Abstract
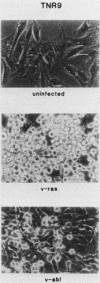
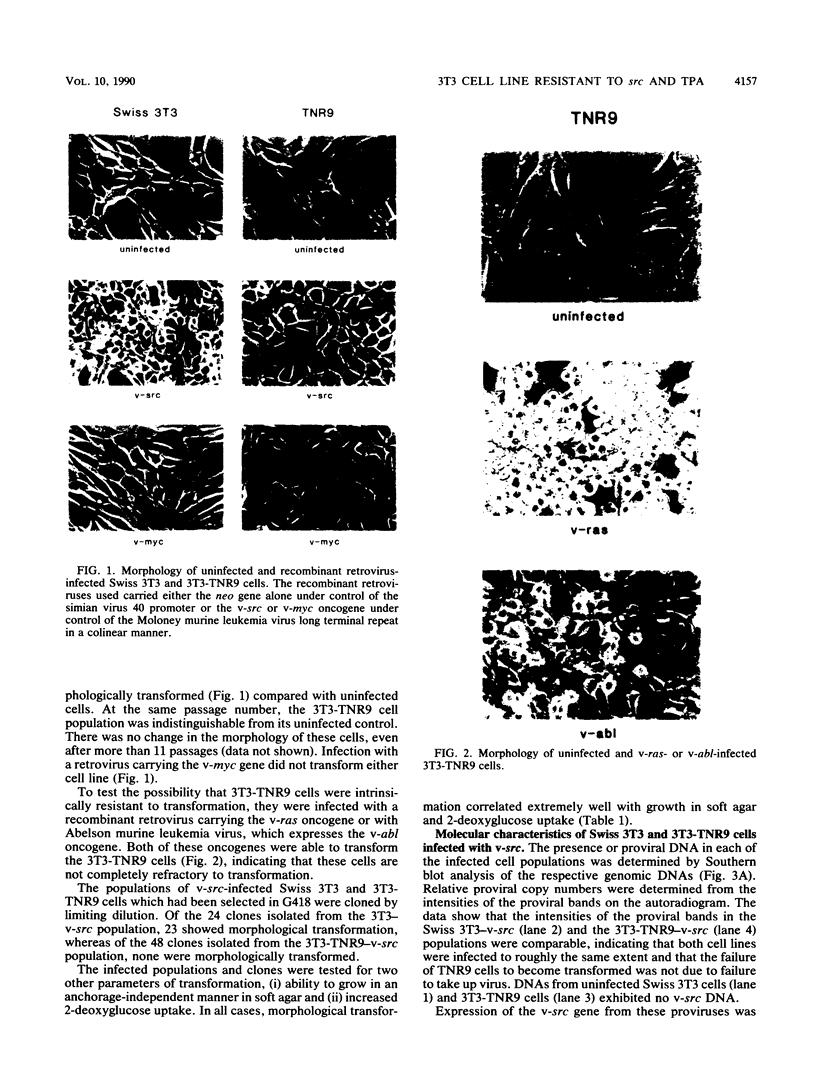
To study the relationship between oncogenesis by v-src and normal cellular signalling pathways, we determined the effects of v-src on 3T3-TNR9 cells, a Swiss 3T3 variant which does not respond mitogenically to tumor promoters such as 12-O-tetradecanoyl-phorbol-13-acetate (TPA). We found that src was unable to transform these variant cells, whether the oncogene was introduced by infection with a murine retrovirus vector or by transfection with plasmid DNA. 3T3-TNR9 cells were not inherently resistant to transformation, since infection with similar recombinant retroviruses containing either v-ras or v-abl did induce transformation. Further analysis of Swiss 3T3 and 3T3-TNR9 cell populations infected with the v-src-containing retrovirus revealed that although the amount of v-src DNA in each was approximately the same, the level of the v-src message and protein and the overall level of phosphotyrosine expressed in the infected variants was much less than in infected parental cells. Cotransfection experiments using separate v-src and neo plasmids revealed a decrease in the number of G418-resistant colonies when transfections of TNR9 cells occurred in the presence of the src-containing plasmid, suggesting a growth inhibitory effect of v-src on 3T3-TNR9 cells, as has also been found for TPA itself. Since v-src cannot transform this variant cell line, which does not respond mitogenically to the protein kinase C agonist TPA, we suggest that src makes use of the protein kinase C pathway as part of its signalling activities.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemà S., Casalbore P., Agostini E., Tatò F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985 Aug 8;316(6028):557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- Anderson D. D., Beckmann R. P., Harms E. H., Nakamura K., Weber M. J. Biological properties of "partial" transformation mutants of Rous sarcoma virus and characterization of their pp60src kinase. J Virol. 1981 Jan;37(1):445–458. doi: 10.1128/jvi.37.1.445-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemann H. P., Erikson R. L. Abnormal protein kinase C down regulation and reduced substrate levels in non-phorbol ester-responsive 3T3-TNR9 cells. Mol Cell Biol. 1990 May;10(5):2122–2132. doi: 10.1128/mcb.10.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R., Martinez R., Nakamura K. D., Weber M. J. A tumor promoter stimulates phosphorylation on tyrosine. Biochem Biophys Res Commun. 1983 Sep 15;115(2):536–543. doi: 10.1016/s0006-291x(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Bishop R., Martinez R., Weber M. J., Blackshear P. J., Beatty S., Lim R., Herschman H. R. Protein phosphorylation in a tetradecanoyl phorbol acetate-nonproliferative variant of 3T3 cells. Mol Cell Biol. 1985 Sep;5(9):2231–2237. doi: 10.1128/mcb.5.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Butler-Gralla E., Herschman H. R. Analysis of phorbol ester receptors in phorbol ester unresponsive 3T3 cell variants. Biochem Biophys Res Commun. 1981 Oct 15;102(3):818–823. doi: 10.1016/0006-291x(81)91611-9. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Butler-Gralla E., Herschman H. R. Glucose uptake and ornithine decarboxylase activity in tetradecanoyl phorbol acetate non-proliferative variants. J Cell Physiol. 1983 Mar;114(3):317–320. doi: 10.1002/jcp.1041140310. [DOI] [PubMed] [Google Scholar]
- Butler-Gralla E., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to the tumor promoter tetradecanoyl-phorbol-acetate. J Cell Physiol. 1981 Apr;107(1):59–67. doi: 10.1002/jcp.1041070108. [DOI] [PubMed] [Google Scholar]
