Summary
Type IV pili (Tfp) are polar surface structures of Pseudomonas aeruginosa required for twitching motility, biofilm formation and adherence. One protein required for the assembly of tfp is FimX, which possesses both GGDEF and EAL domains characteristic of diguanylate cyclases and phosphodiesterases respectively. In this work we demonstrate that FimX has phosphodiesterase activity towards bis-(3′-5′)-cyclic dimeric guanosine monophosphate (c-di-GMP), but does not show diguanylate cyclase activity. Instead, the imperfect GGDEF domain of FimX likely serves to activate phosphodiesterase activity when bound to GTP, as has recently been described for the Caulobacter crescentus composite GGDEF-EAL protein, CC3396. Bacteria expressing FimX in which either the GGDEF or EAL domain is deleted or mutated have phenotypes indistinguishable from a ΔfimX strain, demonstrating the importance of both domains to function. Previous work has shown that FimX localizes to the bacterial pole. In this work we show that restriction of FimX to a single pole requires intact GGDEF and EAL domains. Deletion of the amino-terminal REC domain of FimX, which contains a putative polar localization signal, results in a protein that still supports intermediate levels of pilus assembly and function. RFP–FimXΔREC, unlike RFP–FimX, is no longer localized to the bacterial pole, while transmission electron microscopy shows that surface pili can originate from non-polar sites in this mutant. Although ΔfimX mutants show limited in vitro cytotoxicity, they are as virulent as the wild-type strain in a murine model of acute pneumonia.
Introduction
The Gram-negative bacterium Pseudomonas aeruginosa is a ubiquitous environmental organism which causes a variety of opportunistic infections in human hosts. These include severe acute infections in individuals with local or systemic compromise of host defences, such as burn patients, persons on mechanical ventilators, or patients receiving chemotherapy. P. aeruginosa also causes chronic infections associated with significant morbidity and mortality in patients with cystic fibrosis. P. aeruginosa can infect a broad spectrum of model organisms, including plants, nematodes, insects and mice, and employs a large set of virulence factors to enable colonization, persistence and dissemination in these varied sites. These include surface organelles, namely type IV pili (tfp) and flagella, which play important roles in motility, adhesion and biofilm formation; secreted degradative enzymes and toxins, which lead to tissue damage and cell death; and translocated substrates of the type III secretion system (TTSS), which disrupt host signal transduction pathways and cause cytotoxicity and necrosis (Salyers and Whitt, 2002). The expression of these virulence factors is regulated in response to incompletely understood environmental cues. The proteins which sense and respond to such environmental stimuli are of great interest, both as keys to understanding basic pathogenic mechanisms of Pseudomonas and as targets for therapies that might disrupt pathogenesis.
Type IV pili are thin, flexible filaments localized to the poles of P. aeruginosa (recently reviewed in Mattick, 2002). Through a cycle of extension, attachment and retraction, tfp mediate bacterial movement over solid surfaces in a process called twitching motility (Bradley, 1980; Merz et al., 2000). Tfp also serve as receptors for bacteriophages (Bradley, 1980), facilitate bacterial attachment to eukaryotic cell surfaces (Woods et al., 1980; Doig et al., 1988; Chi et al., 1991; Comolli et al., 1999a), and contribute to biofilm formation (O’Toole and Kolter, 1998; Klausen et al., 2003a,b). Tfp are polymers composed of pilin, the product of the pilA gene (Sastry et al., 1985). The assembly of functional pili on the bacterial surface requires approximately 40 gene products (Mattick, 2002). Many of these proteins are required for assembly (extension) and retraction of the pilus; however, another group of proteins appears to play regulatory roles, controlling either the expression of proteins required for tfp assembly or regulating tfp-dependent motility in response to environmental cues. Tfp expression appears to be co-ordinately regulated with that of other virulence factors primarily via Vfr, a cAMP-binding protein which affects not only tfp and flagellar biosynthesis, but also the expression of Type II general secretory pathway substrates, TTSS substrates and apparatus, and quorum sensing. Other regulatory proteins involved in tfp assembly include the sensor kinase/response regulator pairs PilS/PilR and FimS/AlgR, the complex multidomain regulator ChpA (Whitchurch et al., 2004), and FimL, a recently described regulator which may function by controlling vfr expression (Whitchurch et al., 2005).
In this work we focus on FimX, a protein which appears to have a regulatory role in surface assembly of pili (Huang et al., 2003). As noted by Huang et al., FimX possesses GGDEF (aa 260–424, E-value 3.8e−12) and EAL (aa 439–683, E-value 3.4e−41) domains (http://pfam.wustl.edu), which are found in more than 2000 Gram-positive and Gram-negative bacterial proteins (Romling et al., 2005). A significant number of GGDEF and EAL domain proteins described to date appear to play roles in the co-ordinate regulation of motility, adherence, and complex multicellular phenotypes such as biofilm formation, colony morphology and autolysis. GGDEF and EAL domain proteins also appear to be relevant to host-pathogen interactions. A number of proteins with GGDEF domains have now been shown act as diguanylate cyclases (DGCs), carrying out the synthesis of bis-(3′-5′)-cyclic dimeric guanosine monophosphate (c-di-GMP) from two molecules of GTP (Paul et al., 2004). The EAL domain has recently been shown to be sufficient for the hydrolysis of c-di-GMP into linear dimeric GMP, pGpG, and thus possesses phosphodiesterase-A (PDE-A) activity (Schmidt et al., 2005). Although many proteins possess both GGDEF and EAL domains, all such dual domain proteins tested for enzymatic activity in vitro appear to show only one activity; however, there are in vivo observations which suggest that GGDEF-EAL domain proteins may exhibit both (Romling et al., 2005). The amino-terminus of FimX contains REC (E-value 0.0018) and PAS domains (E-value 4.9e−6). REC, or CheY domains, are commonly found in response regulators of two component phosphorelays, and characteristically have an aspartate residue that serves as a phosphoryl acceptor (Hoch and Silhavy, 1995). The REC domain of FimX (aa 8–127) lacks a candidate catalytic aspartate, suggesting that this domain is unlikely to be activated by phosphorylation; however, a possible polar localization domain, as mapped by Huang et al. (2003), resides at the end of the REC domain. PAS domains (aa 144–208) are commonly found in signalling proteins and appear to allow oxygen and redox potential to be sensed (Zhulin and Taylor, 1997).
We have constructed and analysed a series of domain deletion and point mutants of FimX. We report here that both GGDEF and EAL domains of FimX appear to contribute to its biological function in P. aeruginosa. Purified FimX exhibits in vitro phosphodiesterase activity which is strongly stimulated in the presence of GTP, but lacks detectable DGC activity. Mutation of the EAL domain abolishes phosphodiesterase activity; mutation of the GGDEF domain does likewise. Lastly, we present data demonstrating that intact GGDEF and EAL domains are required for the observed localization of FimX to a single bacterial pole.
Results
ΔfimX bacteria have defects in assembly of tfp and in twitching motility
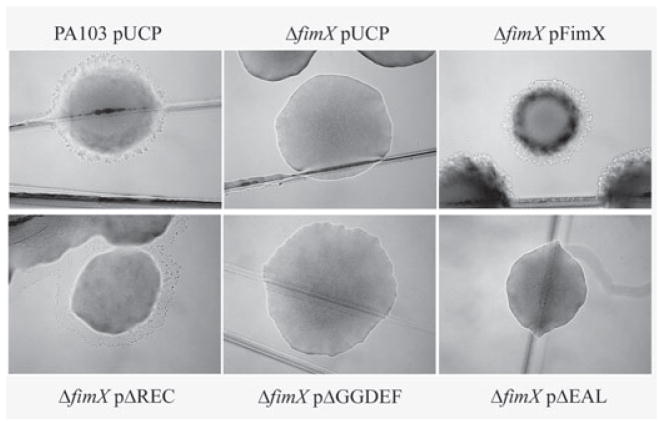
We initially discovered FimX (PA4959) as the site of a transposon insertion that rendered PA103pscJ::SMIT, an invasive, TTSS− strain, no longer invasive for epithelial cells (B.I. Kazmierczak, M.B. Lebron, C. Nelson, R. Kowal, M. Ledizet, J.N. Engel, unpubl. data). We subsequently constructed an unmarked deletion of aa 15–685 of FimX in PA103, a well-characterized clinical isolate of P. aeruginosa that is highly cytotoxic towards cultured epithelial cells. PA103 expresses tfp, surface appendages required for twitching motility, sensitivity to the pilus-specific phage PO4 and biofilm formation (Bradley, 1980; O’Toole and Kolter, 1998). We found that ΔfimX bacteria showed diminished twitching motility as measured by sub-surface stab assays (Table 1), altered colony morphology (Fig. 1) and decreased phage sensitivity (Table 1). These defects were fully complemented by expression of FimX, either from a high-copy number plasmid or from a single copy of the gene integrated at the chromosomal attB site. Our findings corroborate those of Huang et al. who independently discovered FimX in a screen for mutants of PAK that had lost the ability to twitch and published their findings while this work was in progress (Huang et al., 2003). The loss of twitching motility in ΔfimX is due to a failure to assemble surface pili; the pilin subunit, PilA, is still produced in this mutant (Huang et al., 2003).
Table 1.
Tfp-dependent phenotypes of bacterial strains.
| Strain | Efficiency of plaque formationa | Twitching zone (mm)b |
|---|---|---|
| PA103 pUCP | 1.0 | 4.9 ± 0.4 |
| ΔpilA | < 1 × 10−9 | ND |
| ΔfimX pUCP | 0.017 ± 0.008 | 0.7 ± 0.2 |
| ΔfimX pFimX | 1.13 ± 0.16 | 6.1 ± 0.2 |
| ΔfimX pFimXΔREC | 0.73 ± 0.15 | 3.3 ± 0.2 |
| ΔfimX pFimXΔGGDEF | 0.010 ± 0.003 | 3.0 ± 0.6 |
| ΔfimX pFimXΔEAL | 0.0072 ± 0.0051 | 1.8 ± 0.3 |
| ΔfimX pFimX(GDSIF→AASIF) | 0.043 ± 0.015 | 2.5 ± 0.4 |
| ΔfimX pFimX(EVL→AAA) | 0.012 ± 0.009 | 1.6 ± 0.3 |
Efficiency of PO4 plaque formation expressed relative to strain PA103. Mean ± SD of three to six independent experiments.
Twitching motility zone diameter, as measured by subsurface stab assay, performed with six replicates. Strains were assayed three to five times; mean ± SD of a typical experiment are shown.
Fig. 1.
FimX is required for normal colony morphology and twitching motility. PA103 and ΔfimX strains were streaked to LB agar plates supplemented with carbenicillin (200 μg m−1). Colonies were photographed under brightfield illumination at 40μ magnification after incubation at 37°C overnight followed by 24 h incubation at room temperature.
We also constructed an epitope-tagged version of FimX, BB2–FimX, in which the BB2 epitope tag (EVHTNQDPLD) (Brookman et al., 1995) was introduced after the initiating methionine of FimX by PCR. We confirmed that BB2–FimX complemented a ΔfimX mutant fully for phage sensitivity and twitching motility (data not shown). We also constructed BB2 epitope-tagged versions of all mutant alleles of FimX which we subsequently designed, allowing us to determine protein levels for these mutant constructs.
