Summary
Programmed cell death is a necessary part of development and tissue homeostasis enabling the removal of unwanted cells. In the setting of infectious disease, cells that have been commandeered by microbial pathogens become detrimental to the host. When macrophages and dendritic cells are compromised in this way, they can be lysed by pyroptosis, a cell death mechanism that is distinct from apoptosis and oncosis/necrosis. Pyroptosis is triggered by Caspase-1 after its activation by various inflammasomes, and results in lysis of the affected cell. Both pyroptosis and apoptosis are programmed cell death mechanisms, but are dependent on different caspases, unlike oncosis. Similar to oncosis, and unlike apoptosis, pyroptosis results in cellular lysis and release of the cytosolic contents to the extracellular space. This event is predicted to be inherently inflammatory, and additionally coincides with IL-1β and IL-18 secretion. We discuss the role of distinct inflammasomes, including NLRC4, NLRP3 and AIM2, as well as the role of the ASC focus in Caspase-1 signaling. We further review the importance of pyroptosis in vivo as a potent mechanism to clear intracellular pathogens.
Keywords: monocytes/macrophages, Toll-like Receptors/Pattern recognition receptors, Apoptosis/Autophagy, Pyroptosis, Caspase-1
Introduction
In this review we discuss pyroptosis, which is defined as Caspase-1 dependent programmed cell death, resulting in rapid lysis of the affected cell. We begin by placing pyroptosis in the context of other cell death mechanisms.
Cell death was classically viewed as either apoptosis or necrosis, a mechanistically binary perspective that is now known to be an oversimplification. In 1994 E. Faber wrote “there is no field of basic cell biology and cell pathology that is more confusing and more unintelligible than is the area of apoptosis versus necrosis” (1), a view that only seems truer 17 years later. The use of the term necrosis to refer both to a specific type of cell death, and to the pathologic changes at the tissue level after cell death has generated some confusion in the field. The term oncosis has been suggested as an alternative to “necrotic cell death” to refer to cell death that involves cellular swelling, typically caused by ischemia or other insults which impaired cellular ATP generation (2). This preserves the term “necrosis” for the description of histopathology resulting from multiple cell death mechanisms. Although the term oncosis has not been widely used, it is perhaps a more accurate term in discussing cell death, and we will use it herein.
Cell death can be broadly categorized by describing the initiating events, the intermediate changes, the terminal cellular events, and its effect on tissue (see Table 1 for a comparison of apoptosis, pyroptosis, and oncosis). Each cell death pathway may be distinguished on the basis of these four categories. In addition to apoptosis and oncosis, several other forms of cell death have been described, including pyroptosis (3), autophagic cell death (4), NETosis (5), necroptosis (6), mitotic catastrophe (7), and lysosomal membrane permeabilization (8). Most of these modalities have specific initiation events in vitro, and some, but not all, have well defined roles in vivo.
Table 1.
Comparison of Apoptosis, Pyroptosis and Oncosis.
| Apoptosis | Pyroptosis | Oncosis | |
|---|---|---|---|
| Initiating | Programmed | Programmed | Accidental |
| Signaling pathway | Caspase-3/6/7 | Caspase-1 | Non-caspase |
| Terminal event | Non-lytic | Lytic | Lytic |
| Effect on tissue | Non-inflammatory | Inflammatory | Inflammatory |
| Cell types | All | Mϕ and DC | All |
Initiating events in cell death may be programmed, meaning that specific dedicated signaling pathways are involved (as in apoptosis and pyroptosis), or accidental/non-programmed (as in oncosis), in which specific signaling events are not required. Apoptosis was once synonymous with programmed cell death, but now many other forms of programmed cell death have been described (4). It can be initiated by a variety of stimuli arising from receptors on the cell surface (extrinsic pathway) or from detection of events within the cell (intrinsic pathway). Pyroptosis is initiated by a variety of stimuli, including cytosolic flagellin or T3SS rod proteins, phagocytosis of crystals, and opening of pores in the membrane. Oncosis results from chemical, thermal, or radiation damage to cells that cause disruption of cellular processes beyond the point of repair. Such damaged cells will inevitably die. The lines between programmed and non-programmed cell death can become blurred in cases of lower intensity insults, in which cellular enzymatic activities can exacerbate the damage and contribute to cell death. For example, oxidative stress can cause lysosomal disruption and the release of proteases to the cytosol, resulting in proteolysis of cytosolic proteins that induces death. This initiating event could be considered either accidental release of toxic substances, or a pre-set mechanism to detect cellular damage and initiate a cell death response (8).
Intermediate changes observed upon examination of dying cells using bright field, confocal, or electron microscopy reveal differences between apoptotic and necrotic cell death. Apoptosis results in characteristic morphologic changes, including cell shrinkage, nuclear condensation, nuclear fragmentation, and membrane blebbing. In contrast, oncosis results in morphology characterized by cellular rounding, cytoplasmic swelling, dilation of cellular organelles, the absence of chromatin condensation, and disorganized dismantling of intracellular contents (2). While the apoptotic morphology still holds as a specific marker for apoptosis, the necrotic morphology can be observed in several mechanistically distinct forms of cell death.