- Butler-Gralla E., Taplitz S., Herschman H. R. 12-O-tetradecanoylphorbol-13-acetate stimulates release of arachidonic acid, prostaglandin E2 and prostaglandin F2 alpha from TPA nonproliferative variants of 3T3 cells. Biochem Biophys Res Commun. 1983 Feb 28;111(1):194–199. doi: 10.1016/s0006-291x(83)80135-1. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Wendel E. J., Abruzzo G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A., Talbot N., Barbacid M. C127 cells resistant to transformation by tyrosine protein kinase oncogenes. Cell Growth Differ. 1990 Jan;1(1):9–15. [PubMed] [Google Scholar]
- Diamond L., O'Brien S., Donaldson C., Shimizu Y. Growth stimulation of human diploid fibro-blasts by the tumor promoter, 12-0-tetradecanoly-phorbol-13-acetate. Int J Cancer. 1974 May 15;13(5):721–730. doi: 10.1002/ijc.2910130516. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Baird W. M. Tumor promoters and the mechanism of tumor promotion. Adv Cancer Res. 1980;32:1–74. doi: 10.1016/s0065-230x(08)60360-7. [DOI] [PubMed] [Google Scholar]
- Diringer H., Friis R. R. Changes in phosphatidylinositol metabolism correlated to growth state of normal and Rous sarcoma virus-transformed Japanese quail cells. Cancer Res. 1977 Sep;37(9):2979–2984. [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. The effect of phorbol diesters on chicken embryo fibroblasts. Cancer Res. 1977 Sep;37(9):3257–3265. [PubMed] [Google Scholar]
- Erikson R. L., Alcorta D., Bedard P. A., Blenis J., Biemann H. P., Erikson E., Jones S. W., Maller J. L., Martins T. J., Simmons D. L. Molecular analyses of gene products associated with the response of cells to mitogenic stimulation. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):143–151. doi: 10.1101/sqb.1988.053.01.020. [DOI] [PubMed] [Google Scholar]
- Fienup V. K., Jeng M. H., Hamilton R. T., Nilsen-Hamilton M. Relation between the regulation of DNA synthesis and the production of two secreted glycoproteins by 12-O-tetradecanoylphorbol-13-acetate in 3T3 cells and in phorbol ester nonresponsive 3T3 variants. J Cell Physiol. 1986 Nov;129(2):151–158. doi: 10.1002/jcp.1041290205. [DOI] [PubMed] [Google Scholar]
- Frantz C. N., Stiles C. D., Scher C. D. The tumor promoter 12-O-tetradecanoyl-phorbol-13-acetate enhances the proliferative response of Balb/c-3T3 cells to hormonal growth factors. J Cell Physiol. 1979 Sep;100(3):413–424. doi: 10.1002/jcp.1041000305. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Maruyama K., Garrels J. I., Ruley H. E. In vitro establishment is not a sufficient prerequisite for transformation by activated ras oncogenes. Cell. 1986 Feb 14;44(3):409–418. doi: 10.1016/0092-8674(86)90462-9. [DOI] [PubMed] [Google Scholar]
- Heaney M. L., Pierce J., Parsons J. T. Site-directed mutagenesis of the gag-myc gene of avian myelocytomatosis virus 29: biological activity and intracellular localization of structurally altered proteins. J Virol. 1986 Oct;60(1):167–176. doi: 10.1128/jvi.60.1.167-176.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Martin G. S., Shearer M., Critchley D. R., Epstein C. J. Viral transformation of rat myoblasts: effects on fusion and surface properties. Dev Biol. 1976 Jan;48(1):35–46. doi: 10.1016/0012-1606(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Johnson R. M., Wasilenko W. J., Mattingly R. R., Weber M. J., Garrison J. C. Fibroblasts transformed with v-src show enhanced formation of an inositol tetrakisphosphate. Science. 1989 Oct 6;246(4926):121–124. doi: 10.1126/science.2506643. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kozma L. M., Reynolds A. B., Weber M. J. Glycoprotein tyrosine phosphorylation in Rous sarcoma virus-transformed chicken embryo fibroblasts. Mol Cell Biol. 1990 Feb;10(2):837–841. doi: 10.1128/mcb.10.