Construction and expression of domain deletion mutants of FimX
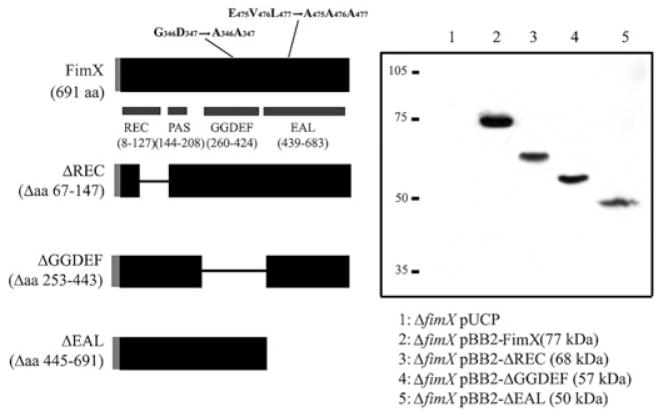
FimX possesses REC (response regulator), PAS, GGDEF and EAL domains, as defined in the Pfam 17.0 (March 2005) database (http://pfam.wustl.edu). In order to test whether these domains are required for FimX function, we constructed a series of deletion mutants lacking aa 67–147 (ΔREC), aa 253–443 (ΔGGDEF) or aa 445–691 (ΔEAL) (Fig. 2A). These mutations were introduced into native FimX and into the BB2 epitope-tagged FimX construct. All constructs were expressed from a high-copy number plasmid (under control of the native promoter) and as a single gene copy integrated into the chromosome at the attB site, also under control of the fimX promoter. As seen in Fig. 2B, all of the domain deletion constructs appear to be stably expressed in PA103 at comparable steady-state levels.
Fig. 2. Construction of FimX domain deletion mutants.
A. Schematic showing domains of FimX. Amino acids deleted in each construct (inclusive) or mutated are indicated. The grey bar represents the amino-terminal BB2 epitope tag present in each construct.
B. Western blot of whole-cell lysates prepared from ΔfimX strains carrying indicated FimX constructs. Samples were normalized to total protein, separated by SDS-PAGE and transferred to PVDF membranes prior to incubation with anti-BB2 mAb. Calculated molecular weights for each construct are given in parentheses. Migration of molecular weight markers is indicated at left of gel.
Both GGDEF and EAL domains are required for twitching motility and phage sensitivity
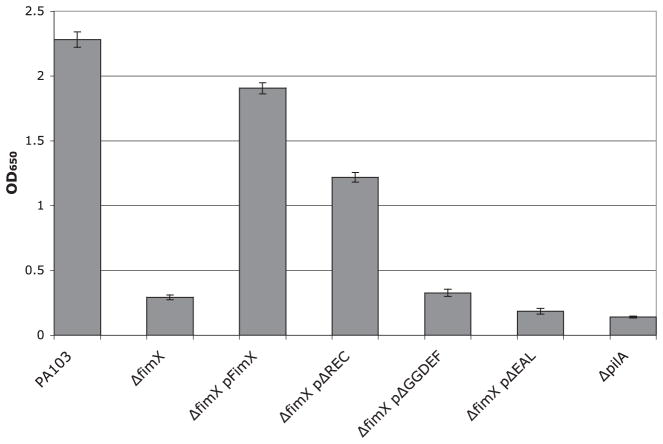
The ΔfimX mutant fails to assemble functional pili. We assayed whether the ΔREC, ΔGGDEF or ΔEAL derivatives of FimX could assemble functional pili. As seen in Table 1 and Fig. 1, the ΔREC mutant appears to restore partial pilus function, as assayed by sensitivity to phage PO4 and by colony morphology. We can also detect surface pili on ΔfimX bacteria expressing FimXΔREC (Fig. 3); however, organized movement of bacteria, as assayed by twitching motility zone size, is not fully complemented. ΔfimX bacteria expressing FimXΔGGDEF have similar levels of surface pilin expression and phage sensitivity as the uncomplemented mutant. Expression of the FimXΔEAL allele reproducibly resulted in decreased levels of surface pili and phage sensitivity as compared with the ΔfimX strain.
Fig. 3.
Total surface pili as determined by ELISA. Bacterial strains were harvested by scraping from LB agar plates after growth overnight at 37°C and resuspended in MEM-lite. Bacterial suspensions were normalized by OD600, then serially diluted. Wells were coated in triplicate with diluted bacteria, fixed, and incubated with primary and secondary antibodies as described in Experimental procedures. Bars indicate mean ± SD of samples diluted to OD600 = 0.003 and are representative of four independent assays.
Point mutations in the GGDEF and EAL motifs recapitulate ΔGGDEF and ΔEAL phenotypes
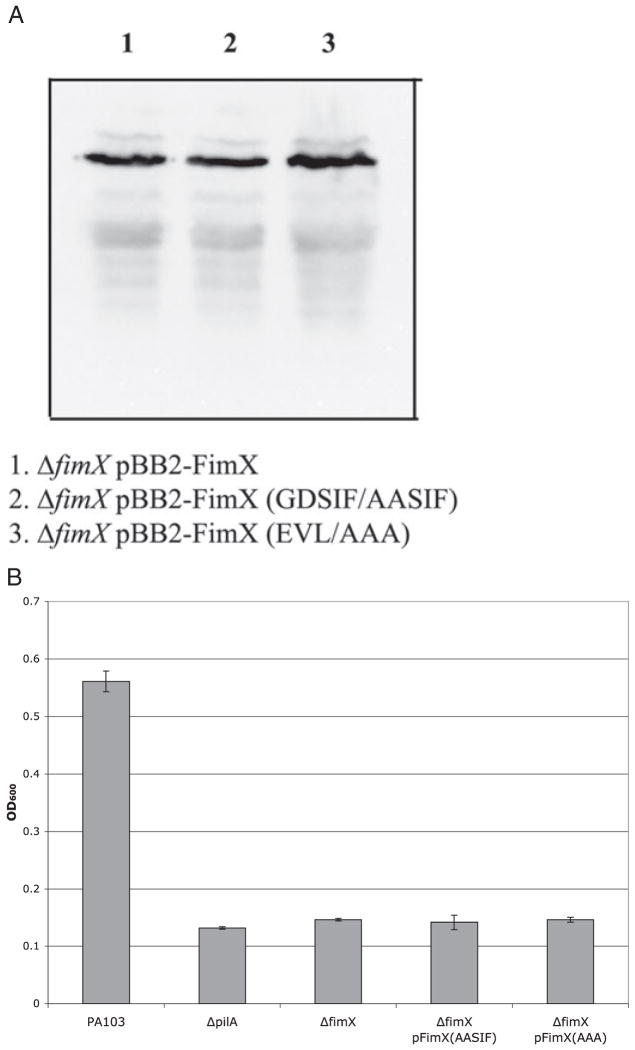
The protein crystal structure of a diguanylate cyclase, PleD, has recently been published (Chan et al., 2004). This structure demonstrates the importance of residues within the GGDEF motif for contacts crucial to substrate binding and catalysis. The corresponding amino acids in FimX are GDSIF, which raises the question of whether this protein is a true DGC. Using site-directed mutagenesis, we substituted alanines for both Gly346 and Asp347 (GDSIF→AASIF) to test the importance of this motif for FimX function. The sequence of FimX at the proposed PDE active site is EVL, which is a variant of EAL found in proteins with documented PDE activity (Schmidt et al., 2005). However, a recent analysis by Gomelsky and colleagues attempted to identify additional sequences within EAL domains which would distinguish whether the proteins were likely to show phosphodiesterase activity (Schmidt et al., 2005). Many of these conserved residues are not present in FimX, raising the question of whether the protein is likely to have an active EAL domain. We therefore mutated the amino acids Glu475, Val476 and Leu477 to alanines (EVL→AAA) to test whether the phosphodiesterase domain of the protein contributes to its activity. Both mutations were introduced into ΔfimX bacteria as native and BB2-tagged proteins expressed under the fimX promoter on a high-copy number plasmid. Steady-state protein levels of both FimX(GDSIF→AASIF) and FimX(EVL→AAA) were comparable with those of wild-type protein (Fig. 4A). However, the phenotypes of bacteria expressing these mutant alleles of FimX were indistinguishable from the null mutant. Neither FimX(GDSIF→AASIF) nor FimX(EVL→AAA) restored twitching activity or PO4 phage sensitivity to ΔfimX bacteria (Table 1). Determination of surface pili by enzyme-linked immunosorbent assay (ELISA) showed that bacteria expressing either point mutant allele of FimX failed to assemble surface pili (Fig. 4B). Thus the GGDEF and EAL domains appear to be critical for FimX function in vivo, and both the EVL and non-canonical GDSIF motifs are functionally essential.
Fig. 4.
A. Expression of FimX point mutants. Whole-cell lysates were prepared from indicated bacterial strains. Samples were normalized to total protein prior to separation by SDS-PAGE, transferred to PVDF membrane and incubated with anti-BB2 mAb as detailed in Experimental procedures.
B. Expression of surface pili as measured by ELISA. Strains were harvested by scraping from agar plates, resuspended in MEM-lite, normalized to OD600, and serially diluted. Wells were coated in triplicate, fixed, and incubated with primary and secondary antibodies as detailed in Experimental procedures. Bars indicate mean ± SD of samples diluted to OD600 = 0.003 and are representative of three independent assays.
FimX exhibits phosphodiesterase, but not DGC, activity in vitro
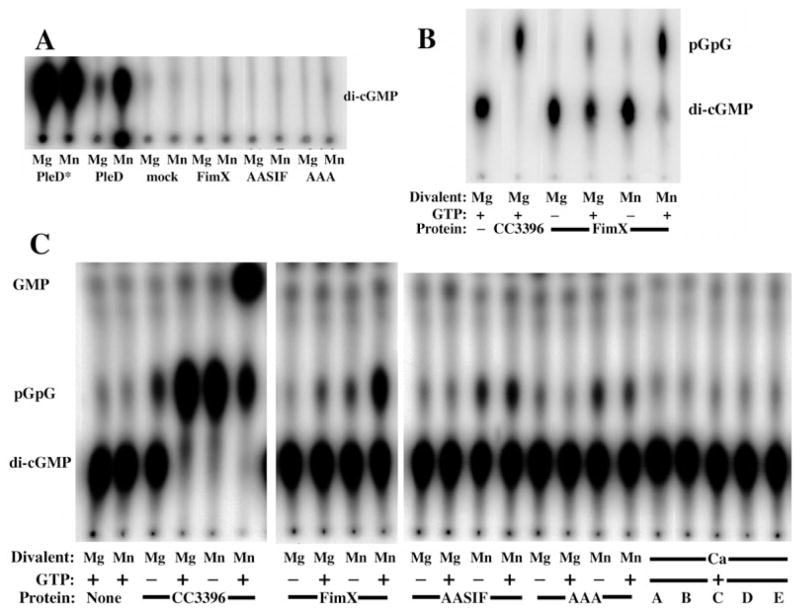
Wild-type FimX, FimX(GDSIF→AASIF) and FimX(EVL→AAA) were expressed as His6 fusion proteins in Escherichia coli, purified by affinity chromatography and assayed to determine whether these proteins possess detectable DGC and/or phosphodiesterase activity. As shown in Fig. 5A, neither wild-type nor mutant alleles of His6-FimX could catalyse c-di-GMP formation. We were readily able to detect DGC activity of the Caulobacter crescentus proteins PleD-His6 and PleD*-His6; mutations in the latter allele render it about 100-fold more active a DGC than wild-type PleD (Paul et al., 2004). FimX activity was assayed in the presence of several divalent cations in addition to Mg2+, including Mn2+, Zn2+ and Ca2+, and in the presence of the reducing agent dithiotreitol (1 μM−10 mM) (data not shown); however, we did not detect c-di-GMP production under any of these conditions.
