Abstract
Objective
To carry out a preliminary phytochemical, acute oral toxicity and antihepatotoxic study of the roots of Paeonia officinalis (P. officinalis) L.
Methods
Preliminary phytochemical investigation was done as per standard procedures. Acute oral toxicity study was conducted as per OECD 425 guidelines. The antihepatotoxic activity of aqueous extract of root of P. officinalis was evaluated against carbon tetrachloride (CCl4) induced hepatic damage in rats. Aqueous extract of P. officinalis at the dose levels of 100 and 200 mg/kg body weight was administered daily for 14 d in experimental animals. Liver injury was induced chemically, by CCl4 administration (1 mL/kg i.p.). The hepatoprotective activity was assessed using various biochemical parameters like aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum alkaline phosphatase (SALP), total bilirubin and total protein (TP) along with histopathological studies.
Result
Phytochemical screening revealed that the roots of P. officinalis contain alkaloids, tannins, saponins, glycosides, carbohydrates, flavonoids, terpenes, steroids and proteins. The aqueous extract did not cause any mortality up to 2 000 mg/kg. In rats that had received the root extract at the dose of 100 and 200 mg/kg, the substantially elevated AST, ALT, SALP, total bilirubin levels were significantly lowered, respectively, in a dose dependent manner, along with CCl4 while TP levels were elevated in these groups. Histopathology revealed regeneration of the livers in extract treated groups while Silymarin treated rats were almost normal.
Conclusions
The aqueous extract of P. officinalis is safe and possesses antihepatotoxic potential.
Keywords: Paeonia officinalis, Antihepatotoxic, Liver, Peony
1. Introduction
The liver is a vital organ of paramount importance involved in the maintenance of metabolic function and detoxification from the exogenous and endogenous challenges, like xenobiotics, drugs, viral infection and chronic alcoholism. If during all such exposures to these challenges, the natural protective mechanisms of the liver are overpowered, the result is a hepatic injury[1]. Damage to liver is always associated with cellular necrosis and increase in serum levels of many biochemical markers like SGOT, SGPT, ALP and bilirubin[2].
Unavailability of rational therapy in modern medicine and no or very less positive influence of synthetic drugs in liver damage have urged researchers in this field to look for herbal drugs with better hepatoprotective action. Numerous medicinal plants and their formulations are used for liver disorders in ethno medical practices and in traditional system of medicine in India[3]. About 160 phytoconstituents from 101 plants have been reported to possess hepatoprotective activity[4]. In India, about 40 polyherbal commercial formulations are available and prescribed by physicians to treat hepatic disorders[5].
The root of Paeonia officinalis (Ood Saleeb) (P. officinalis) has been used in Unani, Ayurvedic and Homoepathic systems of medicine for years[6],[7]. However, no phytochemical investigation, toxicity study or anti-hepatoprotective study has been carried out on this plant[7].
Carbontetrachloride (CCl4) is a widely used industrial chemical and a potent hepatotoxin. It induces hepatotoxicity by producing free radicals, putting oxidative stress hence causing lipid per oxidation in liver tissues leading to necrotic liver damage[8].
So the present study was undertaken to study the phytochemical, toxicity and antihepatotoxic profile of the roots of P. officinalis against CCl4 induced hepatotoxicity in albino rats.
2. Materials and methods
2.1. Plant material
Dried roots of P. officinalis were obtained from a local Unani Hospital in Kashmir. The plant material was identified and authenticated at Centre for Biodiversity & Taxonomy, University of Kashmir, Srinagar. A sample of the plant material was deposited in the herbarium of the Department of Taxonomy, University of Kashmir under voucher specimen number 1051/KASH for future reference.
2.2. Preparation of extract
Aqueous extract of root of P. officinalis (APO) was prepared by the method given by Alkofahi[9]. Dried P. officinalis roots were pulverized the powdered material (1 kg) was macerated in distilled water for 24 h with occasional shaking and then it was allowed to stand for 18 h. The contents were kept for elution and then filtered. The extract was evaporated to dryness under reduced pressure and controlled temperature (40–50 °C) (yield 100.0 g/kg).
2.3. Preliminary phytochemical screening
The powdered plant material was subjected to preliminary phytochemical screening. The presence of saponins, tannins, alkaloids, flavonoids, anthraquinones, glycosides and reducing sugars were determined by the standard qualitative and quantitative methods[10].