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lim R. W., Varnum B. C., O'Brien T. G., Herschman H. R. Induction of tumor promotor-inducible genes in murine 3T3 cell lines and tetradecanoyl phorbol acetate-nonproliferative 3T3 variants can occur through protein kinase C-dependent and -independent pathways. Mol Cell Biol. 1989 Apr;9(4):1790–1793. doi: 10.1128/mcb.9.4.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Martins T. J., Sugimoto Y., Erikson R. L. Dissociation of inositol trisphosphate from diacylglycerol production in Rous sarcoma virus-transformed fibroblasts. J Cell Biol. 1989 Feb;108(2):683–691. doi: 10.1083/jcb.108.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss P. S., Honeycutt N., Pawson T., Martin G. S. Viral transformation of chick myogenic cells. The relationship between differentiation and the expression of the SRC gene. Exp Cell Res. 1979 Oct 1;123(1):95–105. doi: 10.1016/0014-4827(79)90425-7. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Verma I. M., Hunter T. Detection of a transforming gene product in cells transformed by Moloney murine sarcoma virus. Cell. 1982 Jun;29(2):417–426. doi: 10.1016/0092-8674(82)90158-1. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Weber M. J. Genetics of src: structure and functional organization of a protein tyrosine kinase. Curr Top Microbiol Immunol. 1989;147:79–127. doi: 10.1007/978-3-642-74697-0_3. [DOI] [PubMed] [Google Scholar]
- Parsons S. J., McCarley D. J., Ely C. M., Benjamin D. C., Parsons J. T. Monoclonal antibodies to Rous sarcoma virus pp60src react with enzymatically active cellular pp60src of avian and mammalian origin. J Virol. 1984 Aug;51(2):272–282. doi: 10.1128/jvi.51.2.272-282.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3918–3921. doi: 10.1073/pnas.74.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R., Joseph C., Qureshi S. A., Berg K. L., Foster D. A. Evidence that v-src and v-fps gene products use a protein kinase C-mediated pathway to induce expression of a transformation-related gene. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7017–7021. doi: 10.1073/pnas.86.18.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Wagner E. F., Vanek M., Vennström B. Transfer of genes into embryonal carcinoma cells by retrovirus infection: efficient expression from an internal promoter. EMBO J. 1985 Mar;4(3):663–666. doi: 10.1002/j.1460-2075.1985.tb03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilenko W. J., Shawver L. K., Weber M. J. Down-modulation of EGF receptors in cells transformed by the src oncogene. J Cell Physiol. 1987 Jun;131(3):450–457. doi: 10.1002/jcp.1041310318. [DOI] [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- Whitman M., Kaplan D., Roberts T., Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J. 1987 Oct 1;247(1):165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wilkerson V. W., Bryant D. L., Parsons J. T. Rous sarcoma virus variants that encode src proteins with an altered carboxy terminus are defective for cellular transformation. J Virol. 1985 Aug;55(2):314–321. doi: 10.1128/jvi.55.2.314-321.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke J. A., Linial M. Temperature-sensitive avian sarcoma viruses: a physiological comparison of twenty mutants. Virology. 1973 May;53(1):152–161. doi: 10.1016/0042-6822(73)90474-1. [DOI] [PubMed] [Google Scholar]
- Ziegler S. F., Whitlock C. A., Goff S. P., Gifford A., Witte O. N. Lethal effect of the Abelson murine leukemia virus transforming gene product. Cell. 1981 Dec;27(3 Pt 2):477–486. doi: 10.1016/0092-8674(81)90389-5. [DOI] [PubMed] [Google Scholar]