Fig. 5. FimX exhibits phosphodiesterase activity which is stimulated by GTP, but has no detectable DGC activity.
A. TLC of DGC assay products. Purified hexahistidine-tagged proteins were assayed in the presence of 10 mM MgCl2 or MnCl2 (as indicated) for the ability to synthesize c-di-GMP from GTP. Reactions were stopped after 30 min by the addition of 0.5 M EDTA and assayed by TLC on PEI-cellulose as detailed in Experimental procedures.
B. TLC of phosphodiesterase assay products. Purified hexahistidine-tagged proteins were assayed in the presence of 10 mM MgCl2 or MnCl2 (as indicated), 100 μM GTP (as indicated) and [32P]-c-di-GMP for 30 min. Reactions were stopped by the addition of 0.5 M EDTA and assayed by TLC on PEI-cellulose as detailed in Experimental procedures. No product with Rf(pGpG) was detected when substrate was incubated with MgCl2 and GTP in the absence of protein.
C. Phosphodiesterase assay of wild-type FimX and point mutants. Hexahistidine-tagged proteins were incubated with [32P]-c-di-GMP, 10 mM MgCl2, MnCl2, or CaCl2 (as indicated) and 100 μM GTP (as indicated) for 60 min at 25°C. Reaction products were separated by TLC on PEI-cellulose as above. Lanes A–E correspond to: no protein (A); CC3396 (B); FimX (C); FimX(GDSIF→AASIF) (D); FimX(EVL→AAA) (E).
We used PleD* to synthesize [32P]-c-di-GMP from [32P]-GTP; this was then used as the substrate for phosphodi-esterase assays. As shown in Fig. 5B, C. crescentus CC3396, which served as a positive control, catalysed hydrolysis of c-di-GMP into pGpG. As previously described, the reaction was activated by the addition of 100 μM GTP (Christen et al., 2005). FimX was also able to convert c-di-GMP into pGpG and, like CC3396, its PDE activity was significantly increased by the presence of GTP in the reaction mix. His6-FimX(GDSIF→AASIF) and His6-FimX(EVL→AAA) were also assayed under these conditions. As seen in Fig. 5C, neither mutant protein produced pGpG when assayed in the presence of 10 mM MgCl2, regardless of the presence or absence of GTP. This suggests that FimX may be similar to CC3396, a phosphodiesterase in which a degenerate GGDEF domain (GEDEF) binds GTP and allosterically activates PDE activity resident within the EAL domain (Christen et al., 2005). As observed for CC3396, neither FimX nor the point mutant alleles show PDE-A activity when assayed in the presence of Ca2+, despite the addition of 100 μM GTP (Fig. 5C).
Bobrov and colleagues recently described an assay that utilizes bis(p-nitrophenyl) phosphate as a surrogate substrate for EAL domain proteins (Bobrov et al., 2005). Using this assay, they found that the EAL domain of Yersinia pestis HmsP produced p-nitrophenol in the presence of Mn2+, but not when the divalent cation Mg2+ was used instead. We also examined the effect of Mn2+ on PDE activity of FimX and CC3396, using the substrate c-di-GMP (Fig. 5C). For both CC3396 and FimX, Mn2+ appeared to increase PDE activity, both in the presence and absence of GTP. Interestingly, the FimX point mutants showed a different pattern of response to Mn2+: both showed some pGpG production in the absence of GTP, but were no longer activated by the addition of GTP. As a control, we incubated c-di-GMP with 10 mM MnCl2 in the absence of protein for 60 min and did not detect hydrolysis. Furthermore, the effect of adding MnCl2 varied with each protein assayed. These findings suggested that Mn2+ was likely affecting the interaction of the various PDEs with substrate. Mn2+, however, was neither sufficient to catalyse c-di-GMP breakdown itself, nor acting through a common component present in the reactions (i.e. a co-purified E. coli protein or PleD* that had survived the 10 min 95°C incubation step of c-di-GMP preparation).
Localization of FimX to the pole requires two signals
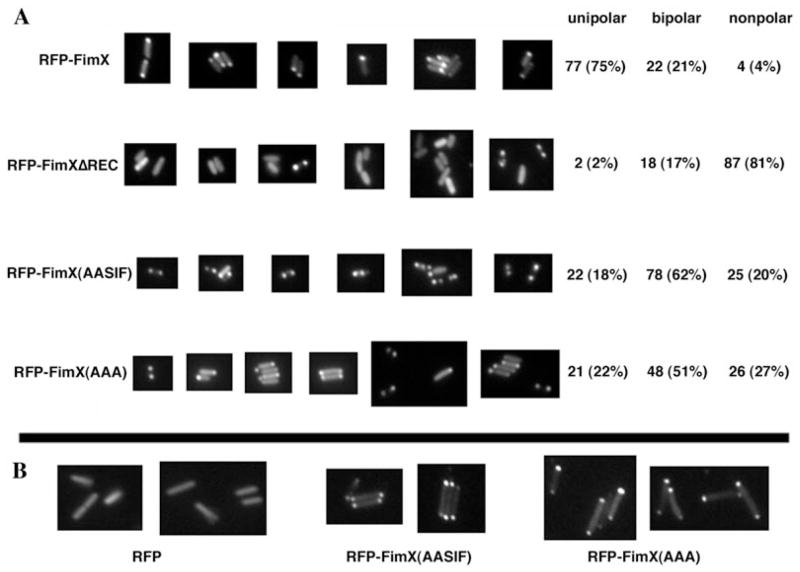
Huang et al. (2003) had demonstrated that a RFP–FimX fusion could be localized a single bacterial pole, and that a truncated fusion protein containing only the amino terminal 154 aa of FimX was correctly localized to the pole. Although no clear consensus sequence for polar localization has been defined, Huang et al. attempted to align the terminal 35 aa of the truncated FimX sequence with amino acid sequences implicated in polar localization of proteins in other bacteria. All of the amino acid residues that they identify as potential determinants of a polar localization signal in FimX are absent in our ΔREC allele, raising the question of whether ΔREC is appropriately localized to the bacterial pole. We addressed this by constructing RFP fusion proteins to FimX and FimXΔREC, which we expressed in ΔfimX bacteria from the pUCP-SK plasmid under control of the fimX promoter. (We could not detect any of the fusion proteins when they were expressed from a single copy of the gene integrated into the chromosomal attB site.) The RFP–FimX fusion restored twitching motility to ΔfimX bacteria, demonstrating that the fusion protein retained function (data not shown). As seen in Fig. 6A, RFP–FimX localized predominantly to one bacterial pole, confirming the observations of Huang et al. Wild-type or ΔfimX bacteria expressing RFP alone showed no polar localization of fluorescence (Fig. 6B and data not shown). Most bacteria failed to localize the RFP–ΔREC fusion to the poles, suggesting that assembly of tfp that supported PO4 phage infection did not absolutely require polar localization of FimX. We also constructed RFP fusions to the two point mutant alleles of FimX to determine whether the putative DGC and PDE activities of FimX influenced localization. The RFP–FimX(GDSIF→AASIF) and RFP–FimX(EVL→AAA) constructs showed predominantly bipolar localization in the ΔfimX background. Chi-square analysis confirmed that the distribution of RFP–FimX was significantly different from that of RFP–ΔREC (χ2 = 147.3, P < 0.0001), of RFP–FimX(GDSIF→AASIF) (χ2 = 75.5, P < 0.0001) and of RFP–FimX(EVL→AAA) (χ2 = 57.56, P < 0.0001). The two point mutants, however, showed similar patterns of distribution (χ2 = 3.153, P = 0.21). As neither point mutant is capable of complementing twitching motility, we hypothesized that the unipolar localization seen for the RFP–FimX might require bacterial motility, in which case the mutant fusion proteins would localize correctly when expressed in the PA103 background. Conversely, unipolar localization might require an enzymatically active FimX protein, in which case the mutant fusion proteins would remain bipolar in PA103. As seen in Fig. 6B, both mutant fusion proteins show a predominantly bipolar distribution in wild-type PA103. We again tallied the localization pattern of the RFP fusions for > 100 bacteria [RFP–FimX(GDSIF→AASIF): unipolar = 26 (23%), bipolar = 75 (66%), non-polar = 12 (11%); RFP–FimX(EVL→AAA): unipolar = 45 (28%), bipolar = 87 (55%), non-polar = 26 (16%)]. Thus expression of the point mutant proteins in a wild-type background is not sufficient to restrict their localization to a single pole. Of note, neither point mutant inhibits twitching motility of wild-type PA103, even when expressed from a high-copy number plasmid (data not shown).
Fig. 6.
Localization of RFP–FimX fusion proteins by indirect immunofluorescence. ΔfimX (panel A) or PA103 (panel B) bacterial colonies expressing indicated RFP–FimX fusion constructs were picked from 2-day-old LB agar plates, resuspended in a drop of ddH2O and spotted onto slides coated with a thin cushion of 1% Gel-Gro gellan. Bacteria were visualized and photographed as detailed in Experimental procedures.
Localization of tfp is altered in bacteria expressing FimXΔREC
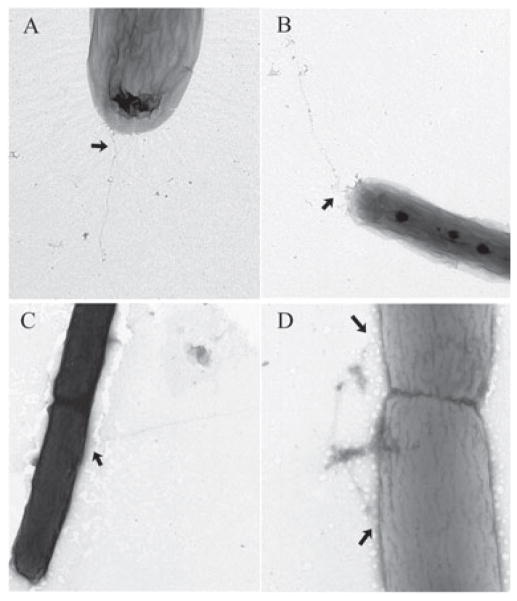
Our observation that RFP–FimXΔREC fails to localize to the poles prompted us to examine whether pili have the same localization in FimXΔREC as in wild-type PA103. PA103 pUCP-SK and ΔfimX pΔREC bacteria were visualized by transmission electron microscopy (TEM) to examine pilus location. Only bacteria which were well-separated from other bacteria were examined, to minimize artifacts caused by a bacterium overlying tfp originating from other cells. As seen in Fig. 7, PA103 bacteria usually have pili that arise at or near the poles; no flagella are seen, as expected for this hypoflagellated, non-swimming strain. In contrast, ΔfimX pFimXΔREC bacteria had pili that originated from non-polar as well as polar sites on the bacterial surface, suggesting that correctly localized FimX may play a role in restricting pilus assembly to the bacterial poles.
