 87.5 | 75.0 |
| DA+TA+FE (800 mg/kg, p.o) | 100.0 | 100.0 | 100.0 | 87.5 | 87.5 |
TA- Thioacetamide, DA - Alloxan treated diabetic, FE - Aqueous extract of F. elephantum. Number of animals in each group are 6-8. The comparison of survival carves done by “Log-rank (Mantel-Cox) Test and Chi-sqaure was found to be P < 0.0001.
2.8. Measurements of parameters
On 9th day of study, the blood was withdrawn by retro-orbital plexus under ether anaesthesia and serum was separated. Rats were euthanatized by cervical dislocation and liver were carefully excised. Weight and volume of livers were measured and livers were preserved in 10% formalin solution. Serum aspartate transaminase (AST), Serum alanine transaminase (ALT) levels and glucose levels were determined, according to the instruction provided in estimation kits (Star diagnostics, Mumbai). Finally histology on liver samples was also carried out for the changes for necrosis, portal inflammation, lobular inflammation, or vascular congestion in the sections of liver are observed.
2.9. Statistical analysis
Mortality data was presented as % survival on each day and analyzed by comparing the survival carves with Log-rank (Mantel-Cox) Test and Chi-sqaure test. The serum and liver parameters were represented as Mean ± SEM and was analyzed by two-way ANOVA followed by Bonferroni tests. P<0.05 was considered significant.
3. Results
3.1. Effect of FE on mortality of rats with diabetes and liver necrosis
Comparison of survival carves by Log-rank (Mantel-Cox) Test and Chi-sqaure showed significant (P<0.000 1) improvement in survival by FE treatment. No mortality was observed in untreated (Group I) or DA (Group II) or TA (Group III) treated rats during the study (Table 1). However, survival in TA treated diabetic animals (in TA+DA) was significantly reduced on day 7 of study (reached to 0% on day 9 of study) (Table 1). On the other hand, FE treatment at 400 and 800 mg/kg, p.o., improved the survival to 75% and 87.5% respectively on day 9 (Table 1).
3.2. Effect of FE on blood glucose level in rats with diabetes and liver necrosis
Two-way ANOVA followed by bonferroni test showed no effect of FE extract in doses tested (400 and 800 mg/kg, p.o.) on serum glucose levels in alloxan induced diabetic rats after 7 days of treatment (Table 2). These results ruled out antihyperglycemic activity of FE.
Table 2. Effect of F. elephantum aqueous extract (FE) on serum glucose levels (mg/dL) in alloxan induced diabetic rats.
| Day of study | Serum glucose levels (mg/dL)±SEM |
||
| DA control | DA+FE (400) | DA+FE (800) | |
| Day 2 (baseline) | 327.65±13.41 | 390.92±48.76 | 408.32±25.57 |
| Day 9 | 349.15±13.67 | 347.13±42.43 | 353.86±27.83 |
TA- Thioacetamide, DA - Alloxan treated diabetic, FE - Aqueous extract of F. elephantum. Data was analyzed by two-way ANOVA followed by “Bonferroni posttests”. Rats were given treatment of alloxan (160 mg/kg, i.p.) and after 48 h later serum glucose levels were measured and taken as baseline values. FE treatment was given from day 2 to day 9 of study (7 day treatment).
3.3. Effect of FE on liver AST, ALT, liver weight and liver volume of rats with liver necrosis in diabetes
Significant (P < 0.001) increase in serum ALT and AST was caused by single dose of thioacetamide (300 mg/kg p.o.) compared to the normal rats (Table 3). FE treatment (at 400 and 800 mg/kg) for 7 days caused significant reversal of AST and ALT values as compared to DA+TA group of rats ((Table 3). However, no such changes occurred in liver weight and liver volume of rats treated with either TA, FE or their combination ((Table 3).
Table 3. Effect of aqueous extract F. elephantum (FE) on physiological parameters of liver on alloxan and/or thioacetamide induced rats.