The terminal cellular event can be characterized as non-lytic or lytic (4). These two categories are intimately linked to the intermediate morphologic changes. Oncosis and pyroptosis result in the lysis of the cell and release of cytosolic contents. Apoptosis ends when cells segment into smaller apoptotic bodies, in a process called blebbing. Apoptotic bodies are membrane bound and contain the contents of the nucleus and cytoplasm, which can be phagocytosed and degraded. Cytosolic contents are not normally released, but if apoptotic bodies are not cleared they will lyse in a process called secondary necrosis (2).
Finally, the effects of cellular death at the tissue level can be considered. Typically apoptosis is non-inflammatory, meaning that neutrophils are not recruited and adaptive immune responses are not initiated. Macrophages migrate towards apoptotic bodies, attracted by “find-me” signals (9), but they do not promote additional inflammatory changes. Apoptotic bodies are cleared without damage to the tissue unless the amount of apoptotic bodies exceeds the removal capacity of the local or recruited macrophages, resulting in secondary necrosis (2). In contrast, oncosis and pyroptosis are inflammatory. Several small molecules and proteins that are normally confined within cells are detected by specific receptors that induce a response characterized by the classical signs of inflammation at the tissue level: rubor (redness), dolor (pain), calor (heat), and tumor (swelling). In areas of extensive cell death, tissue necrosis, also called coagulation necrosis, occurs.
Caspase activation
Caspases play central roles in initiating apoptosis and pyroptosis, but are not involved in other programmed cell death. They are proteases that use active site cysteine residues to cleave target proteins after aspartate residues. Caspases exist in inactive pro forms in the cytosol, and are activated by proteolytic cleavage by other caspases. For example, murine Caspase-1 can be cleaved at six sites: cleavage at D103 and/or D122 removes the CARD domain from the proteolytic domains while cleavage at D296, D308, D313 and D314 separates the p10 and p20 catalytic domain fragments (10). These proteases are broadly classified as apoptotic (Caspase-2, 3, 6, 7, 8, 9, 10) or inflammatory (Caspase-1, 4, 5, 12) (11).
Apoptotic caspases are either initiators or effectors (11). Initiator caspases respond to a specific initiating event that activates the intrinsic or extrinsic pathway. Molecular events occurring inside the cell trigger the intrinsic pathway. For example, DNA damage induces PIDD/RAIDD oligomerization that activates Caspase-2. Alternatively, cytochrome C released from damaged mitochondria is detected by APAF-1, which activates Caspase-9. The extrinsic pathway is triggered by ligation of transmembrane receptors, including TNF receptor, Fas, and TRAIL receptor, resulting in activation of Caspase-8 or -10. Both the intrinsic and extrinsic pathways activate the effector caspases, Caspase-3, -6, and/or -7, which cleave the target molecules that promote apoptosis (12).
In contrast to the apoptotic caspases, initiator and effector functions have not been defined for the inflammatory caspases. Caspase-1 is the best-described inflammatory caspase. It processes the cytokines IL-1β and IL-18 and induces pyroptotic cell death. While Caspase-1 was once thought to also process IL-33, this is no longer believed to be the case(13). Caspase-4 and -5 are also inflammatory caspases, though their functions are less well defined (Caspase-5 is not present in mice, and murine Caspase-4 is also known as Caspase-11). Caspase-12 exists in both truncated and full-length alleles in humans and as a full-length caspase in rodents. The full-length alleles exert inhibitory effects on Caspase-1 (14). Caspase-4 is not required for pyroptosis (10), nor is there any evidence that Caspase-12 plays a role.
Inflammasomes and Apoptosomes
Caspase-9 and Caspase-1 are activated by Nod-like receptors (NLRs) forming the apoptosome and inflammasome, respectively. NLRs are composed of a signaling domain (typically a CARD or Pyrin domain), a nucleotide-binding oligomerization domain (NOD), and a sensor domain (WD40 repeat domain or leucine-rich repeat domain, LRR). The mechanisms of caspase activation were first described for the NLR family member APAF-1. APAF-1 contains a CARD signaling domain and a WD40 repeats domain. In the inactive state, the WD40 repeats interact with and inhibit the NOD domain. When the WD40 repeats bind to cytochrome C, the NOD domain is released, permitting oligomerization of seven APAF-1 monomers into a hub like structure called the apoptosome. The APAF-1 CARD domain recruits pro-Caspase-9 via homotypic interaction with the Caspase-9 CARD domain. This activates Caspase-9 proteolytic activity permitting one Caspase-9 molecule to cleave its neighbor into the active form (15).