2.4. Animals
Albino rats of Wistar strain, both sexes, weighing 125-150 g, were procured from the animal house of Indian Institute of Integrative Medicine (IIIM) canal road, Jammu. The animals were kept in polypropylene cages (6 in each cage) under standard laboratory conditions (12 h light and 12 h dark: day and night cycle) and had a free access to commercial pelleted diet (Ashirwad Industries) and tap water ad libitum. All studies were performed in accordance with the guide for the care and use of laboratory animals, as adopted and promulgated by the Institutional Animal Care Committee, CPCSEA, India (Reg. No. IAEC/PHARM.S/CL/KU/2012). All the chemicals used were of the analytical grade from standard companies and the water used was always the double distilled water.
2.5. Acute oral toxicity study
Acute toxicity study was carried out in vivo in the albino rats. Solutions of the dried extracts were prepared using 2% gum acacia in distilled water. The study was conducted as per Organization of Economic Cooperation and Development (OECD/OCDE)Test guidelines on Acute Oral Toxicity under a computer-guided Statistical Programme-AOT425statPgm, version 1.0. Up and Down Procedure was conducted, using the dose progression of 175 mg/kg p.o., 550 mg/kg p.o. and 2 000 mg/kg p.o. of the aqueous extract[11].
2.6. CCl4 induced hepatotoxicity
The animals were divided into five groups, each group had six animals. Group 1 served as control animals received a single daily dose of gum acacia (p.o., 1 mL of 20 g/L, body weight)[12]. Group 2 received carbon tetrachloride (i.p., 1 mL/kg body weight, 1:1 v/v mixture of CCl4 and olive oil) alone, group 3 received Silymarin suspension (100 mg/kg body weight p.o.) along with CCl4 as in group 2 rats, while group 4 and 5 received orally 100 and 200 mg/kg body weight of aqueous extracts of P. officinalis in 20 g/L gum acacia, respectively along with carbon tetrachloride as in group second. The aqueous extract was given daily while carbon tetrachloride was given every 72 h for 14 d[13].
2.7. Assessment of hepatoprotective activity
After 14 d of drug treatment, the rats were fasted overnight and on the 15th day the rats were anaesthetized with diethyl ether and blood sample from each animal collected by retro-orbital plexus puncture in sterilized centrifuge tubes. The blood samples were allowed to coagulate at 30 °C for 45 min. Serum was separated by centrifugation at 2 500 r/min at 30 °C for 15 min and subjected to biochemical investigations using standard test kits to assess liver function. The following biochemical investigations were carried out in serum: serum alanine transaminase (ALT)[14], serum aspartate transaminase (AST)[15], serum alkaline phosphatase (ALP)[16], total bilirubin[17], and total serum protein[18].
After collecting the blood samples, the animals from all groups were sacrificed by cervical dislocation. The abdomen of the animals was cut open to remove the liver which was washed with normal saline and then fixed in 10% neutral formalin solution to be processed separately for histological observation.
2.8. Histopathological studies
Thin sections (5 µmol/L) were cut and stained with routine hematoxylin and eosin stain for photo microscopic assessment. The initial examination was qualitative, with the purpose of determining histopathological lesions in liver tissue[19].
2.9. Statistical analysis
All the results were expressed as mean±SEM. One-way analysis of variance (ANOVA) was used for the statistical analysis of data. Student's test was used for determining the significance. A probability value of P<0.05 was considered as significant and P<0.01 was considered highly significant.
3. Results
3.1. Phytochemical investigation
Phytochemical screening revealed that the roots of P. officinalis contain alkaloids, tannins, saponins, glycosides, carbohydrates, flavonoids, terpenes, steroids and proteins.
3.2. Acute oral toxicity study
The aqueous extract did not cause any mortality up to 2 000 mg/kg and was considered as safe.
3.3. Biochemical tests
The effect of APO on serum marker enzymes is presented in Table 1. In the present study, CCl4 given in the dose range of 1 mL/kg bodyweight (along with olive oil 1:1) produced a significant rise in AST, ALT, ALP and serum bilirubin levels and a significant fall in the total protein levels on exposure to CCl4, indicating considerable hepatocellular injury. Administration of aqueous extract of P. officinalis at the doses of 100 and 200 mg/kg produced a highly significant fall in the AST, ALT, ALP, total bilirubin levels and a significant rise in the total protein levels in a dose dependent manner.
