Abstract
The aims of this study were twofold: (1) to identify whether peripheral artery disease (PAD) patients had increased muscle concentration of angiogenic VEGF-A, anti-angiogenic VEGF165b or VEGF receptor 1 (VEGF-R1) when compared with control subjects, and (2) to evaluate whether exercise training in PAD patients was associated with changes in muscle concentration of VEGF-A, VEGF165b or VEGF-R1. At baseline, 22 PAD and 30 control subjects underwent gastrocnemius muscle biopsy. Twelve PAD patients were treated with supervised exercise training (SET) and underwent muscle biopsy after 3 weeks and 12 weeks of training and had sufficient tissue to measure VEGF-A, VEGF165b and VEGF-R1 concentrations in skeletal muscle lysates by ELISA. Muscle concentrations of VEGF-A and VEGF165b were similar in PAD patients versus controls at baseline. At both time points after the start of SET, VEGF-A levels decreased and there was a trend towards increased VEGF165b concentrations. At baseline, VEGF-R1 concentrations were lower in PAD patients when compared with controls but did not change after SET. Skeletal muscle concentrations of VEGF-A are not different in PAD patients when compared with controls at baseline. SET is associated with a significant reduction in VEGF-A levels and a trend towards increased VEGF165b levels. These somewhat unexpected findings suggest that further investigation into the mechanism of vascular responses to exercise training in PAD patients is warranted.
Keywords: angiogenesis, peripheral artery disease, vascular endothelial growth factor (VEGF)
Introduction
Peripheral artery disease (PAD) is a prevalent disorder that is caused by atherosclerosis of the arteries of the lower extremity. A common manifestation of PAD is intermittent claudication (IC) which is defined as leg pain that worsens with exertion and improves with rest.1 IC is associated with poor functional capacity and a diminished quality of life.2,3 Patients with PAD are also at a significantly elevated risk of morbidity and mortality when compared with similar patients without PAD.1
With the exception of supervised exercise training (SET), few treatment options have been shown to improve functional capacity in patients with IC. SET has a class IA recommendation in the ACC/AHA guidelines, largely due to the significant improvements in treadmill walking distance and time which typically result.1 Our group has recently published a report on the role of the microvasculature in patients with PAD, suggesting that the number of capillaries per mm2 in skeletal muscle is an important contributor to overall functional performance in patients with IC.4 Vasculogenesis, or the growth of new blood vessels,5,6 occurs with exercise training in healthy adults,7–9 and we recently showed that an increase in capillary supply in ischemic gastrocnemius skeletal muscle occurred prior to improvement in peak oxygen consumption (VO2) in patients with IC treated with SET.10
Vascular endothelial growth factor (VEGF) is perhaps the best known and most extensively studied angiogenic growth factor. In exercise studies of animals and healthy adults, a temporal association exists between increases in VEGF and the number of capillaries and thus VEGF has been implicated as important to exercise-induced skeletal muscle angiogenesis.8,11–13 VEGF, also known as VEGF-A in humans, exists in four major splice variants of 121, 165, 189, and 206 amino acids. In addition, splice variants with a small amino acid change in the 8th exon produce an inhibitory splice variant termed VEGF165b.14 VEGF-A is thought to interact in a complex way with membrane-bound receptors (VEGF-R1 and VEGF-R2) as well as with a soluble splice variant of VEGF-R1, identified as sVEGF-R1. While VEGF-A binds to and activates the membrane-bound VEGF receptors, thus leading to angiogenesis in human cancers and other disease states, VEGF165b inhibits VEGF receptor activation but its role in human conditions has been largely unstudied.15 Recently, a study of patients with systemic sclerosis has documented that while total VEGF165 was elevated in the skin of these patients, the increase was actually in VEGF165b and it was a mechanism for defective angiogenesis and vascular repair in this patient population.15,16 To date, no published human study has investigated protein concentrations of the pro-angiogenic form (VEGF-A), anti-angiogenic form (VEGF165b), and VEGF receptors in the skeletal muscle of patients with IC and the changes in these levels with long-term exercise training in this population.