| Treatment group | AST (U/L) | ALT (U/L) | Liver volume (mL) | Liver weight (g) |
| Normal | 145.38±2.42 | 49.78±1.70 | 7.58±0.03 | 6.57±0.05 |
| TA control | 250.97±4.68aa | 113.98±2.90a | 10.94±0.01 | 8.34±0.02 |
| DA control | 330.83±18.30aa | 265.33±27.29aa | 8.00±0.08 | 6.91±0.09 |
| DA+TA | 1258.00±48.90b,c | 883.67±26.71b,c | 12.52±0.41 | 9.01±0.08 |
| DA+TA+FE (400 mg/kg, p.o) | 632.33±31.86* | 563.83±15.35* | 8.68±0.17 | 7.26±0.03 |
| DA+TA+FE (800 mg/kg, p.o) | 472.50±15.89* | 323.00±20.84* | 7.83±0.20 | 6.72±0.16 |
TA-Thioacetamide, DA-Alloxan treated diabetic, FE-Aqueous extract of F. elephantum. The values are represented as mean±SEM. Data was analyzed by two-way ANOVA followed by “Bonferroni posttests”. a-P<0.05, aa-P<0.001 as compared to normal group, b-P<0.001 as compared to TA and c-P<0.001 as compared to DA, *P<0.001 as compared to (DA+TA) group for respective parameters.
3.4. Effect of FE on liver histopathology of rats with diabetes and liver necrosis
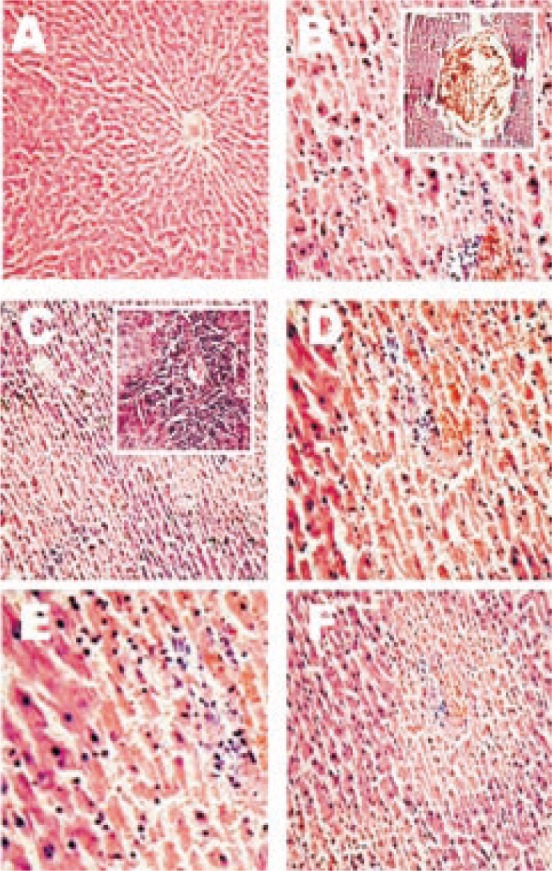
No histopathological changes were observed in liver sections of DA rats. However, severe necrosis (grade 3), portal inflammation (grade 3), lobular inflammation (grade 1), and vascular congestion (grade 1) were observed in livers of T-induced diabetic rats (DA+TA group). These results confirmed TA-induced hepatotoxicity in DA rats. In liver samples of group V and group VI, noticeable decline in severity of necrosis (grade 2 and grade 1 respectively) and lobular inflammation (grade 2) was found (Table 4 and Figure 1). These results are in agreement with results from biochemical (liver function) study and thus confirmed hepatoprotective activity of FE in diabetic rats.
Table 4. Effect of F. elephantum aqueous extract (FE) on thioacetamide (TA) induced liver necorsis in alloxan induced diabetic (DA) rats.
| Treatment | Hepatocellular necrosis | Portal inflammation | Lobular inflammation | Vascular congestion | Hepatocellular regeneration |
| DA control | - | - | - | - | - |
| TA control | +++ | ++ | + | + | + |
| TA+DA+FE (400 mg/kg, p.o) | ++ | + | + | + | + |
| TA+DA+FE (800 mg/kg, p.o) | + | ++ | + | + | + |
TA-Thioacetamide, DA-Alloxan treated diabetic, FE-Aqueous extract of F. elephantum. Photomicrograph of section of livers of rats treated with TA, DA, FE and their combination were taken and microscopically scored for necrotic changes. + mild, ++ moderate and +++ severe. Sections were stained with hematoxylin and eosin (HE) and photographed at magnification of 100 ×.
Figure 1. Effect of F. elephantum aqueous extract (FE) on thioacetamide (TA) induced liver necorsis in alloxan induced diabetic (DA) rats. Photomicrographs shows necrotic and inflammatory changes from livers of (A) DA control (B) DA+TA control (C) FE (400 mg/kg, p.o.) on TA+DA induced and (D) FE (400 mg/kg, p.o.) on TA+DA induced rats. Inset of figure B shows vascular congestion. Inset of figure C shows inflammation of grade 2. Sections were stained with hematoxylin and eosin (HE) and photographed at magnification of 100×.