Table 1. Effect of aqueous extract of the roots of P. officinalis on biochemical parameters against CCl4 induced hepatotoxicity in rats.
| Treatment | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | TB (mg/dL) | TP (g/dL) |
| Normal control (2% gum acacia, 1 mL/kg p.o.) | 88.75±11.14 | 269.14±12.46 | 122.64±9.78 | 1.48±0.11 | 9.97±1.61 |
| Toxic control (CCl4; 1 mL/kg i.p.) | 379.20±26.13b | 768.60±42.15b | 345.49±22.02b | 7.85±0.64b | 5.21±0.56b |
| CCl4 + Silymarin (100 mg/kg p.o.) | 97.88±5.83c | 284.33±5.40c | 141.39±13.08c | 1.83±0.22c | 15.27±1.01ac |
| CCl4 + 100 mg/kg p.o. of aqueous extract | 120.76±7.91ad | 320.17±6.38bd | 184.04±11.67bc | 4.48±0.41bd | 14.78±1.02ad |
| CCl4 + 200 mg/kg p.o. of aqueous extract | 101.53±5.47d | 295.36±2.88d | 153.06±7.62d | 2.91±0.13d | 19.85±0.86ad |
Data expressed in mean±SEM, n=6. a: P<0.05, b: P<0.01, significant differences from the normal control group; c: P<0.05, d: P<0.01, significant differences from the CCl4 group.
3.4. Histopathological observations
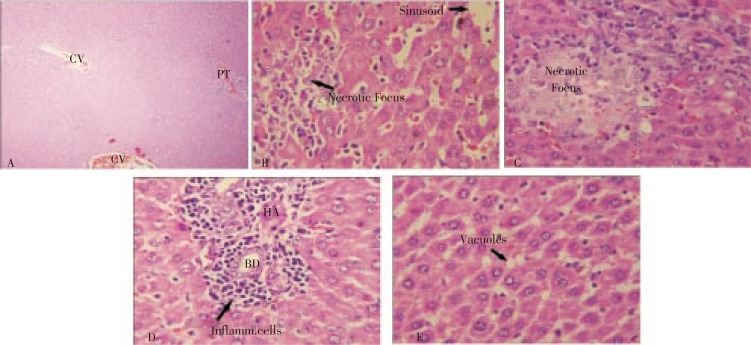
Histopathological examination of the liver slides of rats of normal control showed normal parenchyma and normal portal tract. Livers of the rats administered only CCl4 (toxic control) showed inflammation of the portal triad; fatty change and necrosis of the periportal zone and they also showed necrosis, sinusoidal dilatation, inflammation, hemorrhage and vascular congestion of the centrizonal area. Rat livers treated with CCl4 along with 100 mg/(kg.d) of Silymarin showed almost normal appearance of liver parenchyma (Figure 1).
Figure 1. Histopathology of liver tissues.
(A) Liver from control group rat (CV=central vein, PT=portal triad). (B) Liver from animal treated with CCl4 only. (C) Liver from animal treated with CCl4 and 100 mg/(kg.d) of Silymarin. (D) Liver from animal treated with CCl4 and 100 mg/(kg.d) of aqueous extract of P. officinalis. (E) Liver from animal treated with CCl4 and 200 mg/(kg.d) of aqueous extract of P. officinalis.
However, a necrotic focus was seen in the periportal area. Animals that had received CCl4 along with aqueous extract of 100 mg/(kg.d) of P. officinalis showed periportal inflammation, mild sinusoidal dilatation in the central zone and moderate degree of inflammatory cell infiltration in the portal triad. Liver from animals administered CCl4 along with 200 mg/(kg.d) of aqueous extract of P. officinalis showed showing scattered fatty vacuolation.
4. Discussion
From the results of acute oral toxicity study of the aqueous extract it can be concluded that LD50 of the drug is greater than 2 000 mg/kg bodyweight, which means that even a dose of 2 000 mg/kg bodyweight of aqueous extract of the root powder of P. officinalis is safe for administration.
CCl4 given intraperitoneally, at the dose of 1 mL/kg body weight every 72 h for 14 d, has been reported to produce hepatotoxicity. So, in the present study CCl4 was administered at the dose of 1 mL/kg bodyweight (along with olive oil 1:1) on day one of the study and then every 72 h during 14 d study.
