Abstract
Progesterone can enhance cognitive performance among young and aged mice; however, the mechanisms underlying these effects of progesterone are not well-understood. Aged, mice which lack functional progestin receptors (PRKO), or wildtype mice were administered progesterone (10 mg/kg, SC), or vehicle, and learning/memory was evaluated. Progesterone, compared to vehicle, produced a conditioned place preference in PRKO and wildtype mice. Progesterone improved performance of PRKO and wildtype mice in the object placement, water maze, contextual and cued fear conditioning tasks. PRKO, compared to wildtype, mice performed better in the inhibitory avoidance task, irrespective of progesterone. Thus, progesterone to aged mice enhances performance across a variety of tasks and this may not require actions at PRs.
Keywords: Cognition, Learning, Memory, Estrogen, Estradiol, Aging
Estradiol (E2) and progesterone (P4) may influence cognitive performance. Effects of E2 on learning/memory have received much attention. Yet, P4 also varies over reproductive cycles and may influence cognition. In the water maze, P4 improves performance when administered to ovariectomized (OVX), young adult rats that are E2-primed [30,37]. Among mid-aged rats, forgetting in the water maze is attenuated by P4 and/or E2 after OVX at 14 months [17]. Interestingly, working memory in the radial arm maze was better among 14-month old rats that had been administered P4 and/or E2 following OVX 2 months prior [38]. Yet, better acquisition in the delayed-matching-to-position task was only seen among rats that were administered E2 and/or P4 immediately or 3 but not 10 months after OVX at 13 months of age [18]. Thus, P4, in addition to E2, may enhance cognitive performance of rats; albeit, the nature and duration of steroid deprivation and replacement may influence these effects.
Aged, compared to younger, individuals may differ in their response to P4. We have begun to address this by evaluating the effects of P4 to young and aged mice across cognitive tasks. Several tasks are assessed because they differ in training stimuli, physical requirements and strategies used to perform the task, intertrial-intervals, and have been described in the literature to rely upon functioning of different brain regions. For example, two spatial memory tasks, the water maze and object placement task rely in part upon a functioning hippocampus as determined by lesion studies [5,33]. The water maze requires a high degree of physical activity (swimming), remembering where a hidden escape platform is located in the pool, and a probe trial 24 h after training. The object placement task requires mice to explore objects in different corners of an open field and remember where the objects were during a training trial 4 h before testing. The inhibitory avoidance and conditioned fear tasks may be reliant on the hippocampus and amygdala [22,36] and require associations to be made between footshock and conditioned stimuli (chamber, tone) and testing occurs 24 h post-training trials (single or multiple-parings, respectively). In these tasks, freezing, rather than movement as in water maze and object placement, is required to demonstrate learning/memory. The conditioned place preference (CPP) task requires associations to be made between a compound’s interoceptive effects and the chamber after multiple pairings of these stimuli, is mediated by the nucleus accumbens and projection sites [31], and has a motor component. By using this strategy, we have found that P4 can have memory-enhancing effects among young adult mice in these and other tasks that may be mediated by the hippocampus, prefrontal cortex (PFC), amygdala, nucleus accumbens, and/or cerebellum [15,32]. However, 18–24-month old mice administered P4 only showed better performance in tasks likely mediated by the hippocampus and/or PFC [14]. Aging is associated with disruption of cognitive processes and cell loss in these regions [1,3,33]. Older individuals can be particularly sensitive to confusion and/or cognitive disruption produced by benzodiazepines, which like some progestogens, can have agonist-like actions at GABAA receptors [7,24]. Aged, compared to young rats, had poorer cognitive performance and supraphysiological P4 levels that were improved by OVX [3,4]. Thus, the nature of P4’s effects on cognitive performance may depend upon age, dosing, and actions of P4 at underlying substrates.