Fig. 7.
Visualization of tfp by electron microscopy. PA103 pUCP (panels A and B) and ΔfimX pFimXΔREC (panels C and D) bacteria were grown in LB supplemented with carbenicillin to OD600 = 0.6, then gently diluted 20- to 50-fold in ddH2O prior to preparation and staining of grids, as detailed in Experimental procedures. Images were obtained with a Technai 12 Biotwin at magnifications between 13 000x and 30 000x. Negatives were scanned at 1200 dpi, then cropped, assembled and labelled using Photoshop 7.0. Arrows indicate locations at which pili emerge from the cell body.
FimX is required for in vitro cytotoxicity but not for in vivo virulence
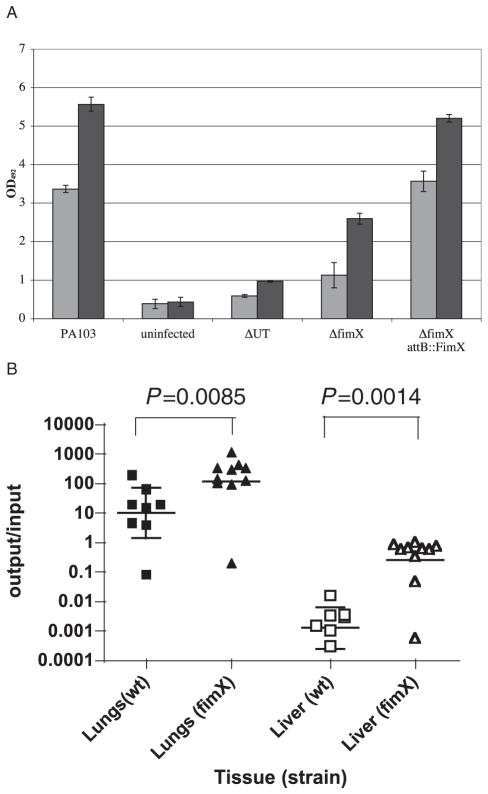
The presence of functional pili is strongly correlated with the ability of bacteria to cause epithelial cell cytotoxicity in vitro (Kang et al., 1997). Whether the pili serve as adhesins, or play a more active role in regulating the TTSS is an area of active debate (Sundin et al., 2002). We tested whether ΔfimX bacteria had any defects in cytotoxicity. As seen in Fig. 8A, ΔfimX bacteria were less cytotoxic than PA103 when assayed at 2 hours post infection (hpi) (c. 33% of wild-type) and 4 hpi (c. 46% of wild-type). This defect could be complemented by transforming ΔfimX with a plasmid expressing FimX under control of its own promoter, or by expressing FimX from an ectopic copy of the gene integrated into the ΔfimX chromosome at the attB site (ΔfimX attB::FimX) (Fig. 8A and data not shown). Rapid cytotoxicity under these conditions is primarily due to ExoU, a phospholipase which is translocated into host cells by the TTSS (Finck-Barbancon et al., 1997; Hauser et al., 1998; Sato et al., 2003). We therefore tested whether the TTSS was intact in ΔfimX by growing this mutant in MinS, which induces TTSS effector production and secretion in vitro (Frank, 1997). ΔfimX produced and secreted ExoU, ExoT, and the translocon components PopB, PopD and PcrV identically to wild-type PA103 under these conditions (data not shown). Thus, deletion of FimX does not appear to affect the function or regulation of the TTSS apparatus and effectors in vitro. ΔfimX growth curves in Luria–Bertani broth (LB) and minimal media [(MinS and Vogel–Bonner minimal (VBM)] were indistinguishable from those of wild-type PA103, arguing that a growth defect of the mutant could not account for the observed reduction in cytotoxicity (data not shown). ΔfimX bacteria were deficient, however, in binding to epithelial cells, a phenotype also described for ΔpilA mutants of P. aeruginosa (Comolli et al., 1999a; Sundin et al., 2002). Thus, in binding assays measuring the percentage of a bacterial inoculum adherent to HeLa cells 1 hpi, we observed that the number of ΔUTΔfimX bacteria bound was about 8% of the ΔUT control (range 2.1–16%, n = 8 independent experiments carried out in triplicate). This is comparable to the efficiency with which ΔpilA bacteria appear to bind HeLa cells, both in our hands (3–5% of isogenic wild-type control) and in published studies (2–4% of isogenic wild-type control as reported by Sundin et al.).
Fig. 8. FimX is required for cytotoxicity towards HeLa cells, but not for virulence in the murine model of acute pneumonia.
A. LDH release measured from infected HeLa cells. Triplicate wells of HeLa cells were infected with indicated bacterial strains at a moi of 10. Culture supernatants were collected at 2 and 4 hpi and assayed for LDH activity. Bars indicate mean ± SD of a representative experiment.
B. Bacterial recovery from infected mice in an acute pneumonia model. Eight- to 10-week-old female C57Bl/6 mice were infected with c. 5 × 105 cfu of PA103 (n = 8) or ΔfimX (n = 10). Mice were euthanized at 16 hpi and the number of bacteria present in lungs and liver were determined as described in Experimental procedures. Results are expressed as the ratio of cfu recovered per g tissue (output) to cfu present in the inoculum (input); each animal is represented by a data point, while the bar shows the geometric mean for each group. The Mann–Whitney test was used to calculate P-values (two-tailed) for each pairwise comparison indicated.
Next, we examined whether ΔfimX bacteria were able to cause disease in the murine acute pneumonia model. Eight- to 10-week-old C57Bl/6 female mice were infected with 5 × 105 PA103 or ΔfimX via the nares, then euthanized at 16 hpi. Both groups of mice appeared ill at this time point, as evidenced by hunched posture, laboured breathing and ruffled fur. ΔfimX bacteria were recovered from infected lungs and livers in numbers greater than those of wild-type PA103 (Fig. 8B). This strongly argues that ΔfimX bacteria are able to utilize the TTSS and its effectors during in vivo infection, because isogenic mutants which do not express positive regulators of TTSS, such as ExsA or RtsM/RetS, are effectively cleared from the lung in this time (Goodman et al., 2004; Laskowski et al., 2004). Of note, ΔpilA P. aeruginosa strains also retain virulence in murine models of acute infection (Comolli et al., 1999b; Kulesekara et al., 2006).
Discussion
Both GGDEF and EAL motifs appear to contribute to FimX function
FimX belongs to the large family of bacterial proteins which contain GGDEF and/or EAL domains. These domains have been shown to possess DGC and phosphodiesterase activity respectively. As such, proteins with DGC or PDE activity have the capacity to regulate the amount of c-di-GMP in the bacterial cell. Biochemical analyses of purified GGDEF and EAL domains have provided additional information regarding sequences important for enzymatic activity. Thus, the DGC PleD of C. crescentus, for which both biochemical (Paul et al., 2004) and structural (Chan et al., 2004) data exist, possesses an active site (A-site) which contains the GGEEF signature motif and which binds c-di-GMP in the PleD/c-di-GMP cocrystal. PleD contains an additional site at which a c-di-GMP dimer was found to bind; binding at this ‘I-site’ appears to inhibit DGC activity allosterically. Amino acid substitutions within the GGDEF motif are poorly tolerated. Thus substitution by alanine of any residue within the GGEEF motif of the Y. pestis HmsT protein results in a complete loss of Y. pestis biofilm formation equivalent to that seen in a HmsT− mutant (Kirillina et al., 2004). Mutation of the GGEEF motif of PleD to DEEEF similarly results in the loss of PleD function vis-a-vis stalk biogenesis (Aldridge and Jenal, 1999) and abolishes in vitro DGC activity of PleD (Paul et al., 2004). This would argue that an imperfect A-site motif, such as the GDSIF sequence present in FimX, is unlikely to catalyse c-di-GMP synthesis. Indeed, we cannot detect DGC activity of purified FimX in vitro.
FimX nonetheless does not tolerate amino acid changes at its imperfect A-site motif, as shown by the loss of function of the FimX(GDSIF→AASIF) allele. Christen et al. (2005) have recently reported that an imperfect A-site motif (GEDEF) in the C. crescentus protein CC3396 is still capable of binding GTP. This protein, which also has an EAL domain, has no demonstrable DGC activity in vitro but does possess phosphodiesterase activity. Interestingly, binding of GTP by CC3396 increases the phosphodiesterase activity of the protein some 40-fold; a mutant version of CC3396, in which the GEDEF motif is mutated to GQNEF, shows only threefold increase of PDE activity in response to GTP (Christen et al., 2005). We have found that FimX also shows phosphodiesterase activity towards c-di-GMP, and that this activity is stimulated by the presence of 100 μM GTP. When the GDSIF motif of FimX is mutated, GTP no longer stimulates PDE activity of FimX, suggesting that this imperfect A-site motif may be playing an analogous role to the GEDEF sequence of CC3396. The PDE-A activity of FimX, CC3396 and the two FimX point mutants also appears to be stimulated more effectively by Mn2+ than by Mg2+. Similar effects of Mn versus Mg on catalytic activity have been described for mammalian phosphodiesterases that hydrolyse cAMP or cGMP (Francis et al., 2000). The biological significance of this finding is not clear, however, as concentrations of Mg2+ are approximately two orders of magnitude greater than those of Mn2+ in bacterial cells (De Medicis et al., 1986).
In a recent publication, Schmidt et al. (2005) compare a number of EAL domain containing proteins and define sequences beyond the EAL motif itself which are conserved among proteins that show phosphodiesterase activity in vitro, but absent from proteins that lack in vitro PDE activity. FimX lacks a number of the residues which appear to characterize active PDEs, though they are present in the majority of P. aeruginosa EAL domain containing proteins. Of note, a very recent publication from Kulesekara et al. (2006) reported that no PDE activity above background could be detected in a whole-cell lysate of PA14 in which FimX was expressed from the low-copy number plasmid pMMB67 under control of the tac promoter. Indeed, only five of the 21 P. aeruginosa EAL domain-containing proteins expressed and assayed in this fashion by these authors showed detectable PDE activity, and none of the five possessed both GGDEF and EAL domains. In our hands, purified His6-FimX is less active than our positive control, CC3396, but nonetheless reproducibly demonstrates PDE activity in the presence of GTP. Like most GGDEF and EAL domain proteins, FimX contains additional domains that are likely to modulate its activity in vivo and perhaps in vitro. We are currently testing the hypotheses that activating signals mediated by the REC and/or PAS domains of the protein modulate its PDE activity.
FimX localizes to the pole in a manner dependent on N-terminal sequences and enzymatic activity
The DGC activity of C. crescentus PleD is increased by phosphorylation of Asp 53, which lies within the N-terminal response regulator domain of this protein (Paul et al., 2004). Phosphorylation is also critical for targeting PleD to the emerging stalked pole; however, DGC activity is not important for subcellular localization, as a PleD allele carrying an inactivating point mutation in the GGEEF motif (PleDGG368DE) is still correctly targeted to the stalked pole (Paul et al., 2004). Thus PleD illustrates two further features of GGDEF/EAL domain proteins. Many contain additional signal transduction domains, which can control DGC or PDE activity temporally, in response to specific cues, and many show subcellular localization, which may serve to restrict production and/or degradation of c-di-GMP spatially. FimX possesses two such amino terminal domains, namely REC (response regulator) and PAS domains. No candidate phosphoacceptor aspartate can be identified in the REC domain. Deletion of the REC domain resulted in a FimX allele which was no longer localized to the bacterial pole. This is most likely because the ΔREC deletion also removes amino acids which may serve as a polar localization signal for FimX (Huang et al., 2003), although our experiments do not rule out an independent signalling function for this domain.