The aims of the present report were twofold: (1) to compare the skeletal muscle concentration of VEGF-A, VEGF165b and VEGF receptor 1 (VEGF-R1) in patients with IC when compared with control patients, and (2) to evaluate whether skeletal muscle VEGF-A, VEGF165b and VEGF-R1 concentrations change with exercise training in patients with IC.
Methods
The patient population, inclusion/exclusion criteria, baseline testing, exercise testing, skeletal muscle biopsy, and skeletal muscle immunohistochemical analysis have been previously described.4,10,17 At baseline, all patients with PAD and control patients underwent similar testing protocols. Patients with PAD were then randomly assigned to supervised exercise training (SET) or home exercise training (HET). All subjects were informed of testing protocols and the potential risks and benefits of participation. Each subject provided written informed consent before enrollment in the study. The Institutional Review Boards at Duke University and the University of Colorado approved the research protocols.
Exercise training
PAD subjects randomized to SET came to medically supervised sessions three times a week until 36 sessions were completed. No subject exceeded 16 weeks to complete the 36 sessions. All subjects exercised on a treadmill at the workload that claudication onset was documented from the baseline cardiopulmonary exercise (CPX) test. Subjects were asked to exercise to near maximal pain utilizing a standardized claudication scale, at which time the subject stepped off the treadmill and rested until claudication pain subsided. Exercise and rest cycles were repeated during each exercise training session until the accumulation of 30–40 minutes of exercise was completed, referring to the actual time walked not including rest breaks. After a subject was able to walk for 8–10 minutes at their initial workload, speed and elevation were increased to elicit claudication again. To provide optimal medical care for subjects not randomized to supervised exercise, subjects in the home exercise group were given identical exercise instructions as the supervised group; however, they were asked to perform the exercise training on their own at home with an exercise prescription following the ACC/AHA guideline recommendation for PAD exercise training (Class I level of evidence A) at the time of the study according to current recommendations. All exercise training for both supervised and home groups were recorded for number of sessions per week and minutes per sessions.
Skeletal muscle analysis
Skeletal muscle samples were snap frozen in liquid nitrogen at the time of biopsy and stored at −80°C. Frozen tissue was homogenized in 2 ml of RIPA lysis buffer (Santa Cruz Biotechnologies) and protein and RNA were extracted at this time. The suspension was centrifuged twice at 8000 g at 4°C for 10 minutes and the protein content of the super-natant was determined by Bio-Rad Dc assay.
VEGF concentrations in skeletal muscle lysates were measured by a sandwich enzyme ELISA (R&D Systems) according to the manufacturer's instruction and the standard curve was made by serial dilution of recombinant human VEGF from 500 pg/ml to 7.8 pg/ml. This kit was designed to detect both VEGF165a and VEGF165b. A similar sandwich ELISA was used to measure the concentrations of VEGF-R1 in skeletal muscle lysates (R&D Systems) using a monoclonal antibody specific to human VEGF-R1 and a standard curve that was made by serial dilution of recombinant human VEGF-R1. VEGF165b was also measured using a Duoset sandwich ELISA (R&D Systems), which is highly specific for VEGF165b and the assay was completed according to the manufacturer's instructions.
Statistical analysis
Differences in categorical variables between control and PAD subjects were determined by chi square analysis. Baseline differences in skeletal muscle measurements between groups for continuous variables were determined by an ANOVA controlling for age and sex. This experiment was designed to compare three time points during exercise training in the PAD subjects: 0 weeks, 3 weeks and 12 weeks. Therefore, a repeated measures analysis was used with post hoc testing. Changes in the number of capillaries per fiber and capillaries per mm2 in skeletal muscle, VEGF-A levels, VEGF165b levels, VEGF-R1 levels and peak VO2 following SET were analyzed. A paired t-test was performed to determine differences in VEGF-A in the IC patients undergoing HET at baseline and after 12 weeks. Bivariate correlations were calculated to determine the relationships between VEGF-A and the number of capillaries per fiber and capillaries per mm2 in patients undergoing SET. Tabular data are presented as means ± SD. A p-value of < 0.05 was considered significant for all tests.
