Abstract
More than 29 million adults in the United States have been diagnosed with hearing loss. Interestingly, elevated homocysteine (Hcy) levels, known as hyperhomocysteinemia (HHcy) is also associated with impaired hearing. However, the associated mechanism remains obscure. The collagen receptor such as discoidin domain receptor 1 and matrix metalloproteinase (MMP) play a significant role in inner ear structure and function. We hypothesize that HHcy increases hearing thresholds by compromise in inner ear vasculature resulted from impaired Hcy metabolism, increased oxidative stress, collagen IVa and collagen la turnover. The treatment with folic acid (FA) protects elevated hearing thresholds and prevents reduction in vessel density by lowering abundant collagen deposition and oxidative stress in inner ear. To test this hypothesis we employed 8 weeks old male wild type (WT), cystathionine-beta-synthase heterozygote knockout (CBS+/−) mice, WT+FA (0.0057 μg/g/day, equivalent to a 400 μg/70 kg/day human dose in drinking water); and CBS(+/−)+FA. The mice were treated for four weeks. The hearing thresholds were determined by recording the auditory brainstem responses. Integrity of vessels was analyzed by perfusion of horseradish peroxidase (HRP) tracer. Endothelial permeability was assessed, which indicated restoration of HRP leakage by FA treatment. A total Hcy level was increased in stria vascularis (SV) and spiral ligament (SL) of CBS+/− mice which was lowered by FA. Interestingly, FA treatment lowered Col IVa Immunostaining by affecting its turnover. The levels of MMP-2, -9, methylenetetrahydrofolate reductase (MTHFR) and cystathione gamma lyase (CSE) were measured by Western blot analysis. The oxidative stress was high in SV and SL of CBS+/− compared to WT however the treatment with FA lowered oxidative stress in CBS+/− mice. These data suggested that hearing loss in CBS+/− mice was primarily due to leakage in inner ear circulation, also partly by induced collagen imbalance, increase in Hey and oxidative stress in inner ear.
Keywords: Oxidative Stress, hearing, auditory brainstem response, cochlea, stria vascularis
Introduction
Elevated plasma homocysteine (Hcy) concentrations called hyperhomocysteinemia (HHcy) are associated with an increased risk of cardiovascular disease. Besides clinical association between cardiovascular compromises, abnormal blood flow being associated with poor hearing, there is a close correlation between plasma Hcy level, and hearing thresholds (Gopinath et al., 2010). Folate is the strongest nutritional and pharmacological determinant of plasma Hcy levels, which also interacts with the genetic variations of methylenetetrahydrofolate reductase (MTHFR). HHcy has also been associated with MTHFR gene point mutation and interestingly polymorphism of MTHFR gene has a significant correlation with poor hearing (Uchida et al., 2011). In addition older population with HHcy are reported to have elevated hearing thresholds (Liu, 1988). The results from animal models suggest that maintaining adequate blood flow to the cochlea is critical to cochlear function and any reduction in blood supply will have a corresponding reduction in cochlear function. This circulatory system pathology may directly affect hearing in a number of ways (Hausler and Levine, 2000). One of the vascular physio-pathological mechanisms described is the increase in blood viscosity, which reduce capillary blood flow and ends up reducing oxygen transport, causing tissue hypoxia, thus causing hearing complaints and hearing loss in patients (Mees and Suckfull, 2002).
The main vascular supply to inner ear is spiral modiolar artery, which splits into the capillary bed of the stria vascularis (SV). The stria vascularis along with the spiral ligament comprises the lateral wall. The lateral wall blood flow of the cochlea has a critical fluid homeostasis and oxygenation (Angelborg et al., 1985; Nakai et al., 1992). The cochlear microcirculation can be impaired through increase in vascular permeability (Quirk and Seidman, 1995), reduced circulation (ischemia) , and thus results in an imbalance in the endolymphatic ion concentration. It has been demonstrated that endolymph is secreted from perilymph by means of trans-epithelial transport systems mainly located in the stria vascularis in the cochlea, which maintains the composition of endolymph (K-rich fluid, almost devoid of Na) and the endolymphatic transepithelial potential (Sterkers et al., 1982a). The perilymph of scala vestibuli, which has the composition of an extracellular fluid, originates from plasma across a blood-perilymph barrier (Sterkers et al., 1982b). This blood-perilymph barrier shares morphological and physiological analogies with the blood-brain barrier as it restricts the entry of electrolytes and small neutral molecules as a function of their molecular weight. Thus this suggests the blood circulation in cochlea is critical for hearing function.
The main vascular supply via spiral modiolar artery (SMA) also plays a major role in the cochlear blood flow and tissue oxygenation (Wangemann, 2002). Here we have not focused our study on the vascular diameter of SMA but rather investigated the microcirculation associated changes due to Hcy metabolic pathway modulations. In general, Ca+2 dependent contractility of the vascular SMCs help to regulate the diameter of the blood vessels (Wangemann, 2002). It is well known that Hcy triggers the Ca+2 activation and contracts the vessels to regulate blood flow (Mujumdar et al., 2000). But capillary blood flow may primarily be regulated by resistance of pre-capillary arteries. Moreover inner ear microcirculation tone is known to depend on ET-1 as an endogenous regulator (Scherer et al., 2001; Scherer and Wangemann, 2002). In-addition, Hcy mediates contractility through ET-1 dependent vasoconstriction (Zhen et al., 2011).
The adult inner ear has an extensive network of basement membrane. The extracellular matrix is chiefly comprised of the type IV collagen chains though the basement membranes of blood vessels in the lateral wall differ when comparing those of the spiral ligament with stria vascularis based on other collagens and ECM proteins. Besides, Cnx30−/− null mice also showed an increased Hcy in the SV resulting in endothelial barrier disruption in the inner ear (Cohen-Salmon et al., 2007). The collagen receptor DDR1 (Discoidin domain receptor 1) null mice reported to have hearing impairment due to severe alterations in the structural and specialized epithelial cells of the cochlea Meyer zum Gottesberge et al., 2008). DDR1 knockout demonstrated an abundant deposition of extracellular matrix within the stria vascularis, moreover animals also manifested increase in accumulation of collagen fiber between cells of the spiral ligament. Irregularity in collagen IV subunits distribution has been implicated in hearing loss (Meyer zum Gottesberge and Felix, 2005). This suggests collagen regulation is essential in maintaining normal hearing functions. Our previous work has shown that the CBS+/− mice have abundant activity of matrix metalloproteinases (MMP) -2, and -9 which in-turn, affect collagen turnover (Kundu et al., 2009a; Munjal et al., 2011). Based on this, we hypothesized that in heterozygous knockout CBS mice with elevated Hey levels resulted in a compromise in cochlear microcirculation which showed improvement with folic acid supplementation.
Materials and Methods
Animals and treatment
All animal and experimental procedures were performed in accordance with National Institute of Health Guidelines for animal research and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Louisville. Eight weeks old male C57BL/6J (Wild Type) and CBS (+/−) heterozygous knockout mice were procured from the Jackson laboratories and genotyped (Kumar et al., 2008). Mice were fed standard mouse diet. All mice had access to de-ionized water in a temperature controlled (22 °C) room with a 12 h light–dark cycle. Folic acid intervention regime was executed as follows: At 8 weeks of age male CBS+/− mice entered a 2 week folic acid intervention study, during which all mice continued to feed ad libitum on standard autoclaved chow. The mice were divided into four groups. Supplemental folic acid was then administered to the animals in their drinking water. (1) Wild type C57BL/6J mice with vehicle treatment (2) Wild type C57BL+6J mice with folic acid treatment (3) CBS+/− heterozygous knockout mice with vehicle treatment (4) CBS+/− heterozygous knockout mice with folic acid treatment. Folic acid dose was administered as per reported previously (Clarke et al., 2006). Folic acid was given at 0.0057 μg/g/day for 4 weeks; dose is equivalent to a 400 μg/70 kg/day human dose, the (USA Food and Drug Administration recommended human daily intake).