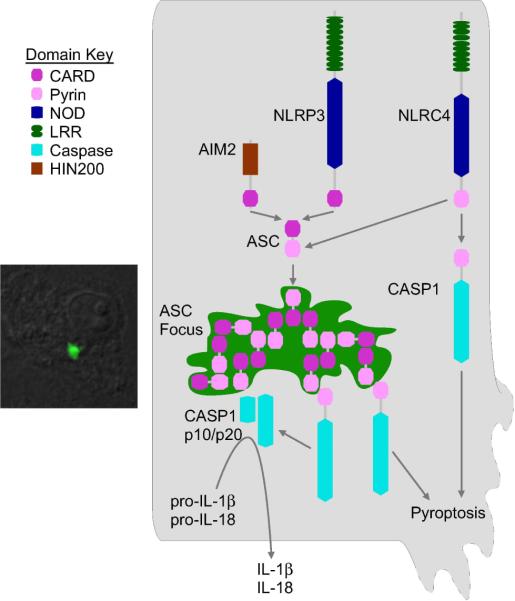
Caspase-1 activation is regulated by protein complexes termed inflammasomes, which are analogous to the apoptosome. Two types of NLR inflammasomes are known. NLRC4 and murine NLRP1b contain CARD domains that directly interact with the Caspase-1 CARD, making these inflammasomes directly analogous to the APAF-1 apoptosome (16). In contrast, NLRP3 contains a Pyrin signaling domain instead of a CARD domain. The Pyrin domain of NLRP3 binds the Pyrin domain of the adaptor protein ASC. ASC (also known as PYCARD) is composed of only a Pyrin and a CARD domain. ASC recruits Caspase-1 via CARD-CARD interactions (17). In an added wrinkle, AIM2 also forms an inflammasome. However, AIM2 is not an NLR. It contains a HIN200 domain and a Pyrin domain that recruits ASC and activates Caspase-1 (18).
Caspase-1 becomes activated in response to numerous stimuli that are detected through distinct inflammasomes. NLRC4 responds to cytosolic flagellin or T3SS rod proteins (16, 19), murine NLRP1b responds to anthrax lethal toxin (20), AIM2 responds to cytosolic DNA (18), and NLRP3 responds to a variety of agonists including crystals (17). Each of these sensors triggers pyroptosis (21).
Pyroptosis
Pyroptosis was first described in 1992 in macrophages infected with Shigella flexneri (22), and shortly thereafter a similar phenotype was observed after infection with S. typhimurium (23, 24). It was termed “apoptosis” based on morphologic changes (apparent blebbing) and the presence of DNA fragmentation, chromatin condensation, and the requirement for Caspase-1 (23–27). At the time, caspase dependence was a hallmark of apoptotic cell death. Pyroptosis was subsequently shown to be distinct from apoptosis (28, 29), and the name was coined in 2001 (30). “Pyro“ originates from the Greek word for fire in reference to the involvement of Caspase-1 and IL-1β in fever and inflammation. “Ptosis” (silent “p”) comes from the Greek word for falling as used for other forms of cell death. To date, pyroptosis has only been described in macrophages and dendritic cells (21, 31) though there is some evidence of Caspase-1 activity in other cell types (32).
Both pyroptosis and apoptosis are forms of programmed cell death that require specific Caspase activity. Unlike apoptosis, pyroptosis occurs after Caspase-1 activation, and does not involve apoptotic caspases (26, 28, 33, 34). In addition, target proteins such as PARP1 and ICAD, which are characteristically cleaved during apoptosis, remain intact during pyroptosis (28, 35, 36). Finally, apoptosis proceeds normally in the absence of Caspase-1 (37, 38). Thus, apoptosis and pyroptosis are distinct forms of programmed cell death.
Several features of pyroptosis seem to overlap with apoptosis, but upon further investigation are found to be distinct. First, during pyroptosis, cells incur DNA damage (22) and become positive in the TUNEL assay (23, 28). However, the nuclear morphology of pyroptotic cells is distinct from apoptotic cells (28, 29), and DNA laddering is not necessarily seen (29). In apoptosis, chromatin undergoes pyknosis, the irreversible condensation of chromatin that localizes to the nuclear membrane (margination). This creates curved half-moon profiles of pyknotic chromatin. The nucleus then breaks up in the process of karyorrhexis (2). In contrast examination of the nucleus in pyroptotic cells reveals chromatin condensation, but the nucleus remains intact and karyorrhexis does not occur (22, 29).