Results
Patient population
The baseline demographic and clinical characteristics of the PAD and control patients are summarized in Table 1. PAD patients were older, more likely to be male, and had lower ankle–brachial index (ABI) measurements at enrollment. Twenty-two PAD subjects were studied at baseline and 12 completed SET with available VEGF-A measures at each time point. Ten PAD subjects were studied at baseline and after 12 weeks of HET.
Table 1.
Baseline demographic and clinical characteristics of PAD and control subjects
| Controls | PAD | |
|---|---|---|
| Number | 30 | 22 |
| Age, years | 53 ± 8 | 67 ± 10* |
| Race, % Caucasian | 63% | 73% |
| Sex (men/women) | 10/20 | 14/8 |
| Body mass index, kg/m2 | 27.3 ± 6.3 | 26.5 ±4.1 |
| Ankle-brachial index | 1.09 ±0.1 | 0.64 ± 0.2* |
| Past or current smoking, % | 29% | 90% |
| Aspirin use, % | 29% | 64%* |
| ACE inhibitor/ARB use, % | 3% | 45% |
| Statin use, % | 6% | 73% |
Values are mean ± SD or percentages.
p < 0.05 between control and PAD patients
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Differences in skeletal muscle VEGF levels among PAD and control subjects at baseline
Prior to exercise training, PAD and control subjects had similar skeletal muscle VEGF-A concentrations and VEGF165b concentrations (Table 2). PAD patients did have significantly lower skeletal muscle VEGF-R1 concentrations when compared with controls (Table 2). Similar to our previous reports in this patient population, patients with IC had significantly fewer capillaries per mm2, significantly fewer capillaries per fiber, and significantly lower peak VO2 when compared with control subjects.4 There was no effect of age or sex by ANCOVA when comparing functional measures, capillaries/mm2, capillaries/fiber, VEGF-A, VEGF165b, or VEGF-R1 concentrations among PAD and control patients.
Table 2.
Differences at baseline in skeletal muscle VEGF concentration, number of capillaries/fiber and capillaries/mm2, and peak VO2 among control and PAD subjects
| VEGF-A | VEGFl65b | VEGF-R1 | Capillary supply | Capillary supply | Peak VO2 | |
|---|---|---|---|---|---|---|
|
| ||||||
| pg VEGF-A/mg protein | pg VEGFl65b/mg protein | pg VEGF-R1/mg protein | Capillaries/mm2 | Capillaries/fiber | ml/kg/min | |
| Control | 116.5 ± 56.6 | 2120.6 ± 819.1 | 508.6 ± 313.8* | 317.6 ± 87.2* | 2.2 ± 0.7* | 25.6 ± 6.6* |
| n = 30 | n = 14 | n = 28 | n = 26 | n = 26 | n = 29 | |
| PAD | 119.3 ± 73.9 | 1720.1 ± 743.5 | 177.3 ± 133.3 | 247.7 ± 69.8 | 1.8 ± 0.6 | 16.9 ± 4.2 |
| n = 22 | n = 17 | n = 20 | n = 22 | n = 22 | n = 22 | |
p < 0.05 between control and PAD patients.
PAD, peripheral artery disease; peak VO2, peak oxygen consumption; pg, picogram; VEGF, vascular endothelial growth factor; VEGF-R1, vascular endothelial growth factor receptor 1.
Changes in skeletal muscle VEGF levels after SET in patients with IC
Figure 1 shows the temporal changes in VEGF-A and VEGF165b in PAD patients who completed 12 weeks of SET. There was a statistically significant decrease in skeletal muscle VEGF-A concentrations after 3 weeks (mean pg VEGF-A/mg protein ± SD; baseline: 137.8 ± 93.5 vs 3 weeks: 75.1 ± 47.5, p < 0.05) and this concentration remained significantly lower after 12 weeks (mean pg VEGF-A/mg protein ± SD; baseline: 137.8 ± 93.5 vs 12 weeks: 79.0 ± 53.0, p < 0.05). Because the assay for VEGF-A measures both the pro-angiogenic and the anti-angiogenic form of VEGF165, we sought to determine if the decrease in total VEGF165 was due to a decrease in the inhibitory form, but this was not the case. PAD patients undergoing SET had a non-significant increase in skeletal muscle VEGF165b levels after 3 weeks (mean pg VEGF165b/mg protein ± SD; baseline: 1835.2 ± 421.1 vs 3 weeks: 2759.0 ± 1572.0, p = 0.34) and 12 weeks (mean pg VEGF165b/mg protein ± SD; baseline: 1835.2 ± 421.1 vs 12 weeks: 3076.1 ± 889.6, p = 0.15). There was no change from baseline in skeletal muscle VEGF-R1 concentrations after supervised exercise training.