In the present study, CCl4 given in dose of 1 mL/kg bodyweight (along with olive oil 1:1) produced a significant rise in AST, ALT, ALP and serum bilirubin levels and a significant fall in the total protein levels on exposure to CCl4, indicating considerable hepatocellular injury. The aqueous extract of P. officinalis was administered at two dose levels of 100 and 200 mg/(kg.d), for 14 d along with CCl4. The results of this experiment reveal that the extract of the root of P. officinalis has a definitive antihepatotoxic effect (P<0.01) against the deleterious effect of CCl4 upon the structure and function of liver as estimated by various parameters. Both doses (100 and 200 mg/kg) of the aqueous extract effectively attenuated the increased levels of AST, ALT, ALP and total bilirubin; effectively increased the total protein levels produced by CCl4 and caused subsequent recovery towards normalization comparable to the control and the standard group animals. The hepatoprotective effect was pronounced more at the dose of 200 mg/(kg.d) than 100 mg/(kg.d).
Histopathological studies also confirm the hepatoprotective role of the aqueous extract of the roots of P. officinalis in antagonizing the deleterious effect of CCl4 on the histology of liver. While CCl4 treated rats showed extensive histological changes, the animals treated with CCl4 and the extract of P. officinalis concurrently at the doses of 100 mg/(kg.d) and 200 mg/(kg.d) showed only moderate to mild changes. The dose of 200 mg/(kg.d) of the aqueous extract was found to be more effective than the dose of 100 mg/(kg.d) in protecting the liver against the hepatocellular injury caused by CCl4.
The roots of P. officinalis contain alkaloids, tannins, saponins, glycosides, terpenes, flavonoids, carbohydrates, steroids and proteins as revealed by phytochemical screening. The LD50 of aqueous extract of the roots of P. officinalis was found to be greater than 2 000 mg/kg. Aqueous extract of the roots of P. officinalis when administered for 14 d showed hepatoprotective/antihepatotoxic effect against CCl4 induced hepatocellular damage in rats. The dose of 200 mg/(kg.d) of P. officinalis was found to be much more hepatoprotective than 100 mg/(kg.d) dose as evidenced by biochemical parameters and marked regenerative activity observed in rat that received 200 mg/(kg.d) dose. However, Silymarin was found to be more effective as a hepatoprotective than the aqueous extracts of the roots of P. officinalis.
Acknowledgments
The research work was supported by the Department of Pharmaceutical sciences, University of Kashmir.
Comments
Background
The root of P. officinalis has been used in Unani, Ayurvedic and Homoepathic systems of medicine for years. However, no phytochemical investigation, toxicity study or anti-hepatoprotective carried out on this plant.
Research frontiers
This drug is being used as a hepatoprotective in the traditional Indian system of medicines and this is the first scientific study as far as toxicity, pharmacognostic and antihepatotoxic activity is concerned.
Related reports
Phytochemical screening revealed that the roots of P. officinalis contain alkaloids, tannins, saponins, glycosides, carbohydrates, flavonoids, terpenes, steroids and proteins. The aqueous extract did not cause any mortality up to 2 000 mg/kg and was considered as safe. The substantially elevated AST, ALT, SALP, total bilirubin levels were significantly lowered in a dose dependent manner, in rats that had received the root extract at the dose of 100 and 200 mg/kg, respectively, along with CCl4 while TP levels were elevated in these regeneration of the livers in extract treated groups while Silymarin treated rats were almost
Innovations and breakthroughs
This is the first scientific study on this herbal drug and proves the traditional claim of the roots being hepatoprotective.
Applications
This drug has been used for years in the traditional system of medicines, however there was no scientific study conducted on this plant so far. This study has checked the toxicity profile, pharmacognostic constituents, and antihepatotoxic activity.