Antibodies and reagents
The following primary antibodies were used; anti MMP-2 (R&D systems, MN, Mineapolis), anti MMP-9 (Abeam Inc.cambridge, MA); anti- p22phox and anti-Nox3 antibodies were purchased from Santa Cruz biotechnology, CA, USA. Antibody to CSE was purchased from Novus biological; Antibody to p47phox was purchased from Cell Signaling (Danvers, MA); Monoclonal anti-GAPDH from Sigma-Aldrich; antibody to MTHFR was from Novus biological. Secondary antibodies used were; rabbit pAb to goat IgG (HRP), goat pAb to rabbit IgG (HRP), goat pAb to mouse IgG (HRP) were from Abeam (Cambridge, MA). Histological staining kits used were Masson's trichrome stain-chromaview (Richard-Allan scientific, Kalamazoo, Ml) and the histological mounting medium used was Permount® (SP15). PCR reagents used were as follows: 2× PCR master mix (PCR Master Mix, 2×: 50 U/ml of Taq DNA polymerase; 400 μM dATP, 400 μM dGTP, 400 μM dCTP, 400 μM dTTP, 3 mM MgCI2 in reaction buffer). Polyvinylidene fluoride (PVDF) membrane was from Bio-Rad (Hercules, CA).
Auditory brainstem response
C57BL/6J (Wild Type), and heterozygous knockout CBS (CBS+/−) male mice were obtained at 8 weeks of age. The hearing assessments of these mice were conducted and mice were grouped in four groups as mentioned above. Folic acid treatment was followed for 4 weeks and auditory threshold of animals were again conducted. Animals were anesthetized intraperitoneally with an injection of 2′,2′,2′ tribromo-ethanol. Core temperature was maintained constant at 37°C using a rectal temperature-controlled heat blanket. For stimulus generation, presentation and data acquisition we used Tucker-Davis-Technologies (TDT) III systems (Tucker-Davis-technologies< Ft Lauderdale, FL, USA) run by BioSig32 software (TDT) routines. Responses were recorded by registration of the potential difference between sub dermal electrodes positioned at the vertex and the mastoid process. A ground electrode was inserted near the tail, inter-electrode impedances were less than 1 kΩ. In general, ABR waveforms were recorded for 10 milliseconds at a sampling rate of 16 kHz using 50-5000 Hz filter settings; waveforms recorded from 1024 stimuli at a frequency of 9 Hz were averaged. ABR waveforms were recorded in decreasing 5-dB sound pressure level (SPL) intervals from the maximum amplitude until no waveforms could be visualized. Mice were presented with 1024 pure-tone stimuli (102 sec; Blackman envelope) at stimulus rates of 19.1/sec. The left and right ears were alternately tested, and ipsilateral EEGs (amplified 200 K) were band passfiltered (300–3000 Hz), parsed with 15 _V artifact rejection, and recorded for 12.5 msec (Intelligent Hearing Systems, Miami, FL).
HRP -tracer experiments
HRP tracer permeability assay was performed in treated and control animals as per the protocol described previously (Sakagami et al., 1984) with some modifications. Briefly, mice were anaesthetized with pentobarbital and carotid was exposed after surgery. The carotid artery was then cannulated with 0-6mm tubing's. The animal was provided with oral intubation and oxygenated in the duration of perfusion. Tube put in carotid was connected to a transducer and animal was gradually perfused with 15mg HRP (Sigma, type II) dissolved in physiological saline for 10 minutes. After 30 minutes of perfusion the inferior vena-cava was punctured and the blood was flushed out by injecting saline through the transducer. Similarly, 4% PFA was perfused through the carotid cannula for the in-vivo tissue fixation. Following the perfusion steps, temporal bone was dissected out and decalcified in 10% EDTA for 4 days. The cochlea was separated and processed for cryo-mold preparation. Eight micron thin sections were taken, dried and washed in PBS. The section was incubated in buffer containing 0.05M Tris-HCl (pH 7.6) and 3,3′-DAB HCl. To this reaction buffer fresh 0.01% of H2O2 was added. The incubation time was 15min sections were than washed and mounted to visualize the distribution of HRP tracer by light microscope. Four sections from each animal were scanned for image analysis. Images were captured by Olympus Fluoview microscope.
Immunohistological staining
Temporal bones were removed from 10-week-old mice and the round and oval windows were opened, together with the small holes in the cochlear apical turn and superior semicircular canal. The samples were fixed by perfusing the perilymphatic space with 4% paraformaldehyde (PFA) from the round to the oval window and immersed in PFA followed by washing with PBS (3 times). The samples were than decalcified with 5% EDTA in PBS for 3 days. Specimens were immersed in 30% sucrose in PBS for 1 day and then frozen in liquid nitrogen. Frozen sections ∼10μm thick were cut from these samples and air dried on slide glasses and stained for specific antigen using peroxidase IHC detection kit (Pierce, IL, USA) as per the manufacturer's instructions with some modifications. Briefly, 10μm sections were washed in BupH™ Tris Buffered Saline twice, 3m each. Antigen retrieval was done by citrate buffer (pH 6.0) in microwave at 95°C. After bringing it to room temperature and brief washing the endogenous peroxiadase reaction was quenched by incubating in peroxidase suppressor. Blocking was done using universal blocker for 30m followed by incubation with appropriate primary antibody for 2hour. Sections were washed and incubated in appropriate HRP conjugated secondary antibody for 30m. Detection of antigen was done with 1X working solution of the DAB/Metal Concentrate (10X) by adding the Stable Peroxide Buffer. Counterstaining was done by hematoxylin, washed and mounted. Visualization was done using Olympus Fluoview and analyzed with Q-Capture pro software.
Digital image analysis
Immunohistochemistry images were analyzed using Image Pro (http://rsb.info.nih.gov/ij/). A custom Image Pro threshold macro was used to quantitatively analyze protein densities and ratios on immunohistochemistry slides. Using the Image Pro interface, this program first split a stained image into its three RGB color channels, enabling threshold to work with gray images. Each RGB color channel signal was manually identified and tissue threshold numbers (an intensity value between 0-255) were used for each image selection. All pixels with intensity values equal to or above the threshold input values were included in the calculations. For this study, we set our signal threshold value manually, at about half the automatically set level of Image Pro's auto threshold function. Using Image Pro's threshold function, we identified the threshold values which, when best combined, represented the entire tissue area. The tissue threshold input must be smaller than the signal threshold input value. Using Image Pro each image was set for measurement of parameters, area, area fraction, and limit to threshold and display label. The threshold macro permitted the user to define the image selection(s). ROI modes: In ROI (Region of Interest) mode, the user could use Image Pro's selection tools (e.g. polygon and ellipse) to manually define particular image selection regions. The rectangle ROI combined the rectangle and ROI modes to create a pixilated overview of an entire image selection. The threshold macro measures and outputs Signal Area, Tissue Area, Signal density, and Signal Ratio values in each of the image. Signal density values were defined as a function of each color channel's signal area divided by tissue area.