RFP–FimX predominantly localizes to a single pole; however, both RFP–FimX(GDSIF→AASIF) and RFP–FimX(EVL→AAA) show predominantly bipolar localization in a ΔfimX background. As these two point mutant alleles do not support twitching motility, we also expressed the RFP fusions in wild-type PA103. RFP–FimX(GDSIF→AASIF) and RFP–FimX(EVL→AAA) remained predominantly bipolar, however, despite being expressed in a background where twitching motility occurred. Thus, residues within the ΔREC domain are required to bring FimX to the poles, and enzymatic activity further restricts FimX to a single pole. We do not know if RFP–FimX resides at the piliated or non-piliated pole.
FimX is required for in vitro cytotoxicity, but not for in vivo virulence
Although ΔfimX bacteria are severely attenuated for cyto-toxicity towards tissue culture cells, the ΔfimX strain is slightly more virulent than PA103 in the murine acute pneumonia model. A similar discordance has been observed for other P. aeruginosa mutants that fail to assemble functional pili and are no longer cytotoxic towards tissue culture cells, but still demonstrate virulence in vivo (Comolli et al., 1999b; Kulesekara et al., 2006). It is not clear why tfp appear dispensable in murine models of acute infection: possibly the absence of tfp diminishes the ability of the innate immune system to recognize and clear P. aeruginosa, as suggested by observations that pili are required for non-opsonic phagocytosis of P. aeruginosa by macrophages and neutrophils (Speert et al., 1986; Kelly et al., 1989).
A common theme emerging from studies of GGDEF and EAL domain proteins is that they often regulate multicellular bacterial behaviour, in large part by altering extracellular matrix components synthesized by bacteria (Romling et al., 2005). Thus, the assembly of other types of adhesive organelles, such as the P. aeruginosa cup fimbriae, appears to be linked to GGDEF/EAL domain proteins (Vallet et al., 2001; D’Argenio et al., 2002), as does the production of exopolysaccharides in a variety of bacterial species (Tal et al., 1998; Ausmees et al., 1999; Zogay et al., 2001; Spiers et al., 2002). Such multicellular behaviours are likely to be very significant for P. aeruginosa colonization and infection of cystic fibrosis patients, in whom bacteria grow as biofilms (Singh et al., 2000). Indeed, P. aeruginosa biofilm formation can be strongly influenced by overexpression of exogenous DGC and PDE proteins (Simm et al., 2004); thus, it is to be expected that some of the many endogenous GGDEF and EAL domain proteins within this organism will also influence biofilm formation. Strain PA103 does not form robust biofilms (T.S. Murray and B.I. Kazmierczak, unpubl. results); thus, evaluating the role of FimX in biofilm formation and in models of chronic lung infection will require introducing the ΔfimX mutation into other strains, as we are doing.
Experimental procedures
Sequence analysis
Sequences of EAL domain-containing proteins were downloaded from the Pseudomonas Genome Project (http://www.pseudomonas.com) (Stover et al., 2000). Multiple sequence alignment was carried out using CLUSTALW (Chenna et al., 2003), available at website http://www.ebi.ac.uk/clustalw/index.html. The alignment was subsequently manually adjusted. The Gluconacetobacter xylinum PdeA1 EAL domain sequence was included in the alignment to allow comparison with the results of Schmidt et al. (2005).
Bacterial strains and media
Bacterial strains were routinely cultured in LB or VBM medium with antibiotics as required at the following concentrations: ampicillin, 100 μg ml−1 for E. coli; carbenicillin, 200 μg ml−1 for P. aeruginosa; gentamicin, 10 μg ml−1 for E. coli or 100 μg ml−1 for P. aeruginosa; tetracycline, 20 μg ml−1 for E. coli or 100 μg ml−1 for P. aeruginosa. All strains were maintained at −80°C as 15% glycerol stocks. Bacteria were grown in MinS media containing 10 mM NTA (Nicas and Iglewski, 1984) to evaluate secretion via the TTSS as previously described (Laskowski et al., 2004). All antibiotics were purchased from Sigma or USB. Bovine serum albumin (BSA), Fraction V was purchased from USB. A list of bacterial strains and plasmids used in this study is shown in Table 2.
Table 2.
Bacterial strains and plasmids.
| Bacterial strain or plasmid | Relevant characteristics | Source |
|---|---|---|
| E. coli XL1 blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10(Tcr)] | Stratagene |
| E. coli S17-1 | Used for mating constructs into P. aeruginosa; thi pro hsdR recA RP4-2 (Tc::Mu) (Km::Tn7) | Simon et al. (1983) |
| E. coli TOP10 | F−mcrAΔ(mrr-hsdRMS-mcrBC) Φ 80lacZΔM15ΔlacX74 recA1 araD139 galU galKΔ(ara-leu)7697rpsL(Strr) endA1 nupG | Invitrogen |
| E. coli BL21 (DE3) pLysS | F−dcm ompT hsdS(rB−mB−) gal λ(DE3) [pLysS Cmr] | Stratagene |
| PA103 | Virulent lung isolate of P. aeruginosa, known type III-secreted effector proteins are ExoT and ExoU | Liu (1966) |
| ΔfimX | PA103 containing an in-frame deletion of aa 15–685 of fimX ORF | This study |
| ΔfimX attB::FimX | FimX under control of its own promoter integrated at attB site of ΔfimX | This study |
| ΔfimX attB::FimXΔREC | FimXΔREC under control of its own promoter integrated at attB site of ΔfimX | This study |
| ΔfimX attB::FimXΔGGDEF | FimXΔGGDEF under control of its own promoter integrated at attB site of ΔfimX | This study |
| ΔfimX attB::FimXΔEAL | FimXΔEAL under control of its own promoter integrated at attB site of ΔfimX | This study |
| ΔUT | PA103 containing in-frame deletions of exoU ORF (aa 330–571) and exoT ORF (aa 36–348) | Garrity-Ryan et al. (2000) |
| ΔUTΔfimX | ΔfimX containing an in-frame deletion of aa 15–685 of fimX ORF | This study |
| ΔpilA | Gmr cassette inserted into pilA in PA103; Gmr | Whitchurch et al. (2005) |
| pGEM-T Easy | E. coli TA cloning vector; Apr | Promega |
| pBluescript-KS | E. coli cloning vector; Apr | Stratagene |
| tdimer2 | RFP dimer cloned in pBluescript-KS | C. Jacobs-Wagner (Yale) |
| pMO012502 | pLA2917 cosmid from PAO1 library containing PA4959 ORF; Tcr | Huang et al. (2000) |
| pUCP-SK | P. aeruginosa—E. coli shuttle vector; Apr(Cbr) | Watson et al. (1996) |
| mini-CTX-2 | Contains attP site for integration at the attB site of the P. aeruginosa chromosome; Tcr | Hoang et al. (1998) |
| pFLP2 | Source of inducible Flp recombinase; Apr(Cbr) | Hoang et al. (1998) |
| pEX100T | Allelic replacement suicide plasmid; Apr(Cbr) sacB oriT | Schweizer and Hoang (1995) |
| pKO–FimX | N- and C-terminal regions flanking FimX ORF cloned in tandem in pEX100T; Apr(Cbr) | This study |
| pFimX (pMLD9) | 2.9 kb BamHI-MfeI fragment of pMO012502 cloned into pUCP-SK; Apr(Cbr) | This study |
| pMLD9x | pFimX with altered MCS; Apr(Cbr) | This study |
| pFimXΔREC | pFimX with sequences encoding aa 67–147 deleted; Apr(Cbr) | This study |
| pFimXΔGGDEF | pFimX with sequences encoding aa 253–443 deleted; Apr(Cbr) | This study |
| pFimXΔEAL | pFimX with stop codon inserted after aa 444; Apr(Cbr) | This study |
| pFimX(GDSIF→AASIF) | pFimX with point mutations G346A D347A; Apr(Cbr) | This study |
| pFimX(EVL→AAA) | pFimX with point mutations E475A V476A L477A; Apr(Cbr) | This study |
| pBB2–FimX | pFimX with BB2 epitope tag inserted after initiating methionine of FimX; Apr(Cbr) | This study |
| pBB2–FimXΔREC | pFimXΔREC with BB2 epitope tag inserted after initiating methionine of FimX; Apr(Cbr) | This study |
| pBB2–FimXΔGGDEF | pFimXΔGGDEF with BB2 epitope tag inserted after initiating methionine of FimX; Apr(Cbr) | This study |
| pBB2–FimΔEAL | pFimXΔEAL with BB2 epitope tag inserted after initiating methionine of FimX; Apr(Cbr) | This study |
| pBB2–FimX(GDSIF→AASIF) | pFimX(GDSIF→AASIF) with BB2 epitope tag inserted after initiating methionine of FimX; Apr(Cbr) | This study |
| pBB2–FimX(EVL→AAA) | pFimX(EVL→AAA) with BB2 epitope tag inserted after initiating methionine of FimX; Apr(Cbr) | This study |
| pRFP | SacI–HindIII fragment of tdimer2 subcloned into pUCP-SK(RFP under control of lac promoter); Apr(Cbr) | This study |
| pRFP–FimX | pFimX with N-terminal RFP dimer fusion; Apr(Cbr) | This study |
| pRFP–FimXΔREC | pFimXΔREC with N-terminal RFP dimer fusion; Apr(Cbr) | This study |
| pRFP–FimX(GDSIF→AASIF) | pFimX(GDSIF→AASIF) with N-terminal RFP dimer fusion; Apr(Cbr) | This study |
| pRFP–FimX(EVL→AAA) | pFimX(EVL→AAA) with N-terminal RFP dimer fusion; Apr(Cbr) | This study |
| pBAD/His | Apr expression plasmid | Invitrogen |
| pET11 | Apr expression plasmid | Stratagene |
| pET21 | Apr expression plasmid | Stratagene |
| pHis6–FimX | pBAD/His; FimX, N-terminal His6 tag | This study |
| pHis6–FimX(GDSIF→AASIF) | pBAD/His; FimX, N-terminal His6 tag | This study |
| pHis6–FimX(EVL→AAA) | pBAD/His; FimX, N-terminal His6 tag | This study |
| pCC2 | pET11; pleD, C-terminal His6 tag | Paul et al. (2004) |
| pRP89 | pET11; pleD*, C-terminal His6 tag | Paul et al. (2004) |
| pMC24 | pET21; CC3396, C-terminal His6 tag | Christen et al. (2005) |
Ap, ampicillin; Cb, carbenicillin; Gm, gentamicin; Km, kanamycin; Tc, tetracycline; Cm, chloramphenicol; Str, streptomycin.
