Abstract
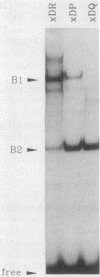
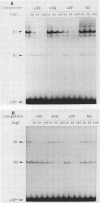
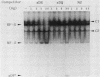
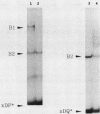
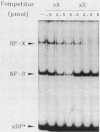
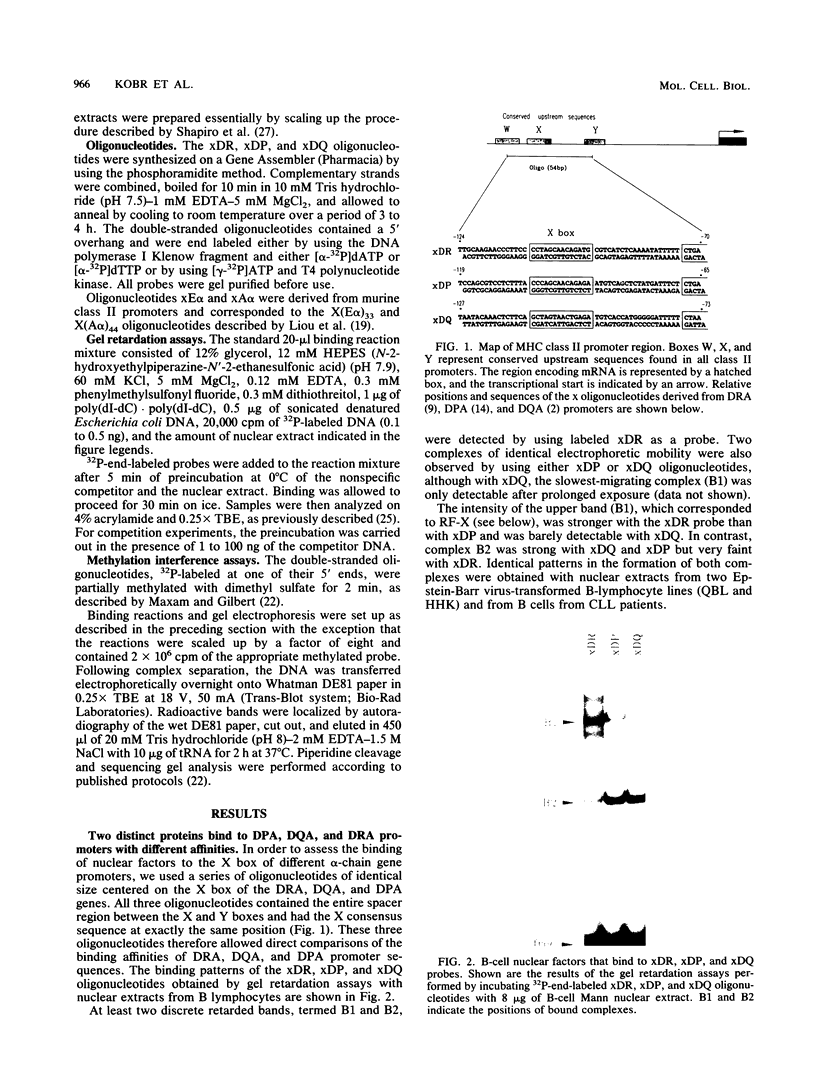
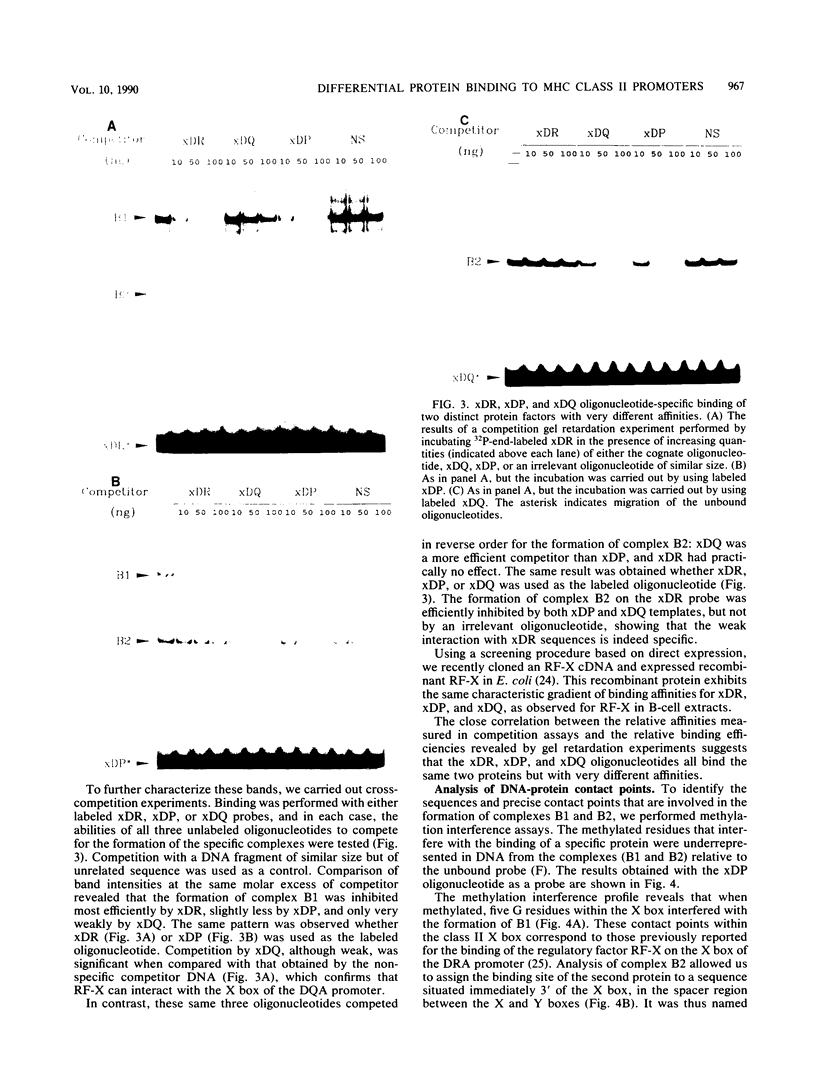
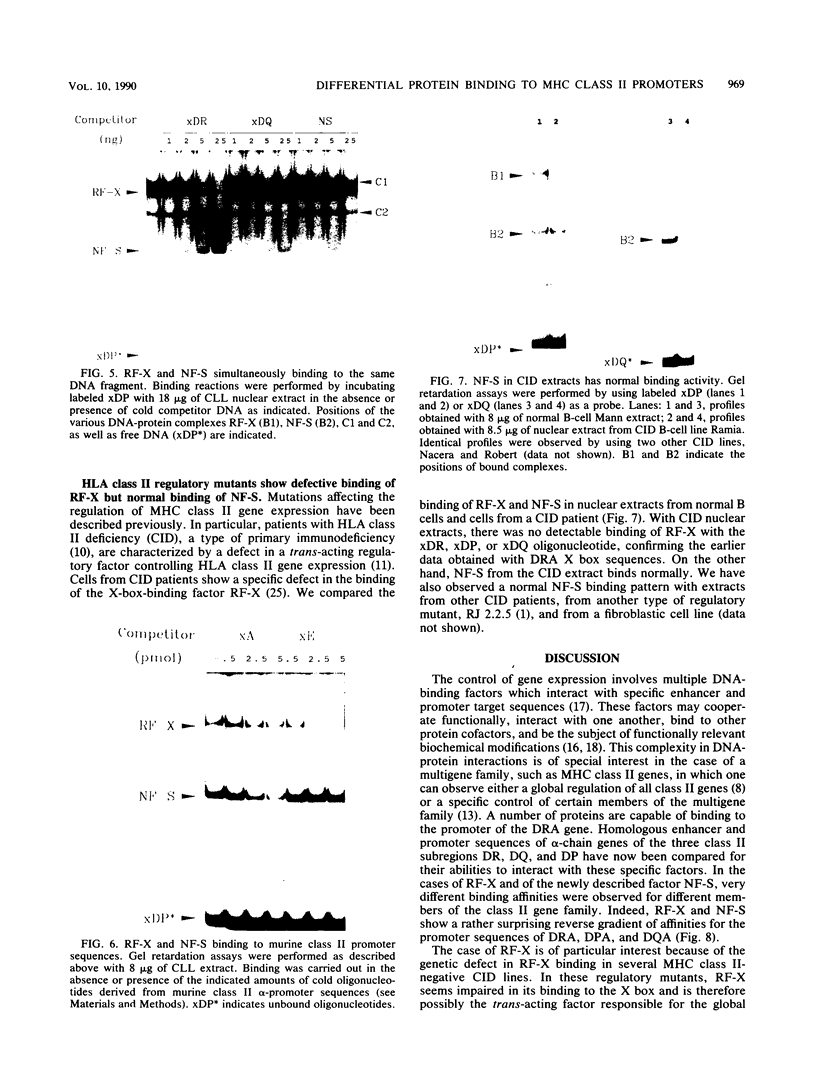
The regulation of major histocompatibility complex (MHC) class II gene expression is a key feature of the control of normal and abnormal immune responses. In humans, class II alpha - and beta-chain genes are organized in a multigene family with three distinct subregions, HLA-DR, -DQ, and -DP. The regulation of these genes is generally coordinated, and their promoters contain highly conserved motifs, in particular the X and Y boxes. We have identified five distinct proteins that bind to specific DNA sequences within the first 145 base pairs of the HLA-DR promoter, a segment known to be functionally essential for class II gene regulation. Among these, RF-X is of special interest, since mutants affected in the regulation of MHC class II gene expression have a specific defect in RF-X binding. Unexpectedly, RF-X displays a characteristic gradient of binding affinities for the X boxes of three alpha-chain genes (DRA greater than DPA much greater than DQA). The same observation was made with recombinant RF-X. We also describe a novel factor, NF-S, which bound to the spacer region between the X and Y boxes of class II promoters. NF-S exhibited a reverse gradient of affinity compared with RF-X (DQA greater than DPA much greater than DRA). As expected, RF-X bound well to the mouse IE alpha promoter, while NF-S bound well to IA alpha. The drastic differences in the binding of RF-X and NF-S to different MHC class II promoters contrasts with the coordinate regulation of HLA-DR, -DQ, and -DP genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accolla R. S. Human B cell variants immunoselected against a single Ia antigen subset have lost expression of several Ia antigen subsets. J Exp Med. 1983 Mar 1;157(3):1053–1058. doi: 10.1084/jem.157.