Isolation of protein
The bony cochlear capsule was dissected out and cochlear tissue from each group was collected in PBS on ice. For Western Blot analysis of matrix proteins, harvested tissue was pooled and minced finely in cacodylic acid buffer (10 mM cacodylic acid pH 5.0, 150 mM NaCl, 1 μM ZnCI2, 20 mM CaCI2, 1.5 mM NaN3, and 0.01% v/v Triton X-100). The minced tissue was kept on rotator at 4°C overnight for the extraction of proteins. The extract was centrifuged at 5,000×g for 10 min and the supernatant was aliquoted and stored in −20°C for further use. For the isolation of total cellular proteins, the harvested cochlea was snap frozen in liquid nitrogen and grounded in 1× RIPA buffer (Tris–HCI 50 mM, pH 7.4; NP-40, 1%; 0.25% Na-deoxycholate, 150 mM NaCI; 1 mM EDTA; 1 mM PMSF; 1 μg/ml each of aprotinin, leupeptin, pepstatin; 1 mM Na3V04; 1 mM NaF) containing 1 mM PMSF and 1 μg complete protease inhibitor (Sigma). The homogenate was kept on ice for 30 min, centrifuged at 10,000×g at 4°C for 15 min and the supernatant was collected. Estimation of protein was done by BCA assay method (Pierce, Rockford, IL) (Kundu et al.,2009a).
Western blotting
Western blot analyses were performed on cochlear tissue homogenates using 10% SDS-PAGE as described previously (Kundu et al., 2009a). BCA method was used to estimate total protein, and 25 μg of protein was loaded in each well of electrophoresis gels. After electrophoresis, proteins were transferred to polyvinylidene difluoride membrane (PVDF). The transferred proteins were processed for immunodetection of specific antigens. Briefly, non-specific sites were blocked with 5% non-fat dry milk in TBS-T (50 mM Tris–HCI, 150 mM NaCI, 0.1% Tween- 20, pH 7.4) for 1 h at room temperature. The blot was then incubated with appropriate primary antibody (at 1:1000 dilutions) in blocking solution according to the supplier's specific instructions. The blots were washed with TBS-T (3 times, 10 min each) and incubated with appropriate HRP- conjugated secondary antibody (at 1:3000 dilutions) for 1 h at room temperature. After washing, ECL Plus substrate (Amersham Biosciences, Pittsburgh, PA) was applied to the blot for 1 min. The blot was developed using X-ray film (RPI Corp, Inc., Mount Prospect, IL) with a Kodak 2000A developer (Eastman Kodak, NY). The blots were stripped and re-probed with GAPDH. The immuno-reactive bands were scanned and densitometricaly analyzed Un-Scan-lt software (Silk Scientific, Orem, UT). The bands were normalized with GAPDH controls.
Semi quantitative RT-PCR
Isolated cochlea from different experimental groups was homogenized in liquid nitrogen and TriZol reagent was used for total RNA extraction. The protocol for mRNA analysis was followed as mentioned previously (Kundu et al., 2009a). Briefly, 2μg total RNA was used for cDNA synthesis and total cDNA pool was amplified by PCR using gene specific primers.
| Gene name | Primer sequence |
| MMP-9 F | 5′-TGTGGGTGGAAATTCAGAAGGT-3′ |
| R | 5′-TTGTTGCCCAGGAAAGTGAAG-3′ |
| MMP2 F | 5′-TGTGGGTGGAAATTCAGAAGGT-3′ |
| R | 5′-TTGTTGCCCAGGAAAGTGAAG-3′ |
| TIMP2 F | 5′-AAGAATCACCCTCCAACCAG -3′ |
| R | 5′-CTTGGTTGAAGCCAGAAACA-3′ |
| TIMP3 F | 5′-GCCCTCCCATATGTATACCC-3′ |
| R | 5′-TAGGCCTCACCTCAAGTCTG-3′ |
| Col4a F | 5′ GCAACGGTACAAAGGGAGAGAG-3′ |
| R | 5′-CTTCATTCCTGGTAACCCTGGTG-3′ |
| Col1a F | 5′- TCCTGGCAACAAAGGAGACA-3′ |
| R | 5′- GGGCTCCTGGTTTTCCTTCT-3′ |
| GAPDH F | 5′-ATGGGAAGCTGGTCATCAAC-3′ |
| R | 5′-TGTGAGGGAGATGCTCAGTG-3′ |
Statistical Analysis
Values are reported as mean ± SEM. Differences between groups were tested by two-way ANOVA. If ANOVA indicated a significant difference (P < 0.05), Tukey's multiple comparison test was used to compare group means and were considered significant if P < 0.05.
Results
Auditory brainstem response
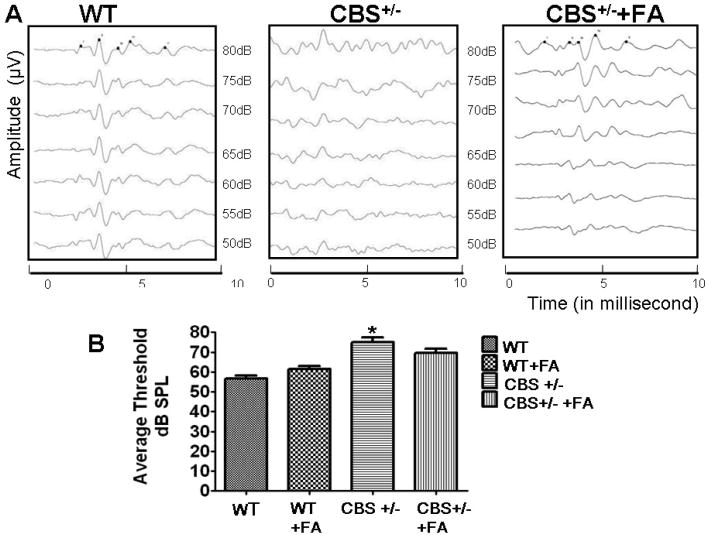
To determine HHcy associated physiological consequences on hearing functions. ABRs were measured in all the four groups. In wild-type mice, auditory response thresholds using tone stimuli at 16 kHz are between 50 and 80 dB SPL (Fig. 1). In contrast, auditory response thresholds from CBS+/− mice are increased significantly between 60 and 85 dB SPL at 16kHz that further indicate severe hearing impairment in HHcy animals. Interestingly, CBS+/− treated with FA demonstrated auditory response thresholds similar to WT+FA group.
Figure.1. Auditory brainstem response recording showing Hcy decreases hearing threshholds.
(A) Representative traces from WT+ FA, CBS+/−, and CBS+/−+ FA mice in response to 16-kHz tone-burst stimuli at decreasing stimuli levels. In the WT + FA mice, multiple ABR peaks can be seen in response to the stimulus at levels down to 55 dB. In the CBS+/s− mice, number of ABR peaks at lower stimulus levels are minimum. (B) Hearing thresholds for click-evoked ABRs between WT, CBS+/− and CBS+/− +FA mice. *P<0.001 vs WT, #P<0.001 vs CBS+/−; n=3 animals/group.