A second feature that is shared between pyroptosis and apoptosis is positive staining with annexin V. Annexin V binds phosphatidyl serine that is normally restricted to the inner leaflet of the cell membrane. During apoptosis, phosphatidyl serine translocates to the outer leaflet, resulting in positive cell surface staining with annexin V. This discriminates apoptotic from live cells (39). During pyroptosis, pores open in the cell membrane, permitting annexin V to enter the cell and stain the inner leaflet of the membrane. In contrast, membrane impermeant dyes such as 7-aminoactinomycin (7-AAD) or propidium iodide (PI) stain pyroptotic cells by entering through the pores, but do not stain apoptotic cells (28, 31, 36, 40). An additional consequence of pore formation in pyroptosis is cellular swelling (36), which can be blocked by extracellular osmoprotectants, or by extracellular glycine (28, 36). Conversely, apoptotic cells shrink. Thus, the mechanisms resulting in positive annexin V staining are different between apoptosis and pyroptosis.
A third apparent similarity between pyroptosis and apoptosis is the formation of spherical membrane bound structures. During apoptosis, the cell blebs, partitioning itself into spherical membrane bound structures known as apoptotic bodies (2). During pyroptosis, increased osmotic pressure results in large spherical protrusions of the membrane as it rips away from the cortical cytoskeleton. Because electron micrographs of the protrusions revealed spherical structures, they were initially thought to be apoptotic blebs (22, 23). However, continued rapid swelling of these protrusions causes coalescence and rupture. This results in the release of cytosolic contents to the extracellular space, commonly measured by assaying for lactate dehydrogenase release (28). This does not occur during apoptosis, since apoptotic bodies are cleared by phagocytosis.
Potential cross-talk between pyroptosis and other cell death pathways
It is possible that there is some cross activation of apoptotic caspases downstream of Caspase-1. Caspase-1 can proteolytically process the apoptotic effector Caspase-7, but not Caspase-3 (34). This may have effects during Legionella infection or septic shock (41, 42). However, since pyroptosis occurs more rapidly than apoptosis, and since Caspase-7 is not required for pyroptosis (34), the physiologic relevance of this processing event remains to be further elucidated. Perhaps in cell types that express Caspase-1 but do not undergo pyroptosis, such as keratinocytes (32), Caspase-1 activation results in apoptosis via Caspase-7. This would be predicted to result in a slower and less inflammatory clearance mechanism to remove the affected cells.
Further cross talk may arise at the agonist level. LPS or S. typhimurium infection has been shown to activate Caspase-3 either independent of Caspase-1 (34) or dependent on Caspase-1 (33). However, we do not observe Caspase-3 activation after LPS stimulation or S. typhimurium infection, and find macrophages to be non-apoptotic and viable after overnight stimulation with LPS or other TLR agonists (Miao and Aderem, unpublished results). Thus the role of Caspase-3 after LPS or Salmonella infection remains to be further elucidated.
In the absence of Caspase-1, alternate cell death pathways may compensate for the lack of pyroptosis. Casp1−/− macrophages infected with S. typhimurium die via alternate cell death pathways that are kinetically slower than pyroptosis. Caspase-2-dependent apoptosis (33) and autophagic cell death (43) have both been implicated as alternate cell death pathways in Casp1−/− macrophages.
Differential Caspase-1 subcellular localization for pyroptosis and cytokine processing
The adaptor protein ASC plays a critical role in connecting pyrin domain connecting inflammasomes (such as NLRP3 and AIM2) to Caspase-1 (44–46). At its simplest level, ASC docks onto the inflammasome hub via pyrin-pyrin interactions, then recruits Caspase-1 via CARD-CARD interactions. Thus, an NLRP3 inflammasome hub containing seven NLRP3 monomers (by analogy to APAF-1) could recruit seven ASC adaptors and seven pro-Caspase-1 proteins. However, the actual signaling complex inside a cell is more complex than this. ASC can interact with itself, recruiting additional ASC molecules via pyrin-pyrin and CARD-CARD interactions (47). This creates a cascade effect where all of the ASC within a cell is recruited to a single subcellular location, which has been called the “ASC focus” or “ASC speck” (10, 48, 49). Because ASC is required for pyroptosis induction via NLRP3 and AIM2, the ASC focus has also been called the “pyroptosome” (50), however given recent results discussed below this term should be discontinued (ASC is not required for pyroptosis in all cases and is more prominently required for cytokine processing). The ASC focus is a large subcellular structure that can be visualized by confocal fluorescent microscopic staining for ASC (Fig. 1), and has a central core with apparent dendrites extending from the center (10, 45, 48–51). The ASC focus recruits Caspase-1, resulting in its activation and proteolysis of pro-Caspase-1 to the mature form via neighboring activated Caspase-1 proteins (45). Caspase-1 proteolysis is required to enable it to cleave pro-IL-1β (10) (Fig. 1). IL-1β is also recruited to the foci via interactions with Caspase-1. IL-1β recruitment is unaffected by the Caspase-1 inhibitor zYVAD-FMK, but was ablated in Casp1−/− macrophages (49). This indicates that the ASC focus recruits and activates Caspase-1 via autoproteolysis, and that Caspase-1 processes pro-IL-1β within the ASC focus.
Figure 1. Role of ASC in cytokine processing and pyroptosis.