Figure 1.
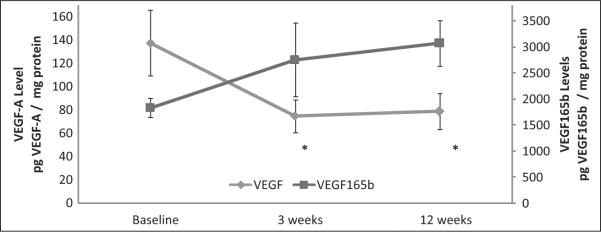
Temporal changes in skeletal muscle VEGF-A and VEGF165b levels in patients with IC who completed 12 weeks of supervised exercise training (SET): n = 11 for VEGF-A measurements; n = 5 for VEGF165b measurements. (VEGF, vascular endothelial growth factor; pg, picogram; *p < 0.05 between baseline and 3-week and baseline and 12-week VEGF-A levels. Note: only 5 IC patients completed 12 weeks of SET and had sufficient tissue to measure VEGF165b levels.)
Changes in skeletal muscle VEGF levels after HET in patients with IC
In PAD patients who completed 12 weeks of HET, there was no observed decrease in skeletal muscle VEGF-A concentrations after 12 weeks (baseline: 97.2 ± 45.3 vs 12 weeks: 91.8 ± 44.3, p = 0.79).
Relationship between VEGF-A concentrations and the number of capillaries per fiber and capillaries per mm2 in the skeletal muscle of patients with IC after 12 weeks of SET
In the patients with IC who completed 12 weeks of SET, the number of capillaries per mm2 increased after 3 weeks and remained significantly higher after 12 weeks (Figure 2). Peak VO2 did not change after 3 weeks (ml/kg/m2 ± SD; baseline: 15.9 ± 3.2 vs 3 weeks: 15.5 ± 3.7, p = 0.41) but increased significantly after 12 weeks of SET (ml/kg/m2 ± SD; baseline: 15.9 ± 3.2 vs 12 weeks: 17.9 ± 3.9, p = 0.001). In the IC patients who completed SET, there was a strong directionally positive relationship between VEGF-A concentrations and the number of capillaries per fiber (r = 0.737, p = 0.006) and capillaries per mm2 (r = 0.727, p = 0.007) (Figure 3). These relationships were not observed at baseline (data not shown).
Figure 2.
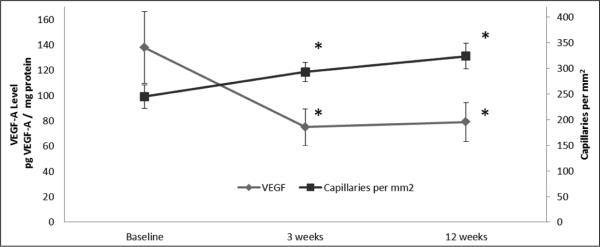
Temporal changes in skeletal muscle VEGF-A levels and the number of capillaries per mm2 in patients with IC who completed 12 weeks of supervised exercise training (SET): n = 11. (VEGF, vascular endothelial growth factor; pg, picogram; *p < 0.05 between baseline and 3-week and baseline and 12-week VEGF-A levels and capillaries per mm2.)
Figure 3.
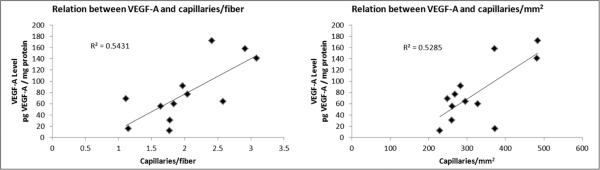
Relation between VEGF-A and the number of capillaries per fiber and capillaries per mm2 in patients with IC who completed 12 weeks of supervised exercise training. (VEGF, vascular endothelial growth factor; pg, picogram.)
