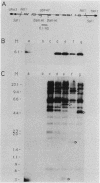
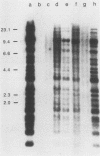
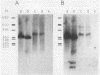
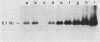
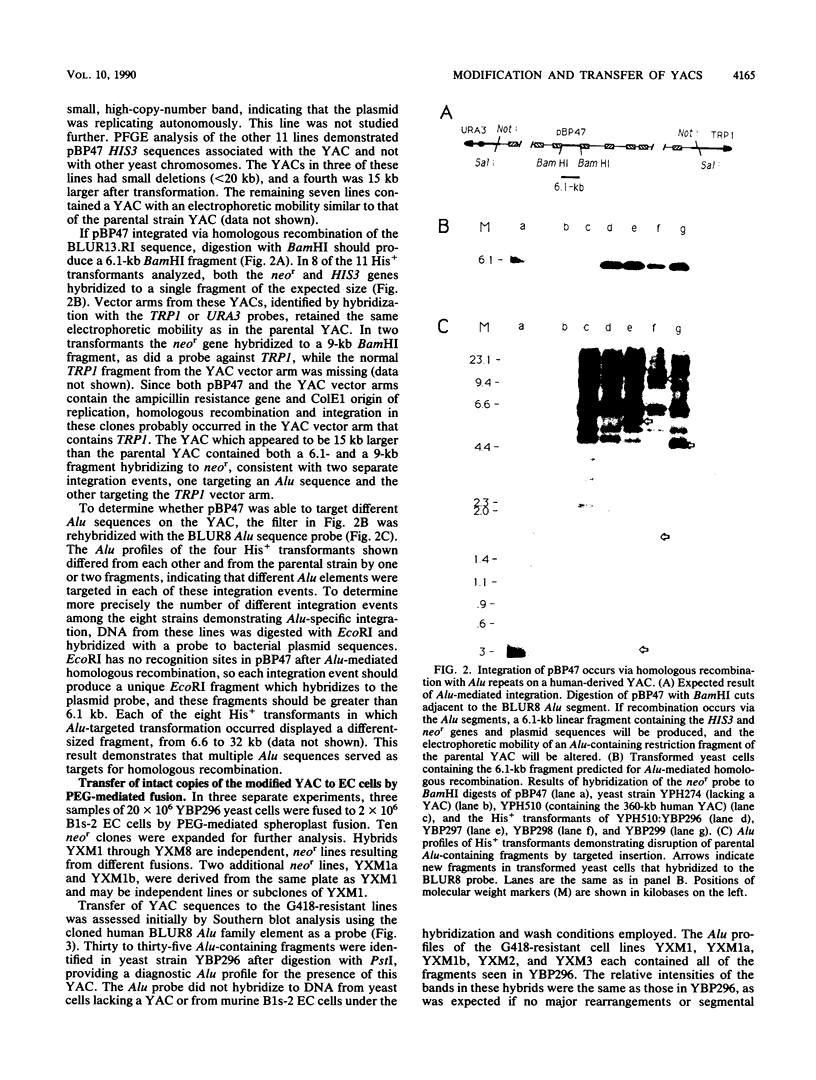
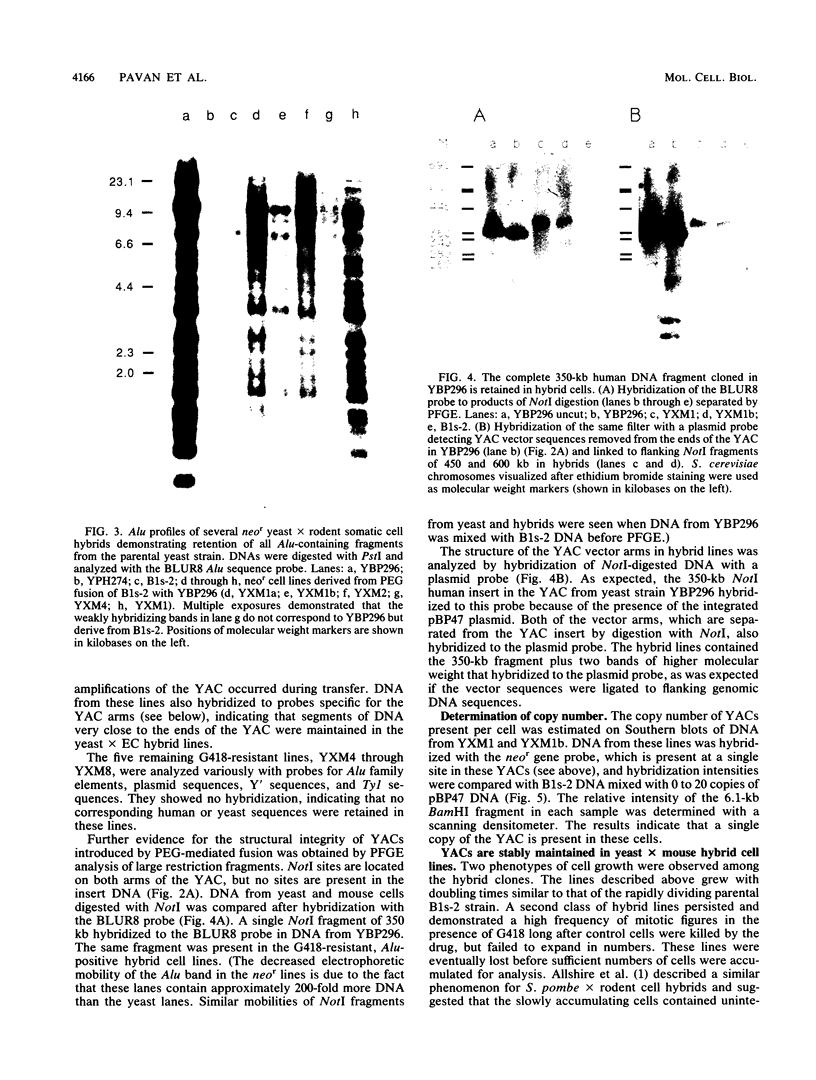
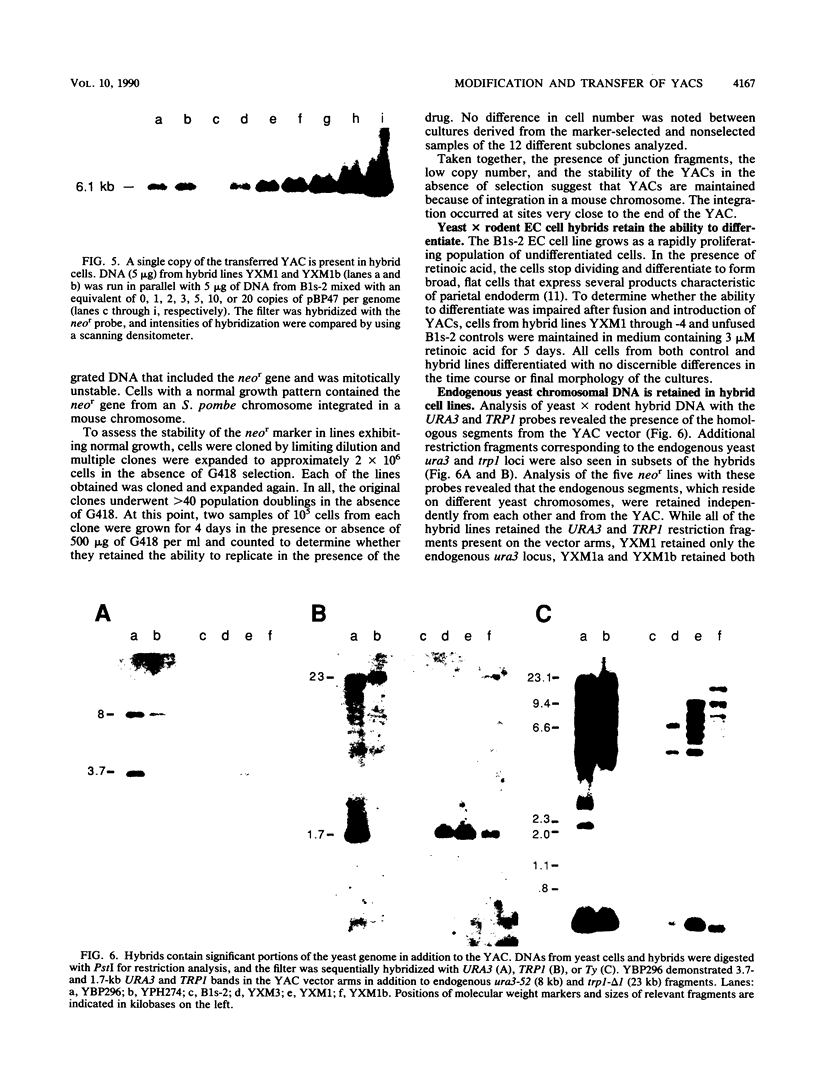
Abstract
A neomycin resistance cassette was integrated into the human-derived insert of a 360-kilobase yeast artificial chromosome (YAC) by targeting homologous recombination to Alu repeat sequences. The modified YAC was transferred into an embryonal carcinoma cell line by using polyethylene glycol-mediated spheroplast fusion. A single copy of the human sequence was introduced intact and stably maintained in the absence of selection for over 40 generations. A substantial portion of the yeast genome was retained in hybrids in addition to the YAC. Hybrid cells containing the YAC retained the ability to differentiate when treated with retinoic acid. This approach provides a powerful tool for in vitro analysis because it can be used to modify any human DNA cloned as a YAC and to transfer large fragments of DNA intact into cultured mammalian cells, thereby facilitating functional studies of genes in the context of extensive flanking DNA sequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allshire R. C., Cranston G., Gosden J. R., Maule J. C., Hastie N. D., Fantes P. A. A fission yeast chromosome can replicate autonomously in mouse cells. Cell. 1987 Jul 31;50(3):391–403. doi: 10.1016/0092-8674(87)90493-4. [DOI] [PubMed] [Google Scholar]
- Bradley A., Evans M., Kaufman M. H., Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984 May 17;309(5965):255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Organization of DNA sequences and replication origins at yeast telomeres. Cell. 1983 Jun;33(2):563–573. doi: 10.1016/0092-8674(83)90437-3. [DOI] [PubMed] [Google Scholar]