All known inflammasomes recruit ASC, resulting in the formation of the ASC focus (microscopic image to left showing ASC focus after L. monocytogenes infection in a macrophage). Caspase-1 is processed in the ASC focus and cleaves pro-IL-1β and pro-IL-18 to their mature, secreted forms. CARD-containing inflammasomes, such as NLRC4, also bind Caspase-1 independent of ASC, and this complex does not require Caspase-1 processing, and triggers pyroptosis. Pyrin-containing inflammasomes, such as AIM2 and NLRP3, trigger pyroptosis through ASC.
In the case of CARD containing inflammasomes, for example NLRC4, the CARD domain can interact directly with Caspase-1 (52), apparently obviating the need for ASC (Fig. 1). However, NLRC4 additionally recruits ASC via CARD-CARD interactions (53) and induces the formation of ASC foci. Thus, NLRC4 (and presumably other CARD containing inflammasomes) can activate Caspase-1 via ASC-dependent and ASC-independent mechanisms. Study of these two mechanisms has recently led to insights into distinct mechanisms for cytokine processing and pyroptosis. After NLRC4 activation, the ASC focus forms and is required for efficient processing and secretion of pro-IL-1β (54). However, Asc−/− macrophages are competent to undergo pyroptosis after activation of NLRC4 (10, 54–59)(and T Bergsbaken and BT Cookson; and C Case and C Roy, personal communication). Thus, the ASC focus is the processing center for IL-1β while it was not required for pyroptosis. Instead, diffuse cytosolic activated Caspase-1 is observed and appears to be the form of Caspase-1 mediating pyroptosis (10) (and T Bergsbaken and BT Cookson; and C Case and C Roy, personal communication). This diffuse Caspase-1 requires catalytic activity, but does not require processing to trigger pyroptosis (10) (Fig. 1). This suggests that diffuse cytosolic NLRC4 inflammasomes activate Caspase-1 via conformational changes, and that these conformational changes do not require proteolysis. Perhaps pro-Caspase-1 undergoes conformational changes while tethered to the inflammasome, and that the protein remains tethered to the diffuse cytosolic inflammasome. Alternately, a conformational change may occur, such as dimerization, releasing activated (but not proteolyzed) Caspase-1 into the cytosol. Thus, NLRC4, and presumably other CARD-containing inflammasomes, can form two functional inflammasomes that are spatially distinct and that have differential processing requirements. The first is simpler and contains only NLRC4 and Caspase-1, is located diffusely in the cytosol, and results in pyroptosis. In the second, NLRC4 triggers formation of the ASC focus, which recruits and activates Caspase-1, resulting in IL-1β (and presumably IL-18) processing and secretion. These two distinct inflammasomes may compete with each other within the cell, as Asc−/− macrophages form pyroptosis-associated pores more rapidly than WT cells (C Case and C Roy, personal communication), suggesting a dynamic interplay between cytokine processing and pyroptosis.
Given this dual inflammasome paradigm for NLRC4, one might think that NLRP3 and AIM2 would not induce pyroptosis because they cannot directly interact with Caspase-1. However, NLRP3 and AIM2 do induce pyroptosis, and they do so through ASC (60–66). This is distinct from NLRC4, which induces pyroptosis independent of ASC. Interestingly, the requirement for proteolytic processing of pro-Caspase-1 is the same between pyrin-containing and CARD-containing inflammasomes. In both cases pro-Caspase-1 must be processed to efficiently cleave pro-IL-1β, but processing is not required for pyroptosis (10). There are several potential mechanisms whereby this may occur. The ASC foci may release activated Caspase-1 to the cytosol. Alternately, the Caspase-1 targets that promote pyroptosis may diffuse to the ASC foci, where they are cleaved. Both of these mechanisms would result in slower onset pyroptosis for NLRP3/AIM2 than NLRC4 inflammasomes. Future work in the field will reveal whether these or alternate mechanisms are at work for pyrin-containing inflammasomes.
Role of pyroptosis in vivo
Pyroptosis has been well defined in vitro, with innumerable reports examining how the inflammasome triggers pyroptosis in cultured macrophages. However, until recently its relevance in vivo was unclear. There were some reports suggested that Caspase-1 may exert effects independent of IL-1β and IL-18 secretion, indirectly suggesting a role for pyroptosis in vivo, but not investigating a role for pyroptosis directly. Recently we provided evidence that pyroptosis occurs in vivo, and that it is an exquisitely potent innate immune effector mechanism used to clear intracellular pathogens. We will summarize these studies in this section.
Casp1−/− mice infected with Francisella novicida (also called F. tularensis subspecies novicida) show increased bacterial burdens compared to wild type mice (67). Administration of both IL-1β and IL-18 neutralizing antibodies into WT mice increased their susceptibility, but did not fully recapitulate the phenotype of Casp1−/− mice. This suggested that the cytokines play a role but that they do not account for the complete phenotype of Casp1−/− mice. The authors hypothesized that pyroptotic cell death may contribute to control of F. novicida, although the occurrence of pyroptosis in vivo and its mechanistic contribution to bacterial clearance was not examined (67). It is also possible that the neutralizing antibodies were not fully penetrant; in this regard results using Il1b-Il18DKO mice will be informative in future studies.