Discussion
The main purpose of our study was to improve our understanding of the skeletal muscle adaptations that occur with SET in patients with IC. VEGF-mediated angiogenesis in peripheral skeletal muscle has been shown to occur in healthy animals and humans and we sought to determine if this occurred in humans with PAD, as no prior study has reported the effects of SET on VEGF-A, VEGF165b and VEGF-R1 in patients with IC. Our study has four new and unique findings: (1) skeletal muscle VEGF-A concentrations are similar and VEGF-R1 concentrations are lower in IC patients when compared with controls; (2) skeletal muscle VEGF-A concentrations actually decline in patients with IC after the onset of SET; (3) skeletal muscle levels of VEGF165b, a previously poorly defined anti-angiogenic growth factor, are similar in PAD and control subjects and these levels tended to increase in patients with IC after performing SET; and (4) there is a significant positive relationship between skeletal muscle VEGF-A concentrations and the number of capillaries per fiber and capillaries per mm2 in IC patients after 12 weeks of SET.
The finding that VEGF-A did not increase and/or that the inhibitory form of VEGF165b failed to decrease following exercise training despite an increase in the number of capillaries is quite unexpected. When these results are compared with the existing literature on VEGF in patients with and without PAD and how these levels change with exercise training, these data suggest a dysfunctional or impaired VEGF response to exercise in PAD. Most human studies of VEGF levels in skeletal muscle have involved healthy subjects, and changes in VEGF levels have been reported with acute and long-term exercise training.8,9,13,18 Comparisons among these studies are limited by differences in study population, measurement techniques, type of exercise intervention, site of skeletal muscle biopsies, timing of biopsies in relationship to exercise performance, and the lack of appreciation that VEGF165 has both a pro-angiogenic and an anti-angiogenic splice variant of the same size that need to be assessed separately. Prior to exercise training, Gavin et al. measured the interstitial concentration of VEGF in vastus lateralis muscle and reported no difference between young and elderly men.9 In older women, skeletal muscle VEGF-A protein levels were found to be lower when compared with younger women, and these lower VEGF-A levels were associated with a lower capillary supply.19 Multiple studies have reported an increase in VEGF mRNA and protein levels at different time points after acute bouts of leg extension or aerobic exercise.9,13,18–22 Two of these studies have described an attenuated VEGF response with long-term exercise when compared with acute exercise, an effect that was hypothesized to be due to skeletal muscle adaptation in the microvasculature and less dependence on VEGF to promote angiogenesis with continued exercise training.18,22
There are no data on the differences in skeletal muscle VEGF concentrations in patients with IC when compared with controls; however, reports from patients with a more severe form of PAD, critical limb ischemia (CLI), have been published. In patients with CLI, skeletal muscle VEGF-A expression was similar to control patients in proximal muscle groups (i.e. at the site of amputation in CLI patients); however, VEGF expression was significantly higher in more distal muscle groups (i.e. foot) in CLI patients undergoing amputation when compared with controls.23,24 Patients with CLI have muscle ischemia at rest, which may be a sufficient stimulus to change VEGF expression. In addition to this CLI study, reports from IC patients undergoing acute and long-term exercise training have been published. In patients with IC undergoing acute exercise training, one group found that skeletal muscle VEGF protein was similar before and after acute bouts of exercise while significant increases in VEGF mRNA expression were seen.25 In a study of both acute and long-term exercise training, Wood et al. reported no change in the plasma VEGF protein concentration after a submaximal exercise test or after long-term exercise training.26 Unfortunately, skeletal muscle samples were not obtained and the authors concluded that `it would be useful to examine the changes in VEGF and receptor expression, and vascular adaptation within the muscle'.