Permeability of stria vascularis and changes in lateral wall
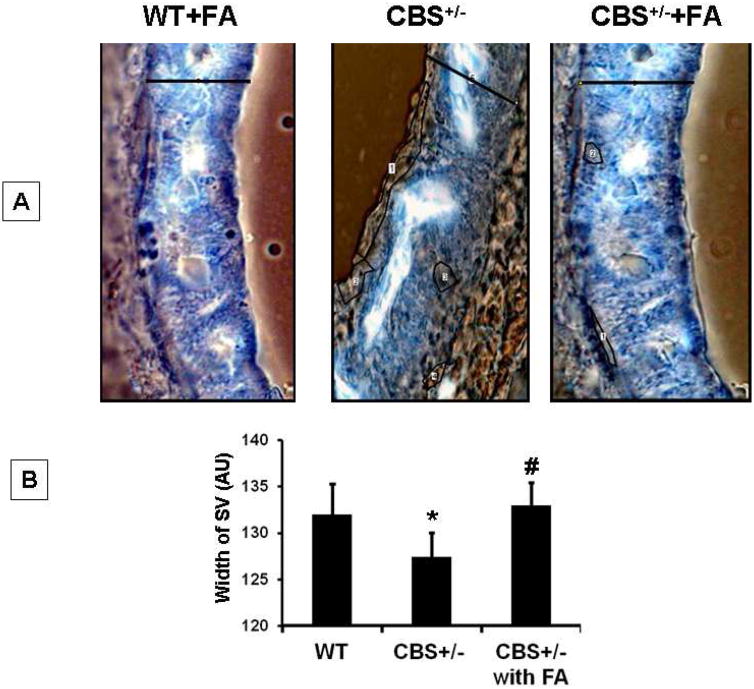
The stria vascularis (SV) is the vascular capillary bed which maintains the endolymphatic voltage of the inner ear. So, the permeability of vessels in the lateral wall was assessed by HRP tracer. Figure 2 demonstrates that in WT+ FA group there are HRP reactions in the spiral ligament which is external to stria vascularis. The endogenous peroxidase reaction was noticeable in the capillaries of SV. In case of CBS +/− group HRP leakage was restored resulting in less peroxidase activity. Thus HRP tracer suggests that FA treatment to CBS+/− mice decreases the permeability ((Figure 2A,B). We analyzed the quantitative parameters of SV, such as width of SV (Figure 2B, using contrast micrograph. Furthermore width of the SV was measured using contrast picture (Figure 2B) and a very prominent thinning of SV was noticed in CBS+/− mice whereas FA treatment restored normal strial thickness (F2,12= 24.9,p>.001)
Figure.2. FA attenuated the HRP leakage in stria vascularis (SV) of CBS+/− mice.
(A) Contrast micrograph of the brightfeild image of HRP infiltration into the stria vascularis (SV) of control and experimental groups. (B) Bar diagram represents quantification of SV diameter using image pro software. *P<0.001 vs WT, #P<0.001 vs CBS+/−; n=4 animals/ group.
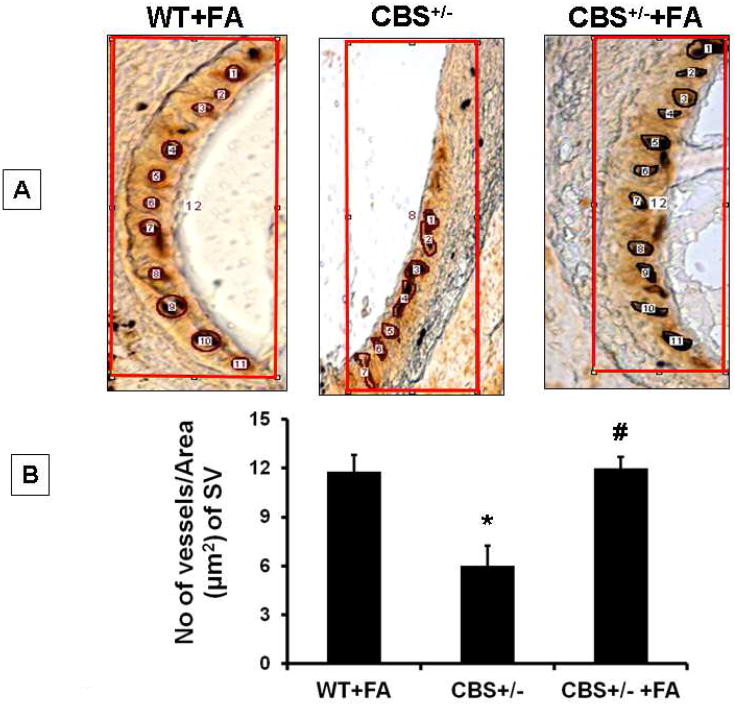
In figure.3, we analyzed the quantitative parameter of SV by counting number of capillaries, using Image Pro tool. Capillaries were identified as “particle” objects and repeated with every image to count the number of capillaries. Folic acid treatment to wild type animal averaged around eleven capillaries in the area counted, whereas CBS+/− mice counted capillaries was a 6 highly reduced (Figure 3). Interestingly, folic acid treatment to CBS+/− mice restored almost wild type count of vessel numbers (≈ 11 capillaries) (F2, 12= 30.1,p>.001).
Figure.3. FA increases capillary density in SV of CBS+/− mice.
(A) Micrographs representing peroxidase activity in the form of brown precipitate in the stria vascularis of mice. (B) Bar diagram represents quantitative estimation of capillary density per unit area in the stria vascularis from the respective groups using image pro software. *P<0.001 vs WT, #P<0.001 vs CBS+/−; n=4 animals/ group.
Regulation of Hcy metabolic pathway
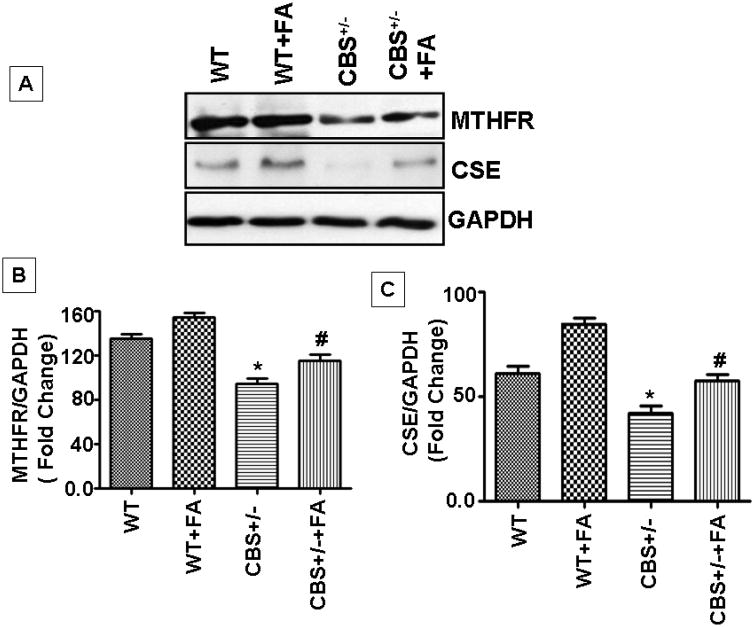
In order to know the metabolic regulation of the Hcy pathway, two crucial enzymes, namely MTHFR and CSE, were analyzed (Figure 4). The MTHFR activity is folate dependent and treatment with folic acid induced an increase from the baseline level of the enzyme in wild type. MTHFR protein levels were significantly reduced in CBS+/− mice as compared with either WT or WT+ FA treatment. MTHFR levels were significantly increased in CBS+/− +FA group with respect to CBS+/− mice (F3,12= 56,p>.001) . In addition, CSE protein expression was significantly low in CBS+/− when compared to WT mice; this may have been due to very low levels of cystathionine, the substrate which is synthesized from Hcy by CBS enzyme. When WT mice were treated with FA there was marginal increase in expression of CSE (F3,12=56,p>.001). It was interesting to find that the enzyme which is dependent on vitamin B6 as co-factor showed marginal increase in levels via folic acid. As a result, we know that in CBS+/− mice the enzyme is inactive, and the conversion of Hcy to cystathionine is not expected (Figure 4).