Strain construction
All PCR primers employed in this study were ordered from Invitrogen, unless otherwise specified. They are listed in Table 3, and are based on the PA01 genome sequence (http://ww.pseudomonas.com) (Stover et al., 2000). All amplifications were carried out with Pfu Turbo polymerase (Invitrogen), using PA103 genomic DNA as template, unless otherwise specified. PCR products were subcloned into pGEM-T Easy (Promega) and sequenced to confirm that no mutations were introduced during amplification. All intermediate cloning steps were performed using XL1-Blue E. coli (Stratagene). Plasmids were introduced into E. coli strains by electroporation (Sambrook et al., 1989) and transformed into chemically competent P. aeruginosa (Mattick et al., 1987) unless otherwise specified.
Table 3.
Primers and oligonucleotides used in this study.
| RFP-F | GCTCATGATGGCCTCCTCCGAGGAC |
|---|---|
| RFP-R | GCTCATGAGGAACAGGTGGTGGCGG |
| fimX-N1 | CTAGGATCCGATATCCGCCAGGTACGCAATGGC |
| fimX-N2 | GATGAATTCCAGAATCAGCAGGCGG |
| fimX-C1 | GATGAATTCTCCTCGGGAGACGAATG |
| fimX-C2 | CGTGAAGCTTGATATCGCTTTCCAGCAGGACAG |
| BB2-A | CGCTGGATCCGCCAGGTACGCAATGG |
| BB2-B | TGGTGTGCACCTCCATGGAAAGGGCTCAGTCCGCG |
| BB2-C | GGAGGTGCACACCAACCAGGACCCGCTCGACGCCATCGAAAAGAAAACCATCCGC |
| BB2-D | CCGGGCTCGAGACACTCGCTGG |
| REC-3 | GCTCACTCGAGTTGCTGCTCGAAGCTCGGTCGACGCCATCAC |
| REC-2 | CGCCGTCGTACGTGGCGG |
| GGDEF-1 | CTCAGCTCGAGCCCGGCGAG |
| GGDEF-2 | GACTCGGTACCCCGCGGATCACCACCTGGATGC |
| GGDEF-4 | CTCGGATCGATCTTGATGAACTGCA |
| GGDEF-5 | GAGCGGTACCCATCGCGATCCTCCAGCAGG |
| EAL | CATAGTAATGAGGTACCTCATTACTATG |
| Ser-up | CGAGTGGTTTAAGGCAACGGTCTTGA |
| Ser-down | AGTTCGGCCTGGTGGAGCAACTCG |
| GDSIF/AASIF forward | GCCGACCTGGCGCGTTTCGCCGCTTCGATCTTCGCCGCC |
| GDSIF/AASIF reverse | GGCGGCGAAGATCGAAGCGGCGAAACGCGCCAGGTCGGC |
| EVL/AAA forward | CGGCGACAGCCACGAGAACTATGCAGCTGCTCTACGCCTCCTCAATCCGCAGGGCC |
| EVL/AAA reverse | GGCCCTGCGGATTGAGGAGGCGTAGAGCAGCTGCATAGTTCTCGTGGCTGTCGCCG |
All primers are listed 5′ to 3 ′.
An unmarked, in-frame deletion of aa 15–685 of FimX in PA103 (ΔfimX) was constructed by allelic exchange (Schweizer and Hoang, 1995). Briefly, N-terminal (500 bp) and C-terminal (790 bp) regions flanking fimX were amplified using the primer pairs fimX-N1/fimX-N2 and fimX-C1/fimX-C2 respectively. The amplified regions were subcloned in tandem into the gene replacement vector pEX100T to generate pKO–FimX. This construct was transformed into E. coli S17-1 and mobilized into PA103 by mating. Carbenecillin-resistant exconjugants (merodiploids) were then resolved by growth on VBM plus 5% sucrose as described previously (Garrity-Ryan et al., 2000). Potential mutants were screened by PCR and confirmed by Southern blot (data not shown). An identical strategy was used to construct ΔUTΔfimX.
Cloning of FimX
A 2.9 kb BamHI-MfeI fragment containing the FimX open reading frame (ORF) plus 400 bp upstream and 421 bp downstream was subcloned from cosmid pMO012502 into the BamHI–EcoRI sites of pUCP-SK to generate pFimX (pMLD9). To facilitate subsequent cloning steps, several restriction sites within the multiple cloning site of pMLD9 were removed by digesting the plasmid with EcoRV and KpnI, blunting the KpnI site, and religating the plasmid. We confirmed that the EcoRV, KpnI and intervening HindIII, ClaI and XhoI sites were no longer present in this vector, which we named pMLD9x.
BB2 epitope-tagged constructs
A BB2 epitope tag (EVHTNQDPLD) was inserted immediately after the initiating methionine of FimX; codon usage was chosen to reflect codon bias within FimX and to create an ApaLI site within the tag sequence. The epitope tag sequence was incorporated into PCR primers BB2-B and BB2-C. Using pMLD9 as a template, the promoter region and amino-terminal portion of FimX (from the BamHI site to an internal XhoI site) were amplified with primer pairs BB2-A/BB2-B and BB2-C/BB2-D. The resulting PCR products were gel-purified, digested with ApaLI and ligated. Products of the ligation reaction were analysed by agarose gel electrophoresis, and the fragment corresponding to the ligation product of BB2-AB plus BB2-CD was cloned into pGEM-T Easy. We confirmed the in-frame incorporation of the BB2 epitope tag and the absence of any other mutations by sequencing. This fragment was then used to replace the BamHI-XhoI fragment of pMLD9x to create pBB2–FimX.
Domain deletion constructs
A deletion (aa 67–147, inclusive) removing the majority of the REC domain was constructed by PCR amplifying a c. 330 bp fragment of FimX which encompasses sequences encoding aa 148–242. The 5′ primer added a XhoI site to the PCR product, while the 3′ end of the PCR product contains a BsiW1 site present in the fimX sequence. This PCR product was subcloned into pGEM-T Easy (Promega), confirmed by sequencing, then used to replace the c. 500 bp XhoI-BsiW1 region within pMLD9x (which contains aa 65–241 of FimX), resulting in pFimXΔREC. pBB2–FimXΔREC was constructed by replacing the XhoI-BsiW1 region of pBB2–FimX with the same PCR product.
The GGDEF domain was deleted as follows. Primers GGDEF-1 and GGDEF-2 were used to amplify a region corresponding to aa 65–252 of FimX; likewise, primers GGDEF-5 and GGDEF-4 amplified the region corresponding to aa 444–618 of FimX. KpnI sites were engineered into primers GGDEF-2 and GGDEF-3. The products of the two PCR reactions, GGDEF 1–2 and GGDEF 5–4, were gel-purified, digested with KpnI and ligated. Products of the ligation reaction were analysed by agarose gel electrophoresis, and the fragment corresponding to the ligation product of GGDEF 1–2 plus GGDEF 5–4 was cloned into pGEM-T Easy. Sequencing confirmed the in-frame deletion of aa 253–443 (inclusive) and the addition of two new amino acids (valine-proline) corresponding to the new KpnI site. This fragment was then used to replace the XhoI-ClaI fragment (encompassing aa 65–618 of FimX) of pMLD9x and pBB2–FimX, resulting in plasmids pFimXΔGGDEF and pBB2–FimXΔGGDEF respectively.
The EAL domain of the protein was deleted by linker mutagenesis. Briefly, pMLD9x and pBB2–FimX were digested with NruI, which cuts these plasmids once at a position corresponding to aa 444 of FimX. An excess of the EAL oligo was then ligated into this blunt site. The EAL oligo inserts three tandem stop codons in frame after aa 444 of FimX, effectively removing the carboxy-terminal EAL domain. A novel KpnI site is also introduced by this mutagenic oligo, which facilitated screening of products of the mutagenesis. The resulting plasmids were named pFimXΔEAL and pBB2–FimXΔEAL.
RFP fusion protein constructs
The RFP control plasmid, pRFP, was constructed by subcloning a SacI–HindIII fragment from tdimer2 into the SacI–HindIII sites of pUCP-SK. This allows the RFP dimer to be constitutively expressed from the lac promoter of pUCP-SK. RFPdimer sequences were introduced as amino-terminal in-frame fusions into pFimX as follows. PCR primers RFP-F and RFP-R were used to amplify RFPdimer from tdimer2; the PCR product was cloned into pGEM-T Easy. A BspHI fragment containing RFPdimer sequences was subcloned into the NcoI site of pFimX. Correct orientation of the insert was confirmed by checking the BamHI/NcoI restriction pattern. The same strategy was used to introduce N-terminal RFP-dimer sequences into pFimXΔREC, pFimX(GDSIF→AASIF) and pFimX(EVL→AAA).
Single-copy complementation
Wild-type FimX, BB2–FimX and each of the domain deletion constructs were cloned into the vector pCTX-2, which was then used to recombine these alleles into the attB site as previously described (Laskowski et al., 2004). Specific details of the subcloning strategy used for each construct are available upon request. Flp recombinase was subsequently introduced into each strain, allowing vector backbone sequences to be removed by virtue of flanking FRT sites (Hoang et al., 1998). Each allele was expressed under control of the native fimX promoter, which was contained within the fragments cloned into pCTX-2. Insertion of each sequence into the attB site was confirmed by PCR amplification using Ser-up and Ser-down primers, followed by diagnostic restriction digests of the resulting PCR products (data not shown).
Construction of point mutants
The EcoR1–HindIII fragment of pFimX, which contains the GGDEF and EAL domains of FimX, was subcloned into pBluescript-KS to create a template for site-directed mutagenesis. Mutagenic oligos GDSIF/AASIF forward and reverse and EVL/AAA forward and reverse (listed in Table 2) were ordered from the DNA Synthesis Facility (Department of Pathology, Yale University) and gel-purified prior to use. PCR was carried out using Pfu Turbo (Invitrogen); annealing and extension conditions are available upon request. PCR products were treated with DpnI, which digests methylated and hemi-methylated DNA prior to transformation into XL1-Blue; this step selectively degrades template DNA. Introduction of the desired mutations was confirmed by sequencing of the entire EcoR1–HindIII insert. We also confirmed that no unwanted mutations were introduced during this process. The mutations were transferred into our series of expression vectors by subcloning; specific details of the strategy used to construct particular vectors are available upon request.
Construction, expression and purification of His6 fusion proteins
Wild-type FimX, FimX(GDSIF→AASIF) and FimX(EVL→AAA) were subcloned in-frame with an amino-terminal 6× His tag in the pBAD/His B vector (Invitrogen); specific details of the subcloning strategy used for each construct are available upon request. The region of the construct spanning the joint between the His6 tag and the FimX ORF was sequenced to confirm that each coding sequence was in frame with the epitope tag. pBAD-His6FimX, pBAD-His6FimX(GDSIF→AASIF) and pBAD-His6FimX(EVL→AAA) were transformed into E. coli Top10 (Invitrogen). Overnight cultures of plasmid carrying strains were diluted 1:100 into LB plus ampicillin and grown at 37°C with vigorous aeration to OD600∼0.8, at which point fusion protein expression was induced for 90 min by the addition of 0.2% arabinose. Bacteria were harvested by centrifugation (7000 g for 20 min), resuspended in 50 mM sodium phosphate pH 7.0/300 mM NaCl/5 mM β-mercaptoethanol/0.2 mg ml−1 PMSF and lysed by passage through a French pressure cell twice (14 000 psi). The lysate was cleared by centrifugation (20 000 g for 30 min) before incubation with Talon™ Metal Affinity Resin (BD Biosciences) at 4°C overnight. Protein was eluted with 150 mM imidazole and fractions assayed by SDS-PAGE. Fractions containing > 95% pure protein were pooled and dialysed against 25 mM Tris-HCl, pH 8.0/250 mM NaCl/5 mM β-mercaptoethanol for 12 h at 4°C. Protein concentrations were determined by BCA assay (Pierce).