In addition to actions at GABAA receptors [7], P4 binds with high affinity to intracellular progestin receptors (PR) [26]. PR knockout (PRKO) mice lack functional PRs [27]. We have previously demonstrated that aged PRKO and wildtype mice have increased sexual and decreased anxiety-like behavior following P4 administration, despite PRKO mice having cortical PR binding at the lower limits of detection [13]. These data suggest that PRs may not be necessary for some of the behavioral effects of P4 in mice. To test the hypothesis that P4’s effects on cognitive performance may be in part independent of PRs, the effects of P4 or vehicle administration to PRKO and wildtype mice that were 20–24 months of age in several cognitive tasks were evaluated.
Methods utilized in this study were pre-approved by the University at Albany IACUC, and were done using adequate measures to minimize pain or discomfort to animal subjects, as outlined in NIH Guide for the Care and Use of Laboratory Animals (#80-23, 1996).
Female (n = 5) and male (n = 4) PRKO mice, and their wildtype controls (female, n = 4; male, n = 6), 20–24-month old at testing were bred in the vivarium at University at Albany-SUNY-Social Sciences Building. Mice were group-housed in same-sex groups (n = 3–4 males/cage; n = 4 or 5 females/cage) with both genotypes represented in each cage to reduce potential housing confounds. Cages were in a room with a reversed 12/12 h light/dark cycle (lights off at 0800) and mice had ad libitum access to rodent chow and tap water in their homecages.
Mice were bred by heterozygous pairings and were from different litters. Genotype was determined by polymerase chain reaction of tail genomic DNA [13,12,29].
Although levels of endogenous P4 in subjects were not determined, mice at this age have very low levels of endogenous levels of progestins [12–14,16]. Male and female mice of each genotype were randomly assigned (n = 2–4/group) to receive subcutaneous (SC) injections of propylene glycol, or P4 (Sigma, 10 mg/kg), which produces P4 levels analogous to that of young mice in behavioral estrus.
Mice were handled/habituated for 1 week prior to behavioral testing [13,12]. Methods are briefly described below [10,14–16]. Indices of motor and/or sensory responses were also evaluated, but there were no effects of any condition on these responses (data not shown). An observer uninformed of the experimental conditions of the mice, and the hypothesis being tested, collected behavioral data. Mice were tested in the CPP task over 12 days. A week later, mice were then tested in the other tasks, once per week, so that there was a week in between vehicle or P4 administration. Mice were tested through tasks in the same order to obviate the potential for stress due to footshock influencing performance in other tasks. This protocol ensured that mice had similar experience with prior exogenous P4 or vehicle administration.
A typical CPP procedure was utilized [14]. Mice were habituated to the chamber in 30-min trials over 2 days where they were allowed to explore both sides of the chamber, which had different flooring (smooth and mesh) on each side. On the 3rd day, baseline preferences of mice for chamber side were determined in a 30-min trial. Mice were then injected with vehicle when mice were placed on the preferred side and P4 before placement on the non-preferred side on eight 30-min trials once per day. Mice were tested for their preference on Day 12. Spending more time in what was originally the non-preferred side of the chamber is an indication of a rewarding effect of P4.
Mice freely explored two identical objects during training [14–16]. Mice were injected with vehicle or P4 immediately after the single 3-min training trial and tested 4 h later in a 3-min trial. The percentage of time spent with the object in the novel location, as a function of total time exploring the novel and familiar locations, is considered an index of spatial memory.
Mice were trained in the water maze in 12 trials, organized into three blocks of 4 trials with a randomized starting position in the maze represented during each of these 4 trials in the block [10,14,15]. In the block, mice had 60 s to find the hidden platform. Each block of trials had a 30 min intertrial-interval. Mice were injected with vehicle or P4 immediately after the last training trial. A probe trial was done 24 h later and the time spent in the quadrant where the platform had been located during training was used as an index of spatial memory.
Mice were trained and tested in the inhibitory avoidance task [10]. Immediately following a single training trial, mice were administered vehicle or P4. For testing, 24 h later, mice are returned to the light side of the chamber and the latency to cross to the shock-associated side of the chamber is recorded. Longer cross-over latencies are indicative of better performance.