We hypothesized that skeletal muscle VEGF-A levels would be lower in IC patients due to a significantly lower number of capillaries in IC patients when compared with controls.4 VEGF-A protein concentrations in skeletal muscle were, in fact, similar between IC and control subjects (Table 2). These findings appear to be in line with reports comparing elderly and young subjects. When compared to the literature in CLI patients, our findings differ – likely because CLI is more severe and was an exclusion criterion in our study. Additionally, these CLI studies measured VEGF mRNA levels while we measured VEGF protein levels as these measurements are not always concordant.27,28
This study demonstrated that VEGF-R1 was significantly lower in patients with IC when compared with control patients. There are three major VEGF receptors but VEGF-R3's role is limited to lymphangiogenesis. Since VEGF-R2 is the dominant VEGF receptor involved in post-natal angiogenesis, a decrease in VEGF-R1 relative to VEGF-R2 could well be viewed as an adaptive response in an attempt to drive the VEGF ligand to VEGF-R2 to increase angiogenesis.29 Alternatively, VEGF-R1 may be simply down-regulated in patients with PAD for reasons yet to be resolved. In the only prior report of VEGF-R1 levels in patients with PAD, VEGF-A and VEGF-R2 (but not VEGF-R1) were diffusely expressed in skeletal myocytes in actively ischemic legs of patients with CLI.30 With exercise training, our patients with IC had an increased number of capillaries per fiber and capillaries per mm2 but they did not have decreased skeletal muscle VEGF-R1 as a mechanism to promote angiogenesis.
Our finding that VEGF-A declines in patients with IC undergoing SET is certainly unexpected. While it is possible that lower skeletal muscle VEGF-A levels in IC patients after SET occurs because the patients have less muscle ischemia due to the effects of the training, this is unlikely as our IC patients continue to have claudication at higher workloads after SET. To verify that VEGF-A did not decrease over time, we used data from IC patients undergoing standard of care (HET) to determine whether VEGF-A levels decrease with all forms of exercise training, and these patients had no difference in VEGF-A after HET. The fact that VEGF-A did not go up with exercise, as is observed in healthy populations, may provide additional evidence for a skeletal muscle myopathy in patients with IC. Of course we were unable to measure all possible growth factors that may be responsible for the observed angiogenesis response, but we were able to refute a primary hypothesis that VEGF-A increases in PAD patients after SET. Further work is needed to better understand the angiogenic processes in skeletal muscle in PAD.
We also directly measured muscle concentrations of the inhibitory splice variant, VEGF165b, with the hypothesis that the decline in VEGF-A (a measurement that includes both VEGF165a and VEGF165b) would be explained by a decline in VEGF165b. This was not the case, and VEGF165b levels actually increased with exercise training (Figure 1). The current data demonstrate that an intervention that leads to improvements in walking measures, peak VO2, and number of capillaries is associated with lower, rather than higher, VEGF-A concentrations in the skeletal muscle of patients with IC, and it is interesting to note that this would be contrary to the approach of trying to increase VEGF-A as a therapeutic tool.
Given prior evidence that VEGF-A was temporally linked to changes in the number of capillaries with exercise training in animals and healthy adults, we explored the relationship between VEGF-A and the number of capillaries per fiber and capillaries per mm2 in our IC patients who completed 12 weeks of SET. We found a strong, statistically significant relationship between VEGF-A and the number of capillaries per fiber and capillaries per mm2 (Figure 3). This was an expected finding, but when taken in the context of lower skeletal muscle VEGF-A levels with exercise training, the strength of this relationship was somewhat surprising. Therefore, the amount of VEGF-A measured following long-term exercise training appears to be related to the number of capillaries, despite the fact that it decreased following exercise training. Further studies will be needed to determine whether VEGF administration can improve the number of capillaries and functional capacity in IC patients undergoing SET.
There are several limitations of this study. First, despite attempts to match the control and IC patients by age and sex in the trial, control patients were younger and more commonly female but, after adjustment, these imbalances had no effect on VEGF measurements in subjects with IC. Second, we had limited amounts of skeletal muscle tissue, and this accounts for the relatively small sample size. Owing to this, we were unable to perform mRNA measurements for comparison with prior skeletal muscle studies, though protein measurements are functionally more important than mRNA measurements. Third, we did not measure the acute effects of exercise with skeletal muscle measurements, and therefore we may have missed changes in VEGF concentrations at various time points after acute bouts of exercise. Last, VEGF expression and function in the human body is a complex process and there is the possibility that unstudied mechanisms, such as sVEGF-R1, could account for some of the variation observed in this study.