- Coulson A., Waterston R., Kiff J., Sulston J., Kohara Y. Genome linking with yeast artificial chromosomes. Nature. 1988 Sep 8;335(6186):184–186. doi: 10.1038/335184a0. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Eichinger D. J., Boeke J. D. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: cell-free Ty1 transposition. Cell. 1988 Sep 23;54(7):955–966. doi: 10.1016/0092-8674(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Garza D., Ajioka J. W., Burke D. T., Hartl D. L. Mapping the Drosophila genome with yeast artificial chromosomes. Science. 1989 Nov 3;246(4930):641–646. doi: 10.1126/science.2510296. [DOI] [PubMed] [Google Scholar]
- Hieter P., Mann C., Snyder M., Davis R. W. Mitotic stability of yeast chromosomes: a colony color assay that measures nondisjunction and chromosome loss. Cell. 1985 Feb;40(2):381–392. doi: 10.1016/0092-8674(85)90152-7. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield J. W., Felix J. S. Rescue of terminally differentiating teratocarcinoma cells by fusion to undifferentiated parental cells. Somatic Cell Genet. 1982 Nov;8(6):743–757. doi: 10.1007/BF01543016. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Norman C. Uranium Enrichment: Heading for a Cliff?: Although the U.S. enrichment business has slashed production costs and is now much more competitive, it still has two tough multibillion dollar problems to overcome. Science. 1987 May 22;236(4804):906–908. doi: 10.1126/science.236.4804.906. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan W. J., Hieter P., Reeves R. H. Generation of deletion derivatives by targeted transformation of human-derived yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1300–1304. doi: 10.1073/pnas.87.