Immediately after training, mice were administered vehicle or P4. Twenty-four hours after a training session (8 pairings of footshock and the conditioned cue 10 s tone), mice are tested in contextual (hippocampus-dependent, with training chamber, no tone) and cued (amygdala-dependent, with training tone, new chamber) testing trials. Freezing behavior is observed for 8 min as an index of learning/memory in this task [14].
Given small sample size and no evidence of a main effect of sex when analyzed by three-way analyses of variance (ANOVA), two-way ANOVAs examined effects of P4 and genotype, collapsed across sex. When the alpha level was p < 0.05, ANOVAs were followed by Fisher’s post-hoc tests to determine group differences.
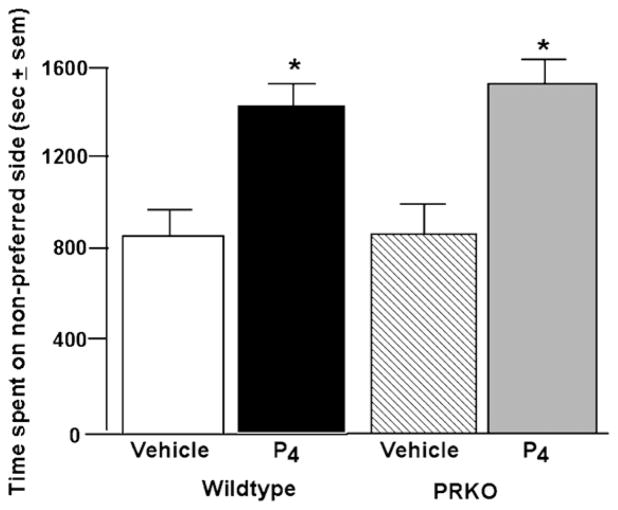
There was no main effect of genotype in the CPP, object placement, water maze, or conditioned fear tasks. In the CPP task, P4 (F(1,16) = 23.29, p < 0.05) increased time spent on the originally non-preferred side of the chamber, compared to vehicle (Fig. 1). In the object placement task, P4 (F(1,16) = 40.07, p < 0.05), compared to vehicle, increased the percentage of time that mice spent with the displaced object (Fig. 2, top left). In the water maze probe trial, P4 (F(1,16) = 48.21, p < 0.05), compared to vehicle, significantly increased the time that mice spent in the hidden platform quadrant (Fig. 2, bottom left). In the conditioned fear task, P4, compared to vehicle, significantly increased the time spent freezing when mice were tested in the same context (F(1,16) = 26.02, p < 0.05; Fig. 2, top right) or with the same cue (F(1,16) = 5.41, p < 0.05; Fig. 2, bottom right) as during training.
Fig. 1.
Effects of P4 in the conditioned place preference task of wildtype (vehicle, n = 4 (2 female and 2 male); P4, n = 6 (2 female and 4 male)) and PRKO (vehicle, n = 5 (3 female and 2 male); P4, n = 5 (3 female and 2 male)) mice. *p < 0.05 compared to respective vehicle.
Fig. 2.
Effects of P4 in the object placement task, water maze, and conditioned contextual fear task of wildtype (vehicle, n = 4 (2 female and 2 male); P4, n = 6 (2 female and 4 male)) and PRKO (vehicle, n = 5 (3 female and 2 male); P4, n = 5 (3 female and 2 male)) mice. *p < 0.05 compared to respective vehicle.
There was no effect of P4 condition in the inhibitory avoidance task. PRKO mice (F(1,16) = 6.21, p < 0.05) had significantly longer cross-over latencies (vehicle: 107 ± 22 SEM; P4: 115 ± 23) than did wildtype mice (vehicle: 39 ± 8 SEM; P4: 84 ± 19).
This study generally supports our a priori hypothesis that P4 would improve memory of aged PRKO and wildtype mice. P4, compared to vehicle, improved CPP, object placement, water maze, contextual and cued fear conditioning performance of aged PRKO and wildtype mice. However, PRKO mice outperformed wildtype mice in the inhibitory avoidance task. Thus, some memory-enhancing effects of P4 may be partly independent of PRs, but the mechanisms for P4 to improve memory performance still need to be elucidated.