In summary, patients with IC have similar levels of skeletal muscle VEGF-A and VEGF165b prior to exercise training when compared with control patients. After 12 weeks of supervised exercise training, skeletal muscle VEGF-A levels decline significantly in patients with IC despite an improvement in walking measures, an increase in capillary density and an improvement in peak VO2. There was a significant relationship between VEGF-A levels and the number of capillaries per fiber and capillaries per mm2 after 12 weeks of SET. Skeletal muscle VEGF165b levels, which have never been reported in patients with PAD, increased after 3 and 12 weeks of SET. There was no change in skeletal muscle VEGF-R1 levels in IC patients performing SET. The results of the current study support the theory that angiogenesis in humans is a complex process that cannot be fully explained by a single growth factor or family of growth factors. Further investigation into the mechanisms involved in skeletal muscle adaptation to supervised exercise training is warranted.
Acknowledgments
Funding This project was supported by R01 HL75752, RO1 HL101200 and 1RO1 HL101200S1 from the National Institute of Health, the National Heart, Lung, and Blood Institute and the Office of Research on Women's Health, Office of the Director to BHA. AO Dokun is also supported by ORG 40745 from the RWJ Foundation, Harold Amos Medical Faculty Development Program.
Footnotes
Conflict of interest None of the authors has any conflicts of interest relative to the intervention.
References
- 1.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 3.Mays RJ, Casserly IP, Kohrt WM, et al. Assessment of functional status and quality of life in claudication. J Vasc Surg. 2011;53:1410–1421. doi: 10.1016/j.jvs.2010.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins JL, Jones WS, Duscha BD, et al. Relationship between leg muscle capillary density and blood flow with endurance capacity in peripheral artery disease. J Appl Physiol. 2011;111:81–86. doi: 10.1152/japplphysiol.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 7.Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977;270:677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavin TP, Drew JL, Kubik CJ, Pofahl WE, Hickner RC. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol (Oxf) 2007;191:139–146. doi: 10.1111/j.1748-1716.2007.01723.x. [DOI] [PubMed] [Google Scholar]
- 9.Gavin TP, Ruster RS, Carrithers JA, et al. No difference in the skeletal muscle angiogenic response to aerobic exercise training between young and aged men. J Physiol. 2007;585:231–239. doi: 10.1113/jphysiol.2007.143198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duscha BD, Robbins JL, Jones WS, et al. Angiogenesis in skeletal muscle precede improvements in peak oxygen uptake in peripheral artery disease patients. Arterioscler Thromb Vasc Biol. 2011;31:2742–2748. doi: 10.1161/ATVBAHA.111.230441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters RE, Rotevatn S, Li P, Annex BH, Yan Z. Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol. 2004;287:C1342–1348. doi: 10.1152/ajpcell.00247.2004. [DOI] [PubMed] [Google Scholar]
- 12.Hoier B, Rufener N, Bojsen-Moller J, Bangsbo J, Hellsten Y. The effect of passive movement training on angiogenic factors and capillary growth in human skeletal muscle. J Physiol. 2010;588:3833–3845. doi: 10.1113/jphysiol.2010.190439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen AH, Nielsen JJ, Saltin B, Hellsten Y. Exercise training normalizes skeletal muscle vascular endothelial growth factor levels in patients with essential hypertension. J Hypertens. 2010;28:1176–1185. doi: 10.1097/HJH.0b013e3283379120. [DOI] [PubMed] [Google Scholar]