A model of sepsis revealed a role for Caspase-1, and this was independent of IL-1β and IL-18. Sarkar et al. used intraperitoneal injection of 5 × 108 cfu/kg of Escherichia coli BL21(DE3) into mice (68). In this model, WT mice succumb to the infection while Casp1−/− mice are resistant. In contrast, Il1b-Il18DKO mice responded similar to WT mice, suggesting a role for pyroptosis. In this report, B cell apoptosis was implicated on the basis of histological appearance of apoptotic bodies in the white pulp that stain for CD79. This was not observed in Casp1−/− mice or mice treated with zVAD-fmk, a generic caspase inhibitor (68). To date it is unclear whether Caspase-1 plays a role in B cells, thus, the observed B cell death may be due to the activity of Caspase-1 within the B cells, or to the activity of Caspase-1 within neighboring phagocytes. Thus, this cell death may have been pyroptosis in B cells, or apoptosis in B cells secondary to pyroptosis in splenic macrophages.
We recently demonstrated that bacterial burden in the draining lymph node after both Legionella pneumophila and Burkholderia thailandensis intraperitoneal infection also showed Caspase-1 dependence that was not replicated in Il1b-Il18DKO mice, again suggesting a role for pyroptosis in vivo. However, we did not directly investigate a role for pyroptosis in these models (59).
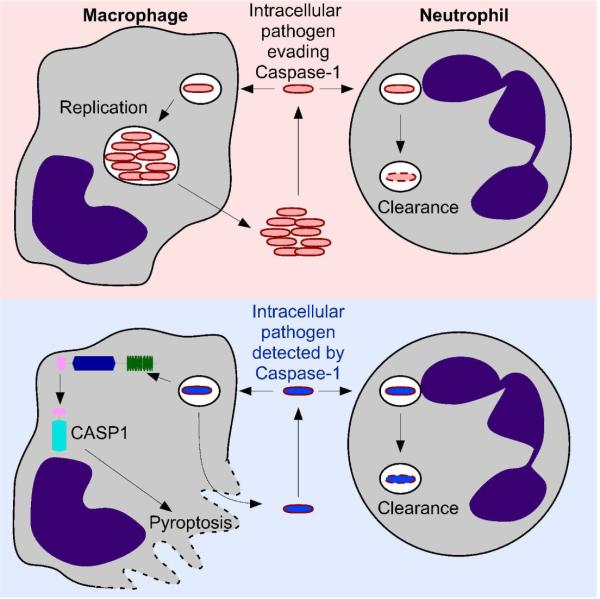
These above reports provided some evidence that Caspase-1 has effects in vivo that cannot be accounted for by IL-1β and IL-18, but did not investigate whether these effects could be attributed to pyroptosis. We recently showed a role for pyroptosis in clearing bacteria in vivo (Fig. 2) by studying the evasion mechanisms that are required for S. typhimurium virulence. S. typhimurium effectively evade detection by the inflammasome by repressing flagellin expression and expressing a T3SS rod protein that is not detected by NLRC4 (for an extensive review on S. typhimurium detection by and evasion of the inflammasome see (69)). We engineered S. typhimurium strains that persistently express flagellin or an NLRC4 detected T3SS rod protein, and found that in both cases the bacteria were rendered severely attenuated via NLRC4-dependent Caspase-1 activation (19, 59). Protection was not observed in Nlrc4−/− or Casp1−/− mice, but surprisingly, was still observed in Il1b-Il18DKO mice (59). We investigated whether pyroptosis plays a role in this clearance response. We found that phagocytes in vivo lost membrane integrity when infected with flagellin expressing bacteria, and that this was dependent upon NLRC4. NLRC4 dependent loss of membrane integrity defines this cell death as pyroptotic and not oncotic. Because there was no clear bactericidal mechanism that would result from lysing an infected cell, we hypothesized that the bacteria were released in a viable state after pyroptosis, and were killed by a secondary phagocyte. Indeed, after induction of pyroptosis, the bacteria were exposed to the extracellular antibiotic gentamicin. We hypothesized that neutrophils were the key phagocyte clearing these bacteria, and thus examined Ncf1−/− mice, which cannot make the p47 component of the NADPH phagocyte oxidase complex. Neutrophils in these mice have defective bactericidal activity. We found that Ncf1−/− mice failed to clear flagellin expressing S. typhimurium. Interestingly, in Ncf1−/− mice, the bacteria were now located predominantly in neutrophils. We concluded that macrophages undergo pyroptosis, releasing the bacteria to the extracellular space, where they are exposed to uptake by neutrophils (59). These data indicate that neutrophils are an important secondary phagocyte in the clearance of bacteria released by pyroptosis and that the NADPH oxidase system is critical to clear bacteria released by pyroptosis. These results were the first demonstration that pyroptosis is an innate immune effector mechanism in vivo (Fig. 2). The potency of pyroptosis in clearing infection is illustrated by the fact that it completely protects an animal from an otherwise lethal infection.