The present results, along with previous findings, suggest that P4 can enhance memory consolidation among aged mice [14–16,19,25]. Administration of P4 to young female mice 1.5 h after training, thereby after memory consolidation, does not improve performance during testing in the object recognition or conditioned fear tasks [15,32]. Here, P4 was administered immediately after training in each task, a regimen which increases circulating and/or central levels of progestogens within minutes with effects sustained for 1–6 h, but not 24 h, [9,15,19]. As such, levels of P4 were likely elevated 24 h after training when mice were tested in the object placement task, but not during testing in the other tasks used. Yet, similar enhancing effects were observed across tasks, except for inhibitory avoidance. Although levels were not measured in these subjects, we have observed endogenous progestogen levels at nadir among similarly aged mice [13], suggesting that the effects observed in the present study were not likely due to endogenous differences in progestogens. The effects of P4 to enhance learning/memory of rodents can be temporally distinct from effects on anxiety, motor and/or sensory performance in this and other studies, as indicated by anxiety task performance, grid crossing, swim speed, and/or response to footshock [6,9,14,15]. Thus, P4, independent of E2, may enhance consolidation when administered post-training and have subsequent effects on learning/memory irrespective of P4 levels at testing in aged wildtype and PRKO mice.
There were consistent effects of P4 to enhance learning/memory among PRKO and wildtype mice. One interpretation of these findings is that actions at PRs are not required for some effects of P4 to enhance cognitive performance. This is consistent with previous studies showing favorable functional responses of rodents to P4 metabolites, which have low affinity for PRs when in physiological concentrations [8,11,21,40,42]. PRKO and wildtype mice respond similarly when administered progestogens and tested for anxiety or sexual behavior [13,12]. However, PRKO mice performed better in the inhibitory avoidance task than did their wildtype counterparts in the present study. In our previous investigation of effects of P4 on learning/memory of aged c57 mice, P4 improved performance in the T-maze, object recognition, water maze, inhibitory avoidance, and contextual fear conditioning tasks, but not cued fear conditioning or CPP [14,15]. Although the present findings that P4 improved performance across these different tasks may be related to differences in background strains (c57 vs 129SV) or housing conditions between studies, we also do not know the extent to which shorter forms of the PR or splice variants may mediate these behavioral responses in wildtype and PRKO mice in this study. We cannot rule out that development effects of PRs influenced these outcomes. Indeed, expression of PRs in the cortex change across development [41]. Although the functional significance of this is not yet established, we do know that aberrations in progestogens during development can alter later functions in hippocampally mediated tasks [7]. Whether these “organizing” effects of P4 require actions at PRs is not known and is the subject of ongoing investigation.
Limitations of the present study need to be considered. First, there were a small number of subjects utilized in this study, due in part to inherent challenges in generating aged, genetically mutant mice. As well, given the small number of mice and that the order that mice were tested in these tasks was not counterbalanced, we need to consider that these factors may have introduced significant error. Second, comparisons of aged subjects to younger counterparts were not conducted in this, as in our prior, studies [14]. The performance of vehicle-administered mice in this study and our prior report with aged mice was comparable. Performance was slightly poorer than that of young control mice in our laboratory, which implies that some of these differences may be attributed to age.
Emerging evidence suggest that P4 may have salient effects across development to alter neural and cognitive processes. Inhibiting formation of progestogens during late gestation may contribute to deleterious birth outcomes [20], which can mediate neural and cognitive function. Among rodents, cognitive performance improves in association with parity and elevated levels of progestogens [23,28,34,35]. Among aged persons with Alzheimer’s disease (AD) or non-AD dementia, levels of progestogens are lower than their age- and hormone-exposure matched counterparts [2,39]. In animal models of AD, there is deficiency in levels of progestogens in the hippocampus and in water maze and object placement task performance [16]. Given the profound implication of progestogens to have effects across the lifespan, future research on its effects and mechanisms is warranted.