- 14.Nowak DG, Amin EM, Rennel ES, et al. Regulation of vascular endothelial growth factor (VEGF) splicing from proangiogenic to anti-angiogenic isoforms: a novel therapeutic strategy for angiogenesis. J Biol Chem. 2010;285:5532–5540. doi: 10.1074/jbc.M109.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dokun AO, Annex BH. The VEGF165b `ICE-o-form' puts a chill on the VEGF story. Circ Res. 2011;109:246–247. doi: 10.1161/CIRCRESAHA.111.249953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manetti M, Guiducci S, Romano E, et al. Overexpression of VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, leads to insufficient angiogenesis in patients with systemic sclerosis. Circ Res. 2011;109:e14–26. doi: 10.1161/CIRCRESAHA.111.242057. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell RG, Duscha BD, Robbins JL, et al. Increased levels of apoptosis in gastrocnemius skeletal muscle in patients with peripheral arterial disease. Vasc Med. 2007;12:285–290. doi: 10.1177/1358863X07084858. [DOI] [PubMed] [Google Scholar]
- 18.Jensen L, Pilegaard H, Neufer PD, Hellsten Y. Effect of acute exercise and exercise training on VEGF splice variants in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2004;287:R397–402. doi: 10.1152/ajpregu.00071.2004. [DOI] [PubMed] [Google Scholar]
- 19.Croley AN, Zwetsloot KA, Westerkamp LM, et al. Lower capillarization, VEGF protein, and VEGF mRNA response to acute exercise in the vastus lateralis muscle of aged vs. young women. J Appl Physiol. 2005;99:1872–1879. doi: 10.1152/japplphysiol.00498.2005. [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson T, Rundqvist H, Norrbom J, Rullman E, Jansson E, Sundberg CJ. The influence of physical training on the angiopoietin and VEGF-A systems in human skeletal muscle. J Appl Physiol. 2007;103:1012–1020. doi: 10.1152/japplphysiol.01103.2006. [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson T, Ameln H, Fischer H, Sundberg CJ, Timmons JA, Jansson E. VEGF-A splice variants and related receptor expression in human skeletal muscle following submaximal exercise. J Appl Physiol. 2005;98:2137–2146. doi: 10.1152/japplphysiol.01402.2004. [DOI] [PubMed] [Google Scholar]
- 22.Richardson RS, Wagner H, Mudaliar SR, Saucedo E, Henry R, Wagner PD. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279:H772–778. doi: 10.1152/ajpheart.2000.279.2.H772. [DOI] [PubMed] [Google Scholar]
- 23.Palmer-Kazen U, Wariaro D, Luo F, Wahlberg E. Vascular endothelial cell growth factor and fibroblast growth factor 2 expression in patients with critical limb ischemia. J Vasc Surg. 2004;39:621–628. doi: 10.1016/j.jvs.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Choksy S, Pockley AG, Wajeh YE, Chan P. VEGF and VEGF receptor expression in human chronic critical limb ischaemia. Eur J Vasc Endovasc Surg. 2004;28:660–669. doi: 10.1016/j.ejvs.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Palmer-Kazen U, Religa P, Wahlberg E. Exercise in patients with intermittent claudication elicits signs of inflammation and angiogenesis. Eur J Vasc Endovasc Surg. 2009;38:689–696. doi: 10.1016/j.ejvs.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Wood RE, Sanderson BE, Askew CD, Walker PJ, Green S, Stewart IB. Effect of training on the response of plasma vascular endothelial growth factor to exercise in patients with peripheral arterial disease. Clin Sci (Lond) 2006;111:401–409. doi: 10.1042/CS20060151. [DOI] [PubMed] [Google Scholar]
- 27.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007;101:948–956. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 28.Waltenberger J, Mayr U, Pentz S, Hombach V. Functional upregulation of the vascular endothelial growth factor receptor KDR by hypoxia. Circulation. 1996;94:1647–1654. doi: 10.1161/01.cir.94.7.1647. [DOI] [PubMed] [Google Scholar]
- 29.Wu FTH, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS. VEGF and soluble VEGF receptor-1 (sFlt-1) distributions in peripheral arterial disease: an in silico model. Am J Physiol Heart Circ Physiol. 2010;298:H2174–2191. doi: 10.1152/ajpheart.00365.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rissanen TT, Vajanto I, Hiltunen MO, et al. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol. 2002;160:1393–1403. doi: 10.1016/S0002-9440(10)62566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