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. H., Crowley M. R., Lorenzon N., Pavan W. J., Smeyne R. J., Goldowitz D. The mouse neurological mutant weaver maps within the region of chromosome 16 that is homologous to human chromosome 21. Genomics. 1989 Oct;5(3):522–526. doi: 10.1016/0888-7543(89)90018-9. [DOI] [PubMed] [Google Scholar]
- Rose M., Grisafi P., Botstein D. Structure and function of the yeast URA3 gene: expression in Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):113–124. doi: 10.1016/0378-1119(84)90172-0. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Traver C. N., Klapholz S., Hyman R. W., Davis R. W. Rapid screening of a human genomic library in yeast artificial chromosomes for single-copy sequences. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5898–5902. doi: 10.1073/pnas.86.15.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumper G., Carbon J. Sequence of a yeast DNA fragment containing a chromosomal replicator and the TRP1 gene. Gene. 1980 Jul;10(2):157–166. doi: 10.1016/0378-1119(80)90133-x. [DOI] [PubMed] [Google Scholar]
- Ward M., Scott R. J., Davey M. R., Clothier R. H., Cocking E. C., Balls M. Transfer of antibiotic resistance genes between yeast and mammalian cells under conditions favoring cell fusion. Somat Cell Mol Genet. 1986 Mar;12(2):101–109. doi: 10.1007/BF01560657. [DOI] [PubMed] [Google Scholar]
- Zimmer A., Gruss P. Production of chimaeric mice containing embryonic stem (ES) cells carrying a homoeobox Hox 1.1 allele mutated by homologous recombination. Nature. 1989 Mar 9;338(6211):150–153. doi: 10.1038/338150a0. [DOI] [PubMed] [Google Scholar]