Peer review
Looking at the biochemical and histopathological results, the work has proven the claim of the traditional system of medicines. The authors have conducted toxicity and pharmacognostic activity, which is very nice and important. I recommend this article to be published.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Latha TB, Srikanth A, Eswar KK, Mastan K, Srinivasa RY, Bhavani B. Comparative hepatoprotective efficacy of kumaryasava and Livfit against carbon tetrachloride induced hepatic damage in rats. Pharmacologyonline. 2009;1:1127–1134. [Google Scholar]
- 2.Wolf PL. Biochemical diagnosis of liver diseases. Ind J Clin Biochem. 1999;14:59–90. doi: 10.1007/BF02869152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haldar PK, Biswas M, Bhattacharya S, Karan T, Ghosh AK. Hepatoprotective activity of Dregea volubilis fruit against paracetamol-induced liver damage in rats. Indian J Pharm Educ Res. 2012;46(1):20–25. [Google Scholar]
- 4.Sreedhar V, Nath LKR, Gopal NM, Raju D, Venugopal K, Sekhar KKR. Hepatoprotective activity of Vitex vestita roots against paracetamol-induced hepatic injury in rats. J Pharm Res. 2011;4(1):288–290. [Google Scholar]
- 5.Bairwa NK, Sethiya NK, Mishra SH. Protective effect of stem bark of Ceiba pentandra linn. against paracetamol-induced hepatotoxicity in rats. Pharmacogn Res. 2010;2(1):26–30. doi: 10.4103/0974-8490.60584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lev E, Amar Z. Practical materia medica of the medieval Eastern Mediterranean according to the Cairo Genizah. Israel: Brill Academic Publishers. 2007:235. [Google Scholar]
- 7.Ahmad F, Tabassum N, Rasool S. Medicinal uses and phytoconstituents of Paeonia officinalis. IRJP. 2012;3(4):85–87. [Google Scholar]
- 8.Dawkins MJR. Carbon tetrachloride poisoning in the liver of the new-born rat. J Path Bact. 1963;85:189. doi: 10.1002/path.1700850118. [DOI] [PubMed] [Google Scholar]
- 9.Alkofahi A, Masaadeh H, Al-Khalil S. Antimicrobial evaluation of some plant extracts of traditional medicine of Jordan. Alex J Pharm Sci. 1996;10:123. [Google Scholar]
- 10.Trease GE, Evans WC. Pharmacognosy. 16th ed. London: Bailliere Tindall Ltd; 2009. pp. 60–75. [Google Scholar]
- 11.Acute Oral Toxicity (AOT) (OECD Test Guideline 425) Statistical Programme (AOT425StatPgm) 2001. Version: 1.0 ( http://www.oecd.org/oecd/pages/home/displaygeneral/0,3380,EN-document-524-nodirectorate-no-24-6775-8,FF.html.
- 12.Eesha BR, Mohanbabu AV, Meena KK, Sarath B, Vijay M, Lalit M, et al. et al. Hepatoprotective activity of Terminalia paniculata against paracetamol induced hepatocellular damage in Wistar albino rats. Asian Pac J Trop Med. 2011;4(6):466–469. doi: 10.1016/S1995-7645(11)60127-2. [DOI] [PubMed] [Google Scholar]
- 13.Verma N, Singh AP, Amresh G, Sahu PK, Rao ChV. Protective effect of ethyl acetate fraction of Rhododendron arboreum flowers against carbon tetrachloride-induced hepatotoxicity in experimental models. Indian J Pharmacol. 2011;43(3):291–295. doi: 10.4103/0253-7613.81518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergmeyer HV, Horder M. IFCC methods for measurement of catalytic concentrations of enzymes. Clin Chim Acta. 1980;105:147–172. [PubMed] [Google Scholar]
- 15.Bergmeyer HV, Bowers GN, Horder M, Mas AW. Optimization of methods for aspartate aminotransferase and alanine amino transferase. Clin Chem Acta. 1978;24:58–73. [PubMed] [Google Scholar]
- 16.Rick W. Klinische chemie und miroskopie. 6th ed. Berlin: Springer Verlag; 1990. p. 294. [Google Scholar]
- 17.Jendrassik L, Grof P. Modified Jendrassik and Grof's method. Biochem. 1938;2(297):81. [Google Scholar]
- 18.Doumas BT. Standards for total serum protein assays-a collaborative study. Clin Chem. 1975;21:1159–1166. [PubMed] [Google Scholar]
- 19.Sabarense CM, Rocha KS, Rosa DD, Martins JH, Pereira MM, Silva FF, et al. et al. A new computational method for hepatic fat microvesicles counting in histological study in rats. Biochem Biophys Res Commun. 2012;418(2):284–289. doi: 10.1016/j.bbrc.2012.01.011. [DOI] [PubMed] [Google Scholar]