Vectors expressing His6 fusions of PleD*, PleD and CC3396 were generously provided by Ralf Paul and Urs Jenal (University of Basel) (Paul et al., 2004; Christen et al., 2005). PleD*-H6, PleD-H6 and CC3396-H6 were expressed and purified from E. coli BL21(DE3) pLysS as described above, except that protein expression was induced at OD600∼0.6 for 45 min by the addition of 0.5 mM IPTG.
Diguanylate cyclase assays
Methods described by Urs Jenal and colleagues were adapted as follows (Paul et al., 2004; Christen et al., 2005). Briefly, 1 μl of α-labelled [32P]-GTP (3.33 pmol, 3000 Ci mmol−1, Amersham) was added to 9 μl of reaction buffer containing 25 mM Tris-HCl, pH 8.0, 250 mM NaCl, 5 mM β-mercaptoethanol, 10 mM MgCl2, MnCl2 or CaCl2 (as indicated) and 15 μM His6-fusion protein (as indicated). Reactions were incubated at 25°C for 10–60 min and stopped by the addition of 2 μl of 0.5 M EDTA. Products were separated by TLC on PEI-cellulose plates (Macherey-Nagel), using 1:1.5 v/v saturated NH4SO4 and 1.5 M KH2PO4, pH 3.6 as the solvent. Products were visualized by exposing TLC plates to film (30 min to 12 h). Autoradiographs were scanned at 300 dpi and assembled into figures using Photoshop 7.0 (Adobe). The product of the PleD*-H6 reaction served as a control for the migration of c-di-GMP in this TLC system.
Synthesis of [32P]-c-di-GMP
[32P]-c-di-GMP was produced enzymatically by incubating 5 μl of α-labelled [32P]-GTP (16.5 pmol, 3000 Ci mmol−1, Amersham) in the presence of 50 μM PleD*-H6, 10 mM MgCl2, 25 mM Tris-HCl, pH 8.0, 250 mM NaCl and 5 mM β-mercaptoethanol for 45 min at 25°C. The protein was precipitated by heating at 95°C for 10 min followed by centrifugation at 20 000 g for 2 min. Conversion of GTP into c-di-GMP was confirmed by TLC on PEI-cellulose plates as described above. The clarified reaction was stored at −20°C; 1 μl was used as substrate per 10 μl PDE assay as described below.
Phosphodiesterase assays
A 10 μl reaction containing hexahistidine-tagged protein (15 μM), 10 mM MgCl2, MnCl2 or CaCl2 (as indicated), 100 μM GTP (as indicated), 25 mM Tris-HCl, pH 8.0, 250 mM NaCl, 5 mM β-mercaptoethanol, and 1 μl of clarified c-di-GMP synthesis reaction (prepared as above) was incubated at 25°C for 10–60 min, then stopped by the addition of 2 μl of 0.5 M EDTA. Reaction products were separated by TLC on PEI-cellulose as described above and visualized by autoradiography. The product of CC3396-H6 incubation served as a positive control for the migration of pGpG in this TLC system. The spot identified as GMP co-migrated with cold GMP (Sigma) by TLC.
Western blotting
Bacterial cultures were grown overnight in LB with appropriate antibiotics. Bacteria were pelleted by centrifugation, then incubated for 10 min in 4% SDS at 100°C. Bacterial lysates were briefly sonicated (10–15 s) before being assayed for total protein concentration using the BCA assay (Pierce). Five to 25 μg of total protein per sample was loaded on 10% gels and separated by SDS-PAGE. Proteins were transferred to PVDF (Immobilon-P, Millipore) and blocked with 1× TBST plus 5% (w/v) dry milk for 30 min prior to incubation with primary antibody. Anti-BB2 hybridoma supernatant (1:100, kindly provided by C. Tschudi, Yale University) or polyclonal antiserum recognizing PA103 PilA (1:1000, kindly provided by J. Engel, UCSF) was diluted into TBST plus 5% (w/v) dry milk and incubated with membranes overnight at 4°C. Horseradish peroxidase-conjugated secondary antibodies (Bio-Rad) were used at a concentration of 1:2000. Membranes were incubated for 1 min in 100 mM Tris-HCl pH 8.5/225 μM coumaric acid (Sigma)/1.25 mM 3-aminophthalhydrazide (Fluka)/0.009% H2O2, and chemiluminescence was detected using an Image Station 2000R running 1D Image Analysis software v. 3.6 (Kodak).
Subsurface stab assay for twitching motility
Bacterial strains were picked from fresh plates and stabbed through LB agar to the agar/plastic interface using a sterile needle as previously described (Huang et al., 2003). Plates were incubated overnight at 37°C, at which time a twitching zone was visible on the plastic surface. The diameter of the twitching zone was measured; for non-circular zones, the longest and shortest dimensions were measured and averaged.
Phage sensitivity
PO4 phage (kindly provided by Patricia Baynham, Thomas More College) was serially diluted and spotted onto soft agar containing wild-type PA103 or indicated isogenic mutants. Plaques were counted following overnight incubation at 37°C. Efficiency of plaque formation was calculated relative to PA103.
Pilin ELISA
Bacteria were grown overnight on LB plates (with supplements or antibiotics as indicated) at 37°C and harvested by scraping. The protocol of Comolli et al. (1999b) was followed with minor modification. Briefly, bacterial suspensions were adjusted in MEM-lite to OD600 = 1.0. Serial dilutions of the suspensions were prepared in MEM-lite and used to coat 96 well ELISA plates in triplicate for 2 h at 37°C. Antigen was fixed with methanol, then exposed to polyclonal antiserum generated against PA103 PilA (1:500) (kindly provided by Joanne Engel, UCSF). ELISA plates were washed three times with ddH2O prior to addition of alkaline phosphatase-conjugated anti-rabbit-IgG (1:1000–1:2000, Bio-Rad). Wells were developed using BluePhos Microwell phosphatase substrate system (KPL, Gaithersburg MD) according to manufacturer’s instructions.
Transmission electron microscopy
Bacteria grown in LB broth plus appropriate antibiotics were gently diluted in ddH2O, then allowed to bind to glow-discharged carbon grids for 1 min. Grids were stained for 2 min in 2% uranyl acetate, washed with ddH2O twice (20 s), then briefly dried before imaging on a Tecnai 12 Biotwin microscope (Center for Cell and Molecular Imaging, Yale University).
Cytotoxicity assays
HeLa cells (ATCC) were cultured and passaged as described previously (Laskowski et al., 2004). HeLa cells were plated in 24 well dishes at 5 × 104 cells well−1 48 h prior to infection. Bacteria were grown in LB as previously described and used at a moi of 10 for infections. Prior to infection, HeLa cells were washed with and placed in MEM-etc. Cells were centrifuged at 800 rpm for 3 min after adding bacteria. Lactate dehydrogenase (LDH) release was assayed at 2 hpi and 4 hpi with the LDH assay kit according to manufacturer’s instructions (Takara).
Binding assays
HeLa cells were cultured and passaged as described above for cytotoxicity assays. Infections were carried out at a moi of 5–10 for 1 h; cells were centrifuged at 800 rpm for 3 min after adding bacteria. Infected cells were washed four times with PBS to remove non-adherent bacteria, then lysed with Hanks Buffered Salt Solution (HBSS) (Invitrogen) plus 0.25% Triton X-100 (USB). Bound plus internalized bacteria were enumerated by plating serial dilutions of the lysate to VBM plates.
Localization of RFP fusion proteins by immunofluorescence microscopy
Bacteria were picked from LB agar plates containing antibiotics as appropriate and gently resuspended in 20 μl of water. Two millilitres of bacterial suspension was spotted onto a microscope slide coated with 1% Gel-Gro (MP Biomedicals) prepared according to manufacturer’s instructions. Bacteria were visualized using an Eclipse TMS 100 microscope and Plan Apo 60XA/1.40 oil immersion lens. Images were obtained with a RT monochrome CCD camera (Spot Diagnostic Instruments) and assembled with Photoshop 7.0 (Adobe).
Murine model of acute pneumonia
Eight- to 10-week-old female C57Bl/6 mice were obtained from NCI and housed under specific pathogen-free conditions. All studies were approved by the Yale University Institutional Animal Care and Use Committee. Infections were carried out as described previously (Laskowski et al., 2004). Briefly, mice were lightly anaesthetized with methoxyflurane (2–4%), then infected intranasally with 40 μl of bacterial suspension, corresponding to c. 5 × 105 cfu of bacteria. The actual dose administered was determined by plating serial dilutions of the inoculum and counting cfu. Mice were euthanized at 16 hpi, and the liver and lungs were removed, homogenized and resuspended in HBSS plus 0.25% Triton X-100. The suspensions were passed through a sterile screen to obtain a uniform suspension and serial dilutions were plated on VBM to determine recovery.
Statistical analysis
Means and standard deviations were calculated using Excel spreadsheet software (Microsoft). T-tests, chi-square analyses and Mann–Whitney statistics were calculated using Prism 4.0 software (GraphPad).
Acknowledgments
This work was supported by start-up funds from Yale University School of Medicine (B.I.K.), and NIH Grants R01 AI054920 (B.I.K.) and T32 AI07210 (T.S.M.). We thank Patricia Baynham, Joanne Engel, Christine Jacobs-Wagner, Herbert Schweizer, Christian Tschudi, Ralf Paul and Urs Jenal for kindly providing phage stock, plasmids, strains and antibodies. We are especially grateful to Urs Jenal for helpful discussions regarding diguanylate cyclase and phosphodiesterase assays. We thank Alison Pardee (Pseudomonas Genetic Stock Center, East Carolina University School of Medicine) for providing cosmid pMO012502. We thank Marc Pypaert (Center for Cell and Molecular Imaging, Yale University) for advice and assistance with TEM.