Acknowledgments
This research was supported in part by grants from NIMH (MH06769801 and MH067698-06) and NSF (IBN03-16083 and IOS-0957148). The assistance of K. Sumida and M. Rhodes is appreciated.
Footnotes
Conflict of interest
Authors have no conflicts of interest to report.
References
- 1.Beason-Held LL, Golski S, Kraut MA, Esposito G, Resnick SM. Brain activation during encoding and recognition of verbal and figural information in older adults. Neurobiol Aging. 2005;26:237–250. doi: 10.1016/j.neurobiolaging.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Bernardi F, Lanzone A, Cento RM, Spada RS, Pezzani I, Genazzani AD, Luisi S, Luisi M, Petraglia F, Genazzani AR. Allopregnanolone and dehydroepiandrosterone response to corticotropin-releasing factor in patients suffering from Alzheimer’s disease and vascular dementia. Eur J Endocrinol. 2000;142:466–471. doi: 10.1530/eje.0.1420466. [DOI] [PubMed] [Google Scholar]
- 3.Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm AC. Ovarian hormones and cognition in the aged female rat. I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci. 2003;117:1395–1406. doi: 10.1037/0735-7044.117.6.1395. [DOI] [PubMed] [Google Scholar]
- 4.Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC. Ovarian hormones and cognition in the aged female rat. II. Progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci. 2004;118:707–714. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- 5.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 6.Farr SA, Flood JF, Scherrer JF, Kaiser FE, Taylor GT, Morley JE. Effect of ovarian steroids on footshock avoidance learning and retention in female mice. Physiol Behav. 1995;58:715–723. doi: 10.1016/0031-9384(95)00124-2. [DOI] [PubMed] [Google Scholar]
- 7.Frye CA. Neurosteroids—from basic research to clinical perspectives. In: Rubin RT, Pfaff DW, editors. Hormones/Behavior Relations of Clinical Importance. Academic Press; San Diego: 2009. pp. 395–416. [Google Scholar]
- 8.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frye CA, Lacey EH. Progestins influence performance on cognitive tasks independent of changes in affective behavior. Psychobiology. 2001;28:550–563. [Google Scholar]
- 10.Frye CA, Rhodes ME, Dudek B. Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Res. 2005;1036:101–108. doi: 10.1016/j.brainres.2004.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frye CA, Sturgis JD. Neurosteroids affect spatial/reference, working, and long-term memory of female rats. Neurobiol Learn Mem. 1995;64:83–96. doi: 10.1006/nlme.1995.1046. [DOI] [PubMed] [Google Scholar]
- 12.Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O’Malley BW, Pfaff DW, Rhodes ME. Progesterone’s effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology. 2006;186:312–322. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- 13.Frye CA, Sumida K, Lydon JP, O’Malley BW, Pfaff DW. Mid-aged and aged wild-type and progestin receptor knockout (PRKO) mice demonstrate rapid progesterone and 3α,5α-THP-facilitated lordosis. Psychopharmacology. 2006;185:423–432. doi: 10.1007/s00213-005-0300-4. [DOI] [PubMed] [Google Scholar]
- 14.Frye CA, Walf AA. Progesterone enhances performance of aged mice in cortical or hippocampal tasks. Neurosci Lett. 2008;437:116–120. doi: 10.1016/j.neulet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frye CA, Walf AA. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol Learn Mem. 2008;90:171–177. doi: 10.1016/j.nlm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frye CA, Walf AA. Effects of progesterone administration and APP-swe + PSEN1Deltae9 mutation for cognitive performance of mid-aged mice. Neurobiol Learn Mem. 2008;89:17–26. doi: 10.1016/j.nlm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 18.Gibbs RB. Effects of ageing and long-term hormone replacement on cholinergic neurones in the medial septum and nucleus basalis magnocellularis of ovariectomized rats. J Neuroendocrinol. 2003;15:477–485. doi: 10.1046/j.1365-2826.2003.01012.x. [DOI] [PubMed] [Google Scholar]