Figure.4. FA treatment induces expression of Hcy metabolic pathway genes in CBS+/− mice cochlea.
MTHFR and CSE expression was induced by FA treatment. In CBS+/− mice MTHFR expression was less as compared to WT. For protein analysis n=4 repetitions from 4 separate animals, each group. *P<0.001 vs WT, #P<0.001 vs CBS+/−; n=4 animals/ group.
Effect of folic acid on oxidative stress
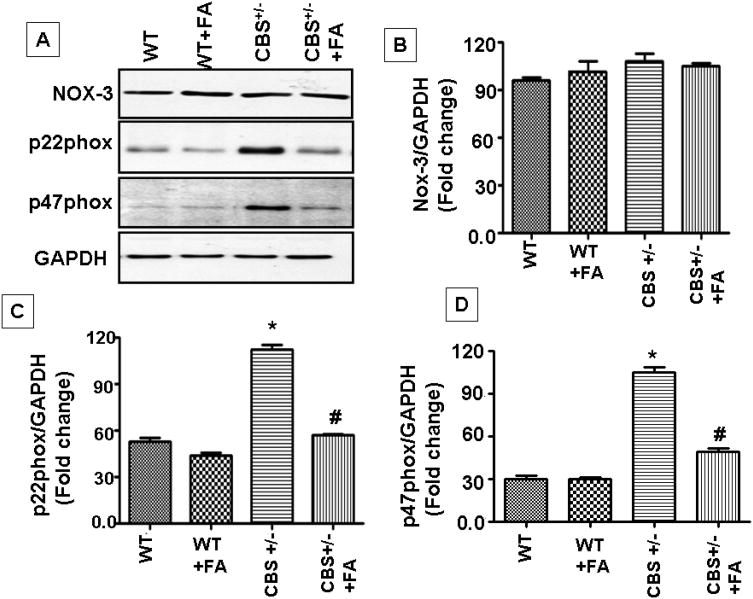
NADPH oxidase-3(NOX-3) mediates OONO− production in the inner ear. Our results showed that in CBS+/− mice, when supplemented with folic acid, NOX-3 protein levels did not alter. We did not found significant difference between the four group (F3, 12 =50, p>.01). Indeed we observed significant up-regulation in p22phox (F3,12=242,p>.001) and p47 phox levels (F3,12=224,p>.001) in CBS+/−, which clearly indicated oxidative stress levels being robust. Folic acid treatment ameliorated oxidative stress (Figure.5).
Figure.5. FA administration resulted in no significant alteration in NOX-3 levels and down-regulation of p22phox, p47phox expression in CBS+/− mice cochlea.
(A) Representative immunoblots for NOX-3, p22phox and p47phox and GAPDH control. Densitometric analysis of (B) NOX-3, (C) p22phox and (D) p47phox protein expression as represented in the bar diagram. All the panels are representative of three independent experiments. *P<0.001 vs WT, #P<0.001 vs CBS+/−; n=4 animals/group.
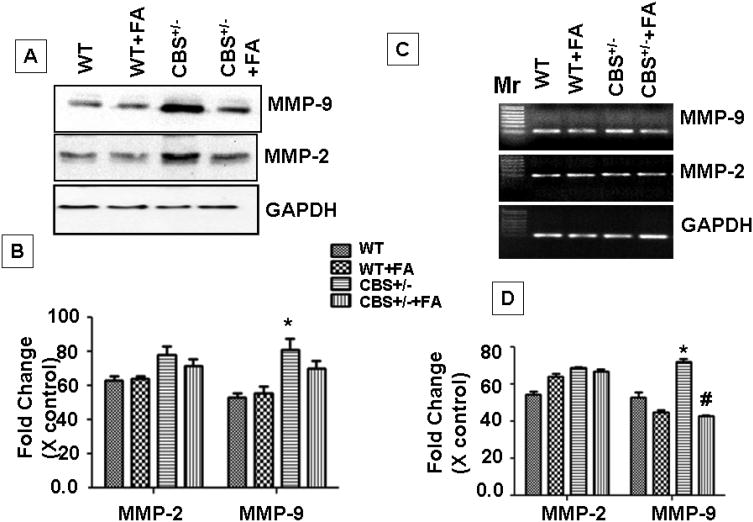
Effect of folic acid on MMP-9
Our results showed that MMP-9 protein and mRNA levels were increased in CBS+/− group as compared to WT. Folic acid treatment down regulated the MMP-9 expression at mRNA (F3,12=72,p>.001) and protein levels in CBS+/ − +FA group as compared to CBS+/− mice (F3,12= 85.1,p>.001). MMP-2 mRNA expression was significantly up-regulated in CBS+/− group as compared to WT (F3,12=20.4,p>.001). MMP-2, protein expression also significantly up-regulated CBS+/− group (F3,12=175.4,p>.001). Folic acid treatment down-regulated the protein expression however mRNA expression was unchanged (Figure 6).
Figure.6. FA administration mitigates expression of MMP-2 and 9 in CBS+/− mice cochlea.
(A) Representative immunoblots for MMP-2, MMP-9 and with GAPDH control. (B) Densitometric analysis of MMP-2 and 9 protein expressions using Image Lab software as shown in the bar diagram. (C) Represented semi quantitative mRNA expression for MMP-2, MMP-9 and with GAPDH control. (D) Densitometric analysis of MMP-2 and 9 mRNA expressions as represented in the bar diagram. *P<0.001 vs WT,Figure.2: FA#P<0.001 vs CBS+/−; n=4 animals/ group.