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Lillie J. W., Korman A. J., Boss J. M., Fréchin N., Guillemot F., Cooper J., Mulligan R. C., Strominger J. L. Structure and expression of HLA-DQ alpha and -DX alpha genes: interallelic alternate splicing of the HLA-DQ alpha gene and functional splicing of the HLA-DQ alpha gene using a retroviral vector. Immunogenetics. 1987;26(1-2):63–73. doi: 10.1007/BF00345456. [DOI] [PubMed] [Google Scholar]
- Basta P. V., Sherman P. A., Ting J. P. Detailed delineation of an interferon-gamma-responsive element important in human HLA-DRA gene expression in a glioblastoma multiform line. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8618–8622. doi: 10.1073/pnas.85.22.8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978 Jun;120(6):1809–1812. [PubMed] [Google Scholar]
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Todd I., Mirakian R., Belfiore A., Pujol-Borrell R. Organ-specific autoimmunity: a 1986 overview. Immunol Rev. 1986 Dec;94:137–169. doi: 10.1111/j.1600-065X.1986.tb01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J. C., Ransom J. T. Molecular mechanisms of transmembrane signaling in B lymphocytes. Annu Rev Immunol. 1987;5:175–199. doi: 10.1146/annurev.iy.05.040187.001135. [DOI] [PubMed] [Google Scholar]
- Collins T., Korman A. J., Wake C. T., Boss J. M., Kappes D. J., Fiers W., Ault K. A., Gimbrone M. A., Jr, Strominger J. L., Pober J. S. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4917–4921. doi: 10.1073/pnas.81.15.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das H. K., Lawrance S. K., Weissman S. M. Structure and nucleotide sequence of the heavy chain gene of HLA-DR. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3543–3547. doi: 10.1073/pnas.80.12.