4. Discussion
Increased incidences of hepatotoxicity have been observed in diabetic patients receiving drug therapies[14]. Type 2 diabetes is known to be predisposing factor for increased hepatotoxicant sensitivity[15]. Therefore, we evaluated effects of FE on liver necrosis caused by TA in diabetic rats. The present study provides evidence for FE protective action of FE against TA-induced liver necrosis in non-diabetic as well as alloxan-induced diabetic (DA) rats.
TA-induced liver damage in animals seems to resemble the important features of human diseases. TA-induced hepatoxicity was known to be potentiated in alloxan[16] or streptozotocin (STZ)[17] induced diabetic rats. The potentiation of liver toxicity by alloxan in rats is more striking than that of STZ[16] and liver function related parameters are is more severe in alloxan model than that of STZ Furthermore, STZ is shown to have resistance is to TA-induced liver injury[18]. Therefore, we used alloxan as a diabetogenic agent in TA-indeced rats as suitable model of HD in the present study.
The dynamic interaction between biotransformation-based liver injury and compensatory tissue repair plays a pivotal role in determining the ultimate outcome of hepatotoxicity initiated by drugs or toxicants. Three possible mechanisms are reported to increase sensitivity of liver necrosis in diabetic animals as compared to non-diabetic animals[19]: (1) Weakning of compensatory liver cell division following hepatotoxicants like TA, and associated liver injury (2) Decreased IL-6 and ERK1/2 MAPK and increased TGFβ1 expression (3) decreased cyclin D1 expression, and lowering of p-pRB, resulted in inhibiting G0/G1 to S-phase clearance of hepatocytes and liver tissue repair. Hepatoprotective effect of FE in the present study might be medicated through these mechanisms.
In the present study, FE showed strong protection against liver necrosis in diabetic rats. FE contains many phytochemcials that can offer protection against liver toxicity[20]. The major among them are flavonoids namely orientin and vitexin. Protective effects on many vital organs were reported by orientin[21],[22] and vitexin[23] by virtue of antioxidant potential[24]. Therefore, protection shown by FE against TA-induced acute liver injury is at least partly contributed by antioxidant activity of orientin and vitexin.
In the present study, FE (leaves extract) did not show antihyperglycemic activity whereas fruits extract is reported to be antidiabetic[10]. However, orientin and vitexin, the major constituents of leaves extract, do not have reports of antidiabetic potential whereas stigmasterol, the major constituent of fruit extract, has been shown glucose regulatory efficacy in diabetic mice[25]. The absence of orientin and vitexin in fruit extract and presence in leaves extract further supports the results of present study.
In conclusion, FE showed hepatoprotective effect against TA-induced liver necrosis in diabetetic rats and can be further explored in prevention HD in DM patients.
Acknowledgments
The authors would like acknowledge Dr. S. S. Kadam, Vice-Chancellor and Dr.K.R. Mahadik, Principal, Poona College of Pharmacy, Bharati Vidyapeeth University, Pune, India for providing necessary facilities to carry out the study. One of the author (PS) received graduate fellowships from All Indian Council for technical education (AICTE) from Government of India during the study.
Footnotes
Conflict of interest statement: Authors have no conflict of interest.
References
- 1.Smith BW, Adams LA. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nat Rev Endocrinol. 2011;7(8):456–465. doi: 10.1038/nrendo.2011.72. [DOI] [PubMed] [Google Scholar]
- 2.Porepa L, Ray JG, Sanchez-Romeu P, Booth GL. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ. 2010;182(11):E526–531. doi: 10.1503/cmaj.092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickman IJ, Macdonald GA. Impact of diabetes on the severity of liver disease. Am J Med. 2007;120(10):829–834. doi: 10.1016/j.amjmed.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen MF. Contribution of defects in glucose production and uptake to carbohydrate intolerance in insulin-resistant subjects. Dan Med Bull. 2008;55(2):89–102. [PubMed] [Google Scholar]