Figure 2. Pyroptosis promotes clearance of intracellular microbes.
Schematic of the role of pyroptosis in clearing intracellular pathogens. Numerous pathogens have the ability to replicate within macrophages (upper panel), eventually being released in greater numbers. Although neutrophils have the capacity to kill many macrophage tropic pathogens, the intracellular niche within the macrophage compartment permits these pathogens to continue a pathogenic replicative cycle. Intracellular pathogens that are detected by an inflammasome (lower panel) activate Caspase-1, resulting in pyroptosis. This releases the bacteria from the macrophage prior to replication, effectively short-circuiting the pathogenic replicative cycle. Released bacteria are thereby exposed to additional clearance mechanisms, including phagocytosis by neutrophils.
Why does pyroptosis function in macrophages and not neutrophils? Many pathogens are able to survive and replicate within macrophages, however very few pathogens are able to do so in neutrophils. This is likely due to intrinsic differences between these two cell types. Macrophages are longer lived, and have reduced microbicidal activity compared to neutrophils, making them a more susceptible target to infection. Neutrophils, on the other hand, are highly microbicidal, and short lived, making them poor targets for intracellular pathogens. Interestingly, while neutrophils express Caspase-1, they did not express NLRC4 (59), suggesting that they do not undergo pyroptosis in response to flagellin or T3SS rod protein contamination of their cytosol. It remains to be determined whether neutrophils will undergo pyroptosis in response to other inflammasome agonists.
Concluding remarks
Pyroptosis can now be viewed as a physiologically important form of cell death, which serves to eject intracellular pathogens from their replicative niche within macrophages. When considering the function of Caspase-1 during infectious and inflammatory diseases, pyroptosis should be considered as a potential mechanism along with IL-1β and IL-18 secretion. For example, resistance to influenza infection conferred by Caspase-1 is likely primarily due to processing and secretion of IL-1β (70–74). On the other hand, Shigella infection is controlled by Caspase-1-dependent IL-18 secretion (75). In contrast, Burkholderia infection may be controlled primarily via pyroptosis (59). In a note of added complexity, S. typhimurium seem to be weakly detected by the inflammasome resulting in some protective effects from IL-18, but they effectively evade pyroptosis, and this evasion is essential for virulence (69). Still other infections are likely to be controlled through a combination of the two cytokines and pyroptosis, and potentially by additional Caspase-1-dependent effector mechanisms that are yet to be discovered.
References
- 1.Farber E. Programmed cell death: necrosis versus apoptosis. Mod Pathol. 1994;7:605–9. [PubMed] [Google Scholar]
- 2.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 3.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duprez L, Wirawan E, Vanden Berghe T, Vandenabeele P. Major cell death pathways at a glance. Microbes Infect. 2009;11:1050–62. doi: 10.1016/j.micinf.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–8. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–32. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153–62. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- 8.Kreuzaler PA, et al. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol. 2011;13:303–9. doi: 10.1038/ncb2171. [DOI] [PubMed] [Google Scholar]
- 9.Chekeni FB, et al. Pannexin 1 channels mediate `find-me' signal release and membrane permeability during apoptosis. Nature. 2010;467:863–7. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8:471–83. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel RM. Caspases at the crossroads of immune-cell life and death. Nat Rev Immunol. 2006;6:308–17. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 12.Mace PD, Riedl SJ. Molecular cell death platforms and assemblies. Curr Opin Cell Biol. 2010;22:828–36. doi: 10.1016/j.ceb.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao W, Hu Z. The enigmatic processing and secretion of interleukin-33. Cell Mol Immunol. 2010;7:260–2. doi: 10.1038/cmi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott AM, Saleh M. The inflammatory caspases: guardians against infections and sepsis. Cell Death Differ. 2007;14:23–31. doi: 10.1038/sj.cdd.4402026. [DOI] [PubMed] [Google Scholar]
- 15.Bao Q, Shi Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 2007;14:56–65. doi: 10.1038/sj.cdd.4402028. [DOI] [PubMed] [Google Scholar]
- 16.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29:275–88. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 17.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010;10:123–30. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- 19.Miao EA, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010;107:3076–80. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–4. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 21.Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105:4312–7. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–9. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 23.Chen LM, Kaniga K, Galan JE. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–15. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 24.Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci U S A. 1996;93:9833–8. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1beta-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun. 1997;65:5165–70. doi: 10.1128/iai.65.12.5165-5170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilbi H, et al. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem. 1998;273:32895–900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 27.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A. 1999;96:2396–401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 29.Watson PR, Gautier AV, Paulin SM, Bland AP, Jones PW, Wallis TS. Salmonella enterica serovars Typhimurium and Dublin can lyse macrophages by a mechanism distinct from apoptosis. Infect Immun. 2000;68:3744–7. doi: 10.1128/iai.68.6.3744-3747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–4. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 31.Edgeworth JD, Spencer J, Phalipon A, Griffin GE, Sansonetti PJ. Cytotoxicity and interleukin-1beta processing following Shigella flexneri infection of human monocyte-derived dendritic cells. Eur J Immunol. 2002;32:1464–71. doi: 10.1002/1521-4141(200205)32:5<1464::AID-IMMU1464>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 32.Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17:1140–5. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 33.Jesenberger V, Procyk KJ, Yuan J, Reipert S, Baccarini M. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J Exp Med. 2000;192:1035–46. doi: 10.1084/jem.192.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamkanfi M, et al. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics. 2008;7:2350–63. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Smith MR, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. Embo J. 1996;15:3853–60. [PMC free article] [PubMed] [Google Scholar]