- 19.Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28:602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Hirst JJ, Palliser HK, Yates DM, Yawno T, Walker DW. Neurosteroids in the fetus and neonate: potential protective role in compromised pregnancies. Neurochem Int. 2008;52:602–610. doi: 10.1016/j.neuint.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Iswari S, Colas AE, Karavolas HJ. Binding of 5α-dihydroprogesterone and other progestins to female rat anterior pituitary nuclear extracts. Steroids. 1986;47:189–203. doi: 10.1016/0039-128x(86)90088-7. [DOI] [PubMed] [Google Scholar]
- 22.Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 23.Kinsley CH, Lambert KG. Reproduction-induced neuroplasticity: natural behavioural and neuronal alterations associated with the production and care of offspring. J Neuroendocrinol. 2008;20:515–525. doi: 10.1111/j.1365-2826.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- 24.Klotz U. Effect of age on pharmacokinetics and pharmacodynamics in man. Int J Clin Pharmacol Ther. 1998;36:581–585. [PubMed] [Google Scholar]
- 25.Lewis MC, Orr PT, Frick KM. Differential effects of acute progesterone administration on spatial and object memory in middle-aged and aged female C57BL/6 mice. Horm Behav. 2008;54:455–462. doi: 10.1016/j.yhbeh.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Lonard DM, O’Malley BW. A contemporary understanding of progesterone receptor function. Mech Ageing Dev. 2004;125:669–678. doi: 10.1016/j.mad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Lydon JP, DeMayo FJ, Conneely OM, O’Malley BW. Reproductive phenotypes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- 28.Macbeth AH, Scharfman HE, Maclusky NJ, Gautreaux C, Luine VN. Effects of multiparity on recognition memory, monoaminergic neurotransmitters, and brain-derived neurotrophic factor (BDNF) Horm Behav. 2008;54:7–17. doi: 10.1016/j.yhbeh.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mani SK, Blaustein JD, O’Malley BW. Progesterone receptor function from a behavioral perspective. Horm Behav. 1997;31:244–255. doi: 10.1006/hbeh.1997.1393. [DOI] [PubMed] [Google Scholar]
- 30.Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm Behav. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- 31.McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 32.McEchron MD, Cheng AY, Gilmartin MR. Trace fear conditioning is reduced in the aging rat. Neurobiol Learn Mem. 2004;82:71–76. doi: 10.1016/j.nlm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 34.Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–115. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawluski JL, Walker SK, Galea LA. Reproductive experience differentially affects spatial reference and working memory performance in the mother. Horm Behav. 2006;49:143–149. doi: 10.1016/j.yhbeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Phillips RG, LeDoux JF. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 37.Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- 38.Sato T, Tanaka K, Ohnishi Y, Teramoto T, Irifune M, Nishikawa T. Effects of estradiol and progesterone on radial maze performance in middle-aged female rats fed a low-calcium diet. Behav Brain Res. 2004;150:33–42. doi: 10.1016/S0166-4328(03)00249-3. [DOI] [PubMed] [Google Scholar]
- 39.Smith CD, Wekstein DR, Markesbery WR, Frye CA. 3α,5α-THP: a potential plasma neurosteroid biomarker in Alzheimer’s disease and perhaps non-Alzheimer’s dementia. Psychopharmacology. 2006;186:481–485. doi: 10.1007/s00213-005-0186-1. [DOI] [PubMed] [Google Scholar]
- 40.Smith HE, Smith RG, Toft DO, Neergaard JR, Burrows EP, O’Malley BW. Binding of steroids to progesterone receptor proteins in chick oviduct and human uterus. J Biol Chem. 1974;249:5924–5932. [PubMed] [Google Scholar]
- 41.Wagner CK. Progesterone receptors and neural development: a gap between bench and bedside? Endocrinology. 2008;149:2743–2749. doi: 10.1210/en.2008-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]