References
- Aldridge P, Jenal U. Cell cycle-dependent degradation of a flagellar motor component requires a novel-type response regulator. Mol Microbiol. 1999;32:379–391. doi: 10.1046/j.1365-2958.1999.01358.x. [DOI] [PubMed] [Google Scholar]
- Ausmees N, Jonsson H, Hoglund S, Ljunggren HG, Lindberg M. Structural and putative regulatory genes involved in cellulose synthesis in Rhizobium leguminosarum bv. trifolii. Microbiology. 1999;145:1253–1262. doi: 10.1099/13500872-145-5-1253. [DOI] [PubMed] [Google Scholar]
- Bobrov AG, Kirillina O, Perry RD. The phosphodiesterase activity of the HmsP EAL domain is required for negative regulation of biofilm formation in Yersinia pestis. FEMS Microbiol Lett. 2005;247:123–130. doi: 10.1016/j.femsle.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Bradley DE. A function of Pseudomonas aeruginosa PAO pili: twitching motility. Can J Microbiol. 1980;26:146–154. doi: 10.1139/m80-022. [DOI] [PubMed] [Google Scholar]
- Brookman JL, Stott AJ, Cheeseman PJ, Burns NR, Adams SE, Kingsman AJ, Gull K. An immunological analysis of Ty1 virus-like particle structure. Virology. 1995;207:59–67. doi: 10.1006/viro.1995.1051. [DOI] [PubMed] [Google Scholar]
- Chan C, Paul R, Samoray D, Amiot NC, Giese B, Jenal U, Schirmer T. Structural basis of activity and allosteric control of diguanylate cyclase. Proc Natl Acad Sci USA. 2004;101:17084–17089. doi: 10.1073/pnas.0406134101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi E, Mehl T, Nunn D, Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect Immun. 1991;59:822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen M, Christen B, Folcher M, Schauerte A, Jenal U. Identification and characterization of a cyclic di-GMP specific phosphodiesterase and its allosteric control by GTP. J Biol Chem. 2005;280:30829–30837. doi: 10.1074/jbc.M504429200. [DOI] [PubMed] [Google Scholar]
- Comolli J, Waite L, Mostov K, Engel JN. The interaction of Pseudomonas aeruginosa pili and asialo-GM1 stimulates epithelial cell cytotoxicity and bacterial internalization. Infect Immun. 1999a;67:3207–3214. doi: 10.1128/iai.67.7.3207-3214.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comolli J, Hauser A, Waite L, Whitchurch C, Mattick J, Engel J. PilU and PilT are required for cytotoxicity and virulence of Pseudomonas aeruginosa. Infect Immun. 1999b;67:3625–3630. doi: 10.1128/iai.67.7.3625-3630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argenio DA, Calfee MW, Rainey PB, Pesci EC. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J Bacteriol. 2002;184:6481–6489. doi: 10.1128/JB.184.23.6481-6489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Medicis E, Paquette J, Gauthier JJ, Shapcott D. Magnesium and manganese contact of halophilic bacteria. Appl Environ Microbiol. 1986;52:567–573. doi: 10.1128/aem.52.3.567-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P, Todd T, Sastry PA, Lee KK, Hodges RS, Paranchych W, Irvin RT. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect Immun. 1988;56:1641–1646. doi: 10.1128/iai.56.6.1641-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SMJ, et al. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- Francis SH, Turko IV, Grimes KA, Corbin JD. Histidine-607 and histidine-643 provide important interactions for metal support of catalysis in phosphodiesterase-5. Biochemistry. 2000;39:9591–9596. doi: 10.1021/bi000392m. [DOI] [PubMed] [Google Scholar]
- Frank DW. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;4:621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- Garrity-Ryan L, Kazmierczak B, Kowal R, Comolli J, Hauser A, Engel J. The arginine finger domain of ExoT is required for actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect Immun. 2000;68:7100–7113. doi: 10.1128/iai.68.12.7100-7113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AL, Kulasekara BR, Rietsch A, Boyd D, Smith RS, Lory S. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Develop Cell. 2004;7:745–754. doi: 10.1016/j.devcel.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Hauser AR, Kang PJ, Engel J. PepA, a novel secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- Hoch JA, Silhavy TJ, editors. Two-Component Signal Transduction. Washington, DC: American Society for Microbiology Press; 1995. [Google Scholar]
- Huang B, Whitchurch CB, Croft L, Beatson SA, Mattick JS. A minimal tiling path cosmid library for functional analysis of the Pseudomonas aeruginosa PAO1 genome. Microb Comp Genomics. 2000;5:189–203. doi: 10.1089/omi.1.2000.5.189. [DOI] [PubMed] [Google Scholar]
- Huang B, Whitchurch CB, Mattick JS. FimX, a multidomain protein connecting environmental signals to twitching motility in Pseudomonas aeruginosa. J Bacteriol. 2003;185:7068–7076. doi: 10.1128/JB.185.24.7068-7076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang PJ, Hauser AR, Apodaca G, Fleiszig S, Wiener-Kronish J, Mostov K, Engel JN. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol Microbiol. 1997;24:1249–1262. doi: 10.1046/j.1365-2958.1997.4311793.x. [DOI] [PubMed] [Google Scholar]
- Kelly NM, Kluftinger JL, Pasloske BL, Paranchych W, Hancock RE. Pseudomonas aeruginosa pili as ligands for nonsopsonic phagocytosis by fibronectin-stimulated macrophages. Infect Immun. 1989;57:3841–3845. doi: 10.1128/iai.57.12.3841-3845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirillina O, Fetherston JD, Bobrov AG, Abney J, Perry RD. HmsP, a putative phosphodiesterase, and HmsT, a putative diguanylate cyclase, control Hms-dependent biofilm formation in Yersinia pestis. Mol Microbiol. 2004;54:75–88. doi: 10.1111/j.1365-2958.2004.04253.x. [DOI] [PubMed] [Google Scholar]
- Klausen M, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol Microbiol. 2003a;50:61–68. doi: 10.1046/j.1365-2958.2003.03677.x. [DOI] [PubMed] [Google Scholar]
- Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol. 2003b;48:1511–1524. doi: 10.1046/j.1365-2958.2003.03525.x. [DOI] [PubMed] [Google Scholar]
- Kulesekara H, Lee VT, Brencic A, Liberati N, Urbach J, Miyata S, et al. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc Natl Acad Sci USA. 2006;103:2839–2844. doi: 10.1073/pnas.0511090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski MA, Osborn E, Kazmierczak BI. A novel sensor kinase-response regulator hybrid regulates Type III secretion and is required for virulence in Pseudomonas aeruginosa. Mol Microbiol. 2004;54:1090–1103. doi: 10.1111/j.1365-2958.2004.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PV. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis: identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966;116:481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Mattick JS. Type IV pili and twitching motility. Annu Rev Microbiol. 2002;56:289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- Mattick JS, Bills MM, Anderson BJ, Dalrymple B, Mott MR, Egerton JR. Morphogenetic expression of Bacteroides nodosus fimbriae in Pseudomonas aeruginosa. J Bacteriol. 1987;169:33–41. doi: 10.1128/jb.169.1.33-41.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz AJ, So M, Sheetz MP. Pilus retraction powers bacterial twitching motility. Nature. 2000;407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- Nicas TI, Iglewski BH. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect Immun. 1984;45:470–474. doi: 10.1128/iai.45.2.470-474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa bio-film development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- Paul R, Weiser S, Amiot NC, Chan C, Schirmer T, Giese B, Jenal U. Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev. 2004;18:715–727. doi: 10.1101/gad.289504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U, Gomelsky M, Galperin MY. C-di-GMP: the dawning of a novel bacterial signalling system. Mol Microbiol. 2005;57:629–639. doi: 10.1111/j.1365-2958.2005.04697.x. [DOI] [PubMed] [Google Scholar]
- Salyers AA, Whitt DD, editors. Bacterial Pathogenesis: A Molecular Approach. Washington, DC: American Society for Microbiology Press; 2002. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sastry PA, Finlay BB, Pasloske BL, Parachych W, Pearlstone JR, Smillie LB. Comparative studies of the amino acid and nucleotide sequences of pilin derived from Pseudomonas aeruginosa PAK and PAO. J Bacteriol. 1985;164:571–577. doi: 10.1128/jb.164.2.571-577.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Frank DW, Hillard CJ, Feix JB, Pankhaniya RR, Moriyama K, et al. The mechanism of action of the Pseudomonas aeruginosa encoded type III cytotoxin, ExoU. EMBO J. 2003;22:2959–2969. doi: 10.1093/emboj/cdg290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AJ, Ryjenkov DA, Gomelsky M. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol. 2005;187:4774–4781. doi: 10.1128/JB.187.14.4774-4781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer HP, Hoang TT. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene. 1995;158:15–22. doi: 10.1016/0378-1119(95)00055-b. [DOI] [PubMed] [Google Scholar]
- Simm R, Morr M, Kader A, Nimtz M, Romling U. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol Microbiol. 2004;53:1123–1134. doi: 10.1111/j.1365-2958.2004.04206.x. [DOI] [PubMed] [Google Scholar]
- Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology. 1983;1:784–791. [Google Scholar]
- Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- Speert DP, Loh BA, Cabral DA, Salit IE. Nonopsonic phagocytosis of nonmucoid Pseudomonas aeruginosa by human neutrophils and monocyte-derived macrophages is correlated with bacterial piliation and hydrophobicity. Infect Immun. 1986;53:207–212. doi: 10.1128/iai.53.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers AJ, Kahn SG, Bohannon J, Travisano M, Rainey PB. Adaptive divergence in experimental populations of Pseudomonas fluorescens. I. Genetic and phenotypic bases of wrinkly spreader fitness. Genetics. 2002;161:33–46. doi: 10.1093/genetics/161.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C, Pham X, Erwin A, Mizoguchi S, Warrener P, Hickey M, et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Sundin C, Wolfgang M, Lory S, Forsberg A, Frithz-Lindsten E. Type IV pili are not specifically required for contact dependent translocation of exoenzymes by Pseudomonas aeruginosa. Microb Pathog. 2002;33:265–277. doi: 10.1006/mpat.2002.0534. [DOI] [PubMed] [Google Scholar]
- Tal R, Wong HC, Calhoon R, Gelfand D, Fear AL, Volman G, et al. Three cdg operons control cellular turnover of cyclic di-GMP in Acetobacter xylinum: genetic organization and occurrence of conserved domains in isoenzymes. J Bacteriol. 1998;180:4416–4425. doi: 10.1128/jb.180.17.4416-4425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet I, Olson JW, Lory S, Lazdunski A, Filloux A. The chaperone/usher pathways of Pseudomonas aeruginosa: identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc Natl Acad Sci USA. 2001;98:6911–6916. doi: 10.1073/pnas.111551898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AA, Alm RA, Mattick JS. Construction of improved vectors for protein production in Pseudomonas aeruginosa. Gene. 1996;172:163–164. doi: 10.1016/0378-1119(96)00026-1. [DOI] [PubMed] [Google Scholar]
- Whitchurch CB, Leech AJ, Young MD, Kennedy D, Sargent JL, Bertrand JJ, et al. Characterization of a complex chemosensory signal transduction system which controls twitching motility in Pseudomonas aeruginosa. Mol Microbiol. 2004;52:873–893. doi: 10.1111/j.1365-2958.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- Whitchurch CB, Beatson SA, Comolli JC, Jakobsen T, Sargent JL, Bertrand JJ, et al. Pseudomonas aeruginosa fimL regulates multiple virulence functions by intersecting with Vfr-modulated pathways. Mol Microbiol. 2005;55:1357–1378. doi: 10.1111/j.1365-2958.2005.04479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DE, Straus DC, Johanson WG, Berry VK, Bass JA. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980;29:1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhulin IB, Taylor BL. PAS domain S-boxes in archaea, bacteria and sensors for oxygen and redox. Trends Biochem Sci. 1997;22:331–333. doi: 10.1016/s0968-0004(97)01110-9. [DOI] [PubMed] [Google Scholar]
- Zogay X, Nimtz M, Rohde M, Bokranz W, Romling U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol. 2001;39:1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]