- 36.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–25. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 37.Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–11. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 38.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–3. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 39.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–20. [PubMed] [Google Scholar]
- 40.Silveira TN, Zamboni DS. Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis. Infect Immun. 2010;78:1403–13. doi: 10.1128/IAI.00905-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akhter A, et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 2009;5:e1000361. doi: 10.1371/journal.ppat.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamkanfi M, et al. Caspase-7 deficiency protects from endotoxin-induced lymphocyte apoptosis and improves survival. Blood. 2009;113:2742–5. doi: 10.1182/blood-2008-09-178038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernandez LD, Pypaert M, Flavell RA, Galan JE. A Salmonella protein causes macrophage cell death by inducing autophagy. J Cell Biol. 2003;163:1123–31. doi: 10.1083/jcb.200309161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119–22. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 45.Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J Immunol. 2003;171:6154–63. doi: 10.4049/jimmunol.171.11.6154. [DOI] [PubMed] [Google Scholar]
- 46.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–25. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 47.Masumoto J, Taniguchi S, Sagara J. Pyrin N-terminal homology domain- and caspase recruitment domain-dependent oligomerization of ASC. Biochem Biophys Res Commun. 2001;280:652–5. doi: 10.1006/bbrc.2000.4190. [DOI] [PubMed] [Google Scholar]
- 48.Masumoto J, et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999;274:33835–8. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- 49.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–55. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandes-Alnemri T, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richards N, et al. Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J Biol Chem. 2001;276:39320–9. doi: 10.1074/jbc.M104730200. [DOI] [PubMed] [Google Scholar]
- 52.Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276:28309–13. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 53.Geddes BJ, et al. Human CARD12 is a novel CED4/Apaf-1 family member that induces apoptosis. Biochem Biophys Res Commun. 2001;284:77–82. doi: 10.1006/bbrc.2001.4928. [DOI] [PubMed] [Google Scholar]
- 54.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–8. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 55.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–75. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki T, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Nunez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–9. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 58.Case CL, Shin S, Roy CR. Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun. 2009;77:1981–91. doi: 10.1128/IAI.01382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–42. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–13. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–8. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dowds TA, Masumoto J, Zhu L, Inohara N, Nunez G. Cryopyrin-induced interleukin 1beta secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem. 2004;279:21924–8. doi: 10.1074/jbc.M401178200. [DOI] [PubMed] [Google Scholar]
- 63.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–27. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 64.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 65.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 66.Kanneganti TD, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–6. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 67.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–9. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarkar A, et al. Caspase-1 Regulates E. coli Sepsis and Splenic B Cell Apoptosis Independently of IL-1{beta} and IL-18. Am J Respir Crit Care Med. 2006 doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miao EA, Rajan JV. Salmonella and Caspase-1: a complex interplay of detection and evasion. Frontiers in Microbiology. 2011;2 doi: 10.3389/fmicb.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szretter KJ, et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007;81:2736–44. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–8. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kozak W, Zheng H, Conn CA, Soszynski D, van der Ploeg LH, Kluger MJ. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1 beta-deficient mice. Am J Physiol. 1995;269:R969–77. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- 73.Van Der Sluijs KF, et al. Enhanced viral clearance in interleukin-18 genedeficient mice after pulmonary infection with influenza A virus. Immunology. 2005;114:112–20. doi: 10.1111/j.1365-2567.2004.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu B, Mori I, Hossain MJ, Dong L, Takeda K, Kimura Y. Interleukin-18 improves the early defence system against influenza virus infection by augmenting natural killer cell-mediated cytotoxicity. J Gen Virol. 2004;85:423–8. doi: 10.1099/vir.0.19596-0. [DOI] [PubMed] [Google Scholar]
- 75.Sansonetti PJ, et al. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–90. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]