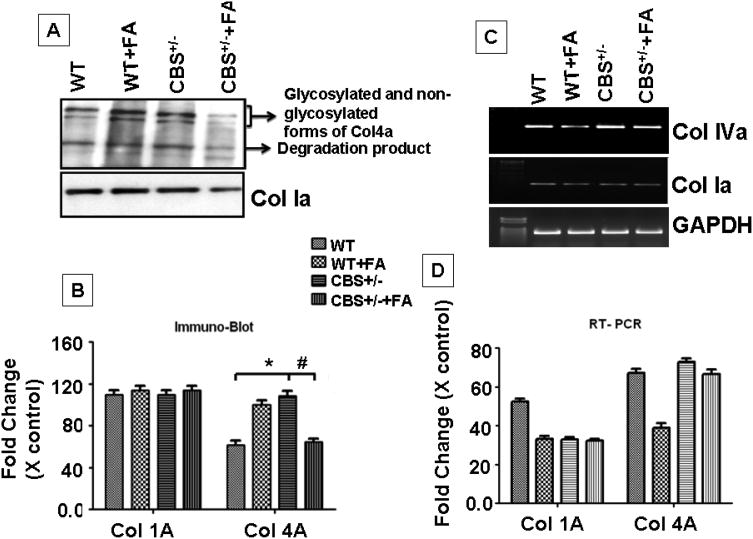
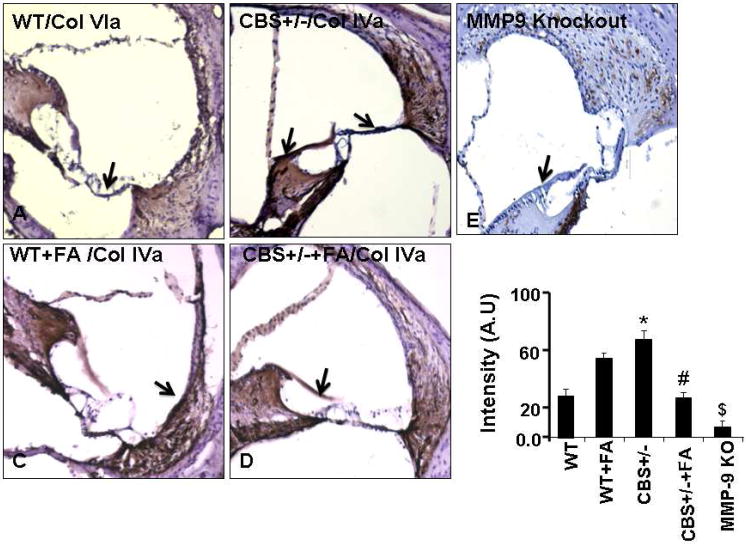
Collagen IV turnover in the lateral wall was mediated by MMP-9
In order to know if the change in the vascular regions of the inner wall related to collagen synthesis. Protein and mRNA expression analysis for collagen 1a and IVa was performed. Interestingly, we observed that the collagen synthesis and degradation was up-regulated by folic acid in wild type mice. A reverse effect was observed in CBS+/− +FA mice. In case of CBS+/− mice when compared to wild type, a higher rate of Col IVa synthesis and degradation was observed, but the turnover rate was significantly reduced by folic acid supplementation (Fig. 7A &B). However, we failed to observe any alteration in Col 1a expression. Folic acid treatment significantly down regulated the ColIVa protein levels (F3,12=32.4,p>.001) and also decreased mRNA expression in CBS+/− + FA compared to CBS+/− mice (F3,12= 44.6,p>.001). Histological staining for Col IV in cochlea sections from wild type and CBS+/− untreated and treated with folic acid were more informative. We observed pale color vessels in lateral wall of WT+FA and CBS+/− + FA group (Fig. 8). Vessels were intensely rich in Col IV staining in CBS+/− group lateral wall in comparison of CBS+/−+FA (Fig.8). We expected that the Col IVa turnover is regulated by collagenase (MMP-9 and MMP-2). Following this rational we used MMP-9 homozygous knockout mice (MMP−/−) and then analyzed Col IV expression (Fig 8). Staining of Col IV specifically would represent the intensity of blood vessels in the lateral wall, and interestingly our observation suggested that Col IV stain was tremendously lower in the lateral wall of MMP-9 KO. Thus, this result suggested that in the lateral wall Col IV deposition was robust increase in CBS+/− mice as compared to CBS+/−+FA. Therefore overall turnover was down-regulated by folic acid. These results suggested that MMP-9 was observed in regulating the level of Col IV in the lateral wall of the cochlea.
Figure.7. FA supplementation reduces collagen turn-over in the cochlear tissue of CBS+/− mice.
(A) Representative immunoblots showing collagen 1a (col1a), collagen VIa (colVIa) and GAPDH protein expression from four groups WT, WT + FA, CBS+/− and CBS+/−+ FA group. (B) Densitometric analysis of col I and colVI immunoblot quantification as shown in the bar diagram. *P<0.001 vs WT,#P<0.001 vs CBS+/−; n=4 animals/ group.
Figure.8. MMP-9 is involved in the ColVIa turnover in the inner ear.
(A) Immunostaining results demonstrate ColVIa expression in the cochlear sections from WT, WT + FA, CBS+/−, CBS+/−+ FA and MMP-9 Knock out group. n=4; three sections from each group were scanned for digital data analysis. *Notice: MMP-9 Knockout mice cochlea was stained for Col4a showed highly reduced deposition for the collagen 4A. (B) Bar diagram represents quantification of immunostaining using image pro software. P<0.001 vs WT,#P<0.001 vs CBS+/−; $ P<0.001 vs CBS+/−; n=4 animals/ group.
Discussion
Despite multiple investigations, very little is known about HHcy associated hearing loss among patients. Previous studies have shown that Hcy induces matrix imbalance, morphologcal changes and oxidative stress in cochlear tissue (Kundu et al., 2009b). However, functional implications and the associated mechanism were not elucidated. The results from our current study have shown that CBS +/− (HHcy) mice develop hearing impairments with significantly increased hearing thresholds indicating poor hearing sensitivity when compared to WT control, whereas mice CBS+/−+ FA treatment displayed no significant differences in hearing compared to control. These finding suggest improved hearing threshold functions through folic acid supplementation in HHcy mice.
Our reports are consistent with recent clinical study that showed HHcy individuals were more likely to develop hearing loss and low serum folate further aggravates the chances of existing hearing defect among the population (Gopinath et al., 2010). The results of this study have provided an overall picture of the mechanism that is involved in Hcy associated hearing impairment.
Previous reports have demonstrated that HHcy has been implicated in the endothelial barrier disruption around the capillary lining of the stria vascularis, causing leakage and hearing defects (Cohen-Salmon et al., 2007). Indeed, folic acid deficiency has been implicated increased arterial permeability in elderly patients (Symons et al., 2006). We have also found increased permeability of blood vessels around the stria vascularis (SV) during HHcy. This increased permeability was mitigated with FA treatment. These findings suggests beneficial role of FA in maintaining capillary vascular integrity around SV.
A recent report from our lab has demonstrated that Hcy levels were significantly reduced upon FA supplementation to CBS+/− mice (Qipshidze et al., 2010). This downregulation in Hcy levels suggests altered activity/expression of Hcy metabolic pathway enzymes. In order to understand the role of FA treatment in expression of Hcy metabolic pathway genes in cochlear tissue, we measured the expression of MTHFR and CSE. Immunoblot results revealed downregulation of Hcy metabolic pathway genes expressed in the cochlea of CBS+/− HHcy mice. This was mitigated with FA supplementation. Furthermore, MTHFR gene mutations have been implicated in sensorineural hearing defect in humans (Capaccio et al., 2005; Uchida et al., 2011).
Collagen turnover, both synthesis and degradation at a controlled rate, is essential for the maintenance of normal auditory processing in the cochlea. Other researchers and we have shown that Hcy increases collagen turnover in various pathological conditions (Miller et al., 2002; Polyzos et al., 2010). Here we report that FA mitigates Hcy induced collagen turn over by decreasing the expression of Col4a and Col1a in the cochlear tissue of CBS+/− mice. Since, our previous report, we have shown that folic acid treatment decreases Hcy levels. This decrease in Collagen turn over may, in part, be due to the decrease in Hcy levels by folic acid. Moreover, abnormality in col IV subunits distribution has been implicated in hearing loss among patients (Meyer zum Gottesberge and Felix, 2005), suggesting the elevation of Col 4a levels may be a consistent marker of hearing defect. Conversely, deficiency in collagen receptors called DDR1 has been associated with decrease in auditory function and substantial structural alterations (Meyer zum Gottesberge et al., 2008). These finding suggest optimum levels of collagen are essential in maintaining matrix turn over in the ear and consequently maintaining proper hearing function.