- 5.Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30(3):734–743. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 6.García-Compean D, Jaquez-Quintana JO, Maldonado-Garza H. Hepatogenous diabetes. Current views of an ancient problem. Ann Hepatol. 2009;8(1):13–20. [PubMed] [Google Scholar]
- 7.Kamat CD, Khandelwal KR, Bodhankar SL, Ambavade SD, Mhetre NA. Hepatoprotective activity of leaves of Ferronia elephantum Correa (Rutaceae) against carbontetrachloride-induced liver damage in rats. J Nat Remedies. 2003;3(2):148–154. [Google Scholar]
- 8.Balani I. Surgical treatment of nephrolithiasis of the distopic kidney. Klin Khir. 1998;(4):53–54. [PubMed] [Google Scholar]
- 9.Chitra V. Hepatoprotective and antioxidant activities of fruit pulp of limonia acidissima linn. Int J Health Res. 2010;2(4):361–8. [Google Scholar]
- 10.Mishra A, Garg GP. Antidiabetic activity of fruit pulp of Feronia elephantum Corr. Pharmacogn J. 2011;3(20):27–32. [Google Scholar]
- 11.Gupta R, Johri S, Saxena A. Effect of ethanolic extract of Feronia elephantum Correa fruits on blood glucose levels in normal and streptozotocin-induced diabetic rats. Nat Prod Rediance. 2009;8(1):32–36. [Google Scholar]
- 12.Umar A, Ahmed QU, Muhammad BY, Dogarai BB, Soad SZ. Anti-hyperglycemic activity of the leaves of Tetracera scandens Linn. Merr. (Dilleniaceae) in alloxan induced diabetic rats. J Ethnopharmacol. 2010;131(1):140–145. doi: 10.1016/j.jep.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Manjusha M, Patil KM, Zambare GN, Khandelwal KR, Bodhankar SL. Hepatoprotective activity of aqueous extract of leaves of Feronia elephantum correa against thioacetamide and allyl alcohol intoxication in rats. Toxicol Int. 2004;11:69–74. [Google Scholar]
- 14.Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: Risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15(3):280–288. doi: 10.3748/wjg.15.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pooranaperundevi M, Sumiyabanu M, Viswanathan P, Sundarapandiyan R, Anuradha C. Insulin resistance induced by high-fructose diet potentiates carbon tetrachloride hepatotoxicity. Toxicol Indian Health. 2010;26(2):89. doi: 10.1177/0748233709359273. [DOI] [PubMed] [Google Scholar]
- 16.El-Hawari AM, Plaa GL. Potentiation of thioacetamide-induced hepatotoxicity in alloxan- and streptozotocin-diabetic rats. Toxicol Lett. 1983;17(3-4):293–300. doi: 10.1016/0378-4274(83)90241-2. [DOI] [PubMed] [Google Scholar]
- 17.Sawant SP, Dnyanmote AV, Warbritton A, Latendresse JR, Mehendale HM. Type 2 diabetic rats are sensitive to thioacetamide hepatotoxicity. Toxicol Appl Pharmacol. 2006;211(3):221–232. doi: 10.1016/j.taap.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Shankar K, Vaidya VS, Wang T, Bucci TJ, Mehendale HM. Streptozotocin-induced diabetic mice are resistant to lethal effects of thioacetamide hepatotoxicity. Toxicol Appl Pharmacol. 2003;188(2):122–134. doi: 10.1016/s0041-008x(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 19.Sawant SP, Dnyanmote AV, Mehendale HM. Mechanisms of inhibited liver tissue repair in toxicant challenged type 2 diabetic rats. Toxicology. 2007;232(3):200–215. doi: 10.1016/j.tox.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Intekhab J, Aslam M. Isolation of a flavonoid from Feronia limonia. J Saudi Chem Soc. 2009;13(3):295–298. [Google Scholar]
- 21.Lee HJ, Kim KA, Kang KD, Lee EH, Kim CY, Um BH, et al. The compound isolated from the leaves of Phyllostachys nigra protects oxidative stress-induced retinal ganglion cells death. Food Chem Toxicol. 2010;48(6):1721–1727. doi: 10.1016/j.fct.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 22.Lu N, Zheng XX. Orientin protects cardiomyocytes against reperfusion via mitochondrial calcium uniporter. Appl Mech Mater. 2011;80:757–761. [Google Scholar]
- 23.Dong LY, Chen ZW, Guo Y, Cheng XP, Shao X. Mechanisms of vitexin preconditioning effects on cultured neonatal rat cardiomyocytes with anoxia and reoxygenation. Am J Chin Med. 2008;36(2):385–397. doi: 10.1142/S0192415X08005849. [DOI] [PubMed] [Google Scholar]
- 24.Wu N, Fu K, Fu YJ, Zu YG, Chang FR, Chen YH, et al. Antioxidant activities of extracts and main components of Pigeonpea Cajanus cajan (L.) Millsp.. leaves. Molecules. 2009;14(3):1032–1043. doi: 10.3390/molecules14031032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panda S, Jafri M, Kar A, Meheta BK. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia. 2008;80(2):123–126. doi: 10.1016/j.fitote.2008.12.002. [DOI] [PubMed] [Google Scholar]