Collagen turnover is also regulated by matrix metalloproteinases (MMPs), which are involved in remodeling of ECM in the inner ear. Recent studies demonstrated that MMP-2 and MMP-9 are both expressed within the cochlea and their basal level of activity are required for normal hearing functions (Setz et al., 2011).
In-addition, it has been shown that MMP inhibitors significantly suppress hearing functions by causing hair cell death (Woo et al., 2008). On the other hand elevation in MMP levels contributes to the pathogenesis of childhood hearing loss (Lauhio et al., 2010). Interestingly, our previous reports have shown that HHcy mice displays increase expression of MMP-2 and MMP-9 in cochlear tissue (Kundu et al., 2009a). Our current study demonstrated that Hcy induced MMP-2 and 9 were returned to their normal levels in the CBS+/− mice given FA supplementation. Hcy induced matrix imbalance is more likely to contribute higher collagen turn over which was mitigated by FA treatment to CBS+/−. The role of MMP-9 was further established by our immunohistochemistery images Figure 8 that shows very little Col4a deposition in the chochlear sections from MMP-9 knockout mice.
Interestingly, oxidative stress, implicated as a pathological mechanism in HHcy, which contributes significantly to the noise, ototoxicity- and age-induced hearing loss (Gates and Mills, 2005). NADP oxidase-3 expressed in specific portion of the inner ear has been implicated in the ROS generations that contribute to irreparable hearing loss (Banfi et al., 2004). During HHcy, Hcy undergoes auto-oxidation and triggers systemic ROS generation. In the present study we demonstrated levels of oxidative stress triggered by Hcy around the cochlear tissue was mitigated with FA supplementation. Additionally, we did not observe any significant alteration in the (NOX-3) levels, consistent with our previous reports (Kundu et al., 2009a). Interestingly, we found the NOX subunits p22phox and p47phox protein levels were robustly increased in cochlear tissue of HHcy mice, which was mitigated with FA supplementation. These results showed that FA alleviated Hcy induced oxidative stress and thus improves hearing functions.
Conclusions
Overall these find suggest the promising role of FA supplementation therapy in the hearing defect associated with HHcy. We were also able to delineate the possible mechanism responsible for Hcy-associated hearing impairment.
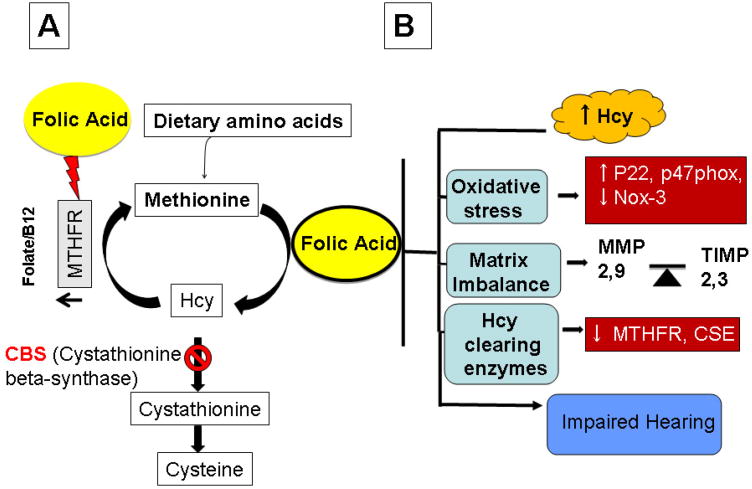
Figure.9. Schematic representation of the proposed hypothesis.
that Hcy induces matrix imbalance in the cochlear tissue which then affects hearing thresh holds. Hcy also induces oxidative stress in the cochlear tissue, in part, by down regulating levels of p22phox and p47phox subunits this in-turn decreases hearing threshold. Folic acid treatment alleviates Hcy induced decrease hearing threshold, by down regulating Hcy-induced oxidative stress, via declining collagen turnover and matrix imbalance.
Highlights.
A total homocysteine (Hcy) levels was increased in stria vascularis (SV) and spiral ligament (SL) of cystathionine-beta-synthase heterozygote knockout (CBS+/−) mice which wwas lowered by folic acid (FA) treatment.
Our current study have shown that CBS +/− (HHcy) mice develop hearing impairments with significantly increased hearing thresholds indicating poor hearing sensitivity when compared to WT control, whereas mice CBS+/−+ FA treatment displayed no significant differences in hearing compared to control.
Folic acid supplementation improved hearing threshold in HHcy mice.
The oxidative stress was high in SV and SL of CBS+/− compared to WT however the treatment with FA lowered oxidative stress in CBS+/− mice.
Hearing loss in CBS+/− mice was primarily due to leakage in inner ear circulation in- part by induced collagen imbalance.
Acknowledgments
This work was supported by NIH grants: HL-71010 and NS-51568 to SCT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Angelborg C, Larsen HC, Slepecky N. Regional cochlear blood flow studied by observation of microspheres in serial sections. Ann Otol Rhinol Laryngol. 1985;94:181–5. doi: 10.1177/000348948509400217. [DOI] [PubMed] [Google Scholar]
- Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–72. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- Capaccio P, Ottaviani F, Cuccarini V, Ambrosetti U, Fagnani E, Bottero A, Cenzuales S, Cesana BM, Pignataro L. Sudden hearing loss and MTHFR 677C>T/1298A>C gene polymorphisms. Genet Med. 2005;7:206–8. doi: 10.1097/01.gim.0000157817.92509.45. [DOI] [PubMed] [Google Scholar]
- Clarke ZL, Moat SJ, Miller AL, Randall MD, Lewis MJ, Lang D. Differential effects of low and high dose folic acid on endothelial dysfunction in a murine model of mild hyperhomocysteinaemia. Eur J Pharmacol. 2006;551:92–7. doi: 10.1016/j.ejphar.2006.08.085. [DOI] [PubMed] [Google Scholar]
- Cohen-Salmon M, Regnault B, Cayet N, Caille D, Demuth K, Hardelin JP, Janel N, Meda P, Petit C. Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci U S A. 2007;104:6229–34. doi: 10.1073/pnas.0605108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–20. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Flood VM, Rochtchina E, McMahon CM, Mitchell P. Serum homocysteine and folate concentrations are associated with prevalent age-related hearing loss. J Nutr. 2010;140:1469–74. doi: 10.3945/jn.110.122010. [DOI] [PubMed] [Google Scholar]
- Hausler R, Levine RA. Auditory dysfunction in stroke. Acta Otolaryngol. 2000;120:689–703. doi: 10.1080/000164800750000207. [DOI] [PubMed] [Google Scholar]
- Kumar M, Tyagi N, Moshal KS, Sen U, Kundu S, Mishra PK, Givvimani S, Tyagi SC. Homocysteine decreases blood flow to the brain due to vascular resistance in carotid artery. Neurochem Int. 2008;53:214–9. doi: 10.1016/j.neuint.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Tyagi N, Sen U, Tyagi SC. Matrix imbalance by inducing expression of metalloproteinase and oxidative stress in cochlea of hyperhomocysteinemic mice. Mol Cell Biochem. 2009a;332:215–24. doi: 10.1007/s11010-009-0194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Tyagi N, Sen U, Tyagi SC. Matrix imbalance by inducing expression of metalloproteinase and oxidative stress in cochlea of hyperhomocysteinemic mice. Molecular and cellular biochemistry. 2009b;332:215–24. doi: 10.1007/s11010-009-0194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauhio A, Rezes S, Tervahartiala T, Sziklai I, Pitkaranta A, Sorsa T. Matrix metalloproteinase-8/collagenase-2 in childhood otitis media with effusion. Ann Med. 2010 doi: 10.3109/07853890.2010.530684. [DOI] [PubMed] [Google Scholar]
- Liu D. Influence of hypertension and coronary heart disease on the hearing in the aged. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1988;23:342–5. 384–5. [PubMed] [Google Scholar]
- Mees K, Suckfull M. Cochlear and vestibular risk at high altitude. Laryngorhinootologie. 2002;81:465–8. doi: 10.1055/s-2002-33290. [DOI] [PubMed] [Google Scholar]
- Meyer zum Gottesberge AM, Felix H. Abnormal basement membrane in the inner ear and the kidney of the Mpv17−/− mouse strain: ultrastructural and immunohistochemical investigations. Histochem Cell Biol. 2005;124:507–16. doi: 10.1007/s00418-005-0027-7. [DOI] [PubMed] [Google Scholar]
- Meyer zum Gottesberge AM, Gross O, Becker-Lendzian U, Massing T, Vogel WF. Inner ear defects and hearing loss in mice lacking the collagen receptor DDR1. Lab Invest. 2008;88:27–37. doi: 10.1038/labinvest.3700692. [DOI] [PubMed] [Google Scholar]
- Miller A, Mujumdar V, Palmer L, Bower JD, Tyagi SC. Reversal of endocardial endothelial dysfunction by folic acid in homocysteinemic hypertensive rats. Am J Hypertens. 2002;15:157–63. doi: 10.1016/s0895-7061(01)02286-5. [DOI] [PubMed] [Google Scholar]
- Mujumdar VS, Hayden MR, Tyagi SC. Homocyst(e)ine induces calcium second messenger in vascular smooth muscle cells. J Cell Physiol. 2000;183:28–36. doi: 10.1002/(SICI)1097-4652(200004)183:1<28::AID-JCP4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Munjal C, Givvimani S, Qipshidze N, Tyagi N, Falcone JC, Tyagi SC. Mesenteric vascular remodeling in hyperhomocysteinemia. Mol Cell Biochem. 2011;348:99–108. doi: 10.1007/s11010-010-0643-y. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Masutani H, Moriguchi M, Matsunaga K, Kato A, Maeda H. Microvasculature of normal and hydropic labyrinth. Scanning Microsc. 1992;6:1097–103. discussion 1103-4. [PubMed] [Google Scholar]
- Polyzos SA, Anastasilakis AD, Efstathiadou Z, Litsas I, Kita M, Panagiotou A, Papatheodorou A, Arsos G, Moralidis E, Barmpalios G, Zafeiriadou E, Triantafillidou E, Makrigiannaki E, Terpos E. Serum homocysteine, folate and vitamin B12 in patients with Paget's disease of bone: the effect of zoledronic acid. J Bone Miner Metab. 2010;28:314–9. doi: 10.1007/s00774-009-0131-1. [DOI] [PubMed] [Google Scholar]
- Qipshidze N, Metreveli N, Lominadze D, Tyagi SC. Folic acid improves acetylcholine-induced vasoconstriction of coronary vessels isolated from hyperhomocysteinemic mice: An implication to coronary vasospasm. J Cell Physiol. 2010 doi: 10.1002/jcp.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk WS, Seidman MD. Cochlear vascular changes in response to loud noise. Am J Otol. 1995;16:322–5. [PubMed] [Google Scholar]
- Sakagami M, Sano M, Tamaki H, Matsunaga T. Ultrastructural study of the effect of acute hyper- and hypotension on the stria vascularis and spiral ligament. Acta Otolaryngol Suppl. 1984;406:256–62. doi: 10.3109/00016488309123046. [DOI] [PubMed] [Google Scholar]
- Scherer EQ, Wonneberger K, Wangemann P. Differential desensitization of Ca2+ mobilization and vasoconstriction by ET(A) receptors in the gerbil spiral modiolar artery. J Membr Biol. 2001;182:183–91. doi: 10.1007/s00232-001-0041-1. [DOI] [PubMed] [Google Scholar]
- Scherer EQ, Wangemann P. ETA receptors in the gerbil spiral modiolar artery. Adv Otorhinolaryngol. 2002;59:58–65. doi: 10.1159/000059236. [DOI] [PubMed] [Google Scholar]
- Setz C, Brand Y, Radojevic V, Hanusek C, Mullen PJ, Levano S, Listyo A, Bodmer D. Matrix metalloproteinases 2 and 9 in the cochlea: expression and activity after aminoglycoside exposition. Neuroscience. 2011;181:28–39. doi: 10.1016/j.neuroscience.2011.02.043. [DOI] [PubMed] [Google Scholar]
- Sterkers O, Saumon G, Tran Ba Huy P, Amiel C. K, Cl, and H2O entry in endolymph, perilymph, and cerebrospinal fluid of the rat. Am J Physiol. 1982a;243:F173–80. doi: 10.1152/ajprenal.1982.243.2.F173. [DOI] [PubMed] [Google Scholar]
- Sterkers O, Saumon G, Tran Ba Huy P, Amiel C. Evidence for a perilymphatic origin of the endolymph: application to the pathophysiology of Meniere's disease. Am J Otolaryngol. 1982b;3:367–75. doi: 10.1016/s0196-0709(82)80012-4. [DOI] [PubMed] [Google Scholar]
- Symons JD, Zaid UB, Athanassious CN, Mullick AE, Lentz SR, Rutledge JC. Influence of folate on arterial permeability and stiffness in the absence or presence of hyperhomocysteinemia. Arterioscler Thromb Vasc Biol. 2006;26:814–8. doi: 10.1161/01.ATV.0000204408.01416.16. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Sugiura S, Ando F, Nakashima T, Shimokata H. Hearing impairment risk and interaction of folate metabolism related gene polymorphisms in an aging study. BMC Med Genet. 2011;12:35. doi: 10.1186/1471-2350-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P. Cochlear blood flow regulation. Adv Otorhinolaryngol. 2002;59:51–7. doi: 10.1159/000059241. [DOI] [PubMed] [Google Scholar]
- Woo MS, Park JS, Choi IY, Kim WK, Kim HS. Inhibition of MMP-3 or -9 suppresses lipopolysaccharide-induced expression of proinflammatory cytokines and iNOS in microglia. J Neurochem. 2008;106:770–80. doi: 10.1111/j.1471-4159.2008.05430.x. [DOI] [PubMed] [Google Scholar]
- Zhen PP, Duan JH, Zhao Q, Hou DD, Wang HX, Hong N, Zhen HX, Wang W. Phytoestrogen alpha-zearalanol improves vascular function in ovariectomized hyperhomocysteinemic rats. Atherosclerosis. 2011;215:309–15. doi: 10.1016/j.atherosclerosis.2010.12.029. [DOI] [PubMed] [Google Scholar]