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburg J. H., Fibbe W. E., Goselink H. M., Van Rood J. J., Jansen J. Human hematopoietic progenitor cells in long-term cultures express HLA-DR antigens and lack HLA-DQ antigens. J Exp Med. 1985 Oct 1;162(4):1359–1369. doi: 10.1084/jem.162.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson K., Widmark E., Jonsson A. K., Servenius B., Sachs D. H., Larhammar D., Rask L., Peterson P. A. Class II genes of the human major histocompatibility complex. Evolution of the DP region as deduced from nucleotide sequences of the four genes. J Biol Chem. 1987 Jun 25;262(18):8778–8786. [PubMed] [Google Scholar]
- Hoeffler W. K., Kovelman R., Roeder R. G. Activation of transcription factor IIIC by the adenovirus E1A protein. Cell. 1988 Jun 17;53(6):907–920. doi: 10.1016/s0092-8674(88)90409-6. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Kakidani H., Ptashne M. GAL4 activates gene expression in mammalian cells. Cell. 1988 Jan 29;52(2):161–167. doi: 10.1016/0092-8674(88)90504-1. [DOI] [PubMed] [Google Scholar]
- Liou H. C., Boothby M. R., Glimcher L. H. Distinct cloned class II MHC DNA binding proteins recognize the X box transcription element. Science. 1988 Oct 7;242(4875):69–71. doi: 10.1126/science.3140376. [DOI] [PubMed] [Google Scholar]
- Matis L. A., Glimcher L. H., Paul W. E., Schwartz R. H. Magnitude of response of histocompatibility-restricted T-cell clones is a function of the product of the concentrations of antigen and Ia molecules. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6019–6023. doi: 10.1073/pnas.80.19.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Reith W., Barras E., Satola S., Kobr M., Reinhart D., Sanchez C. H., Mach B. Cloning of the major histocompatibility complex class II promoter binding protein affected in a hereditary defect in class II gene regulation. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4200–4204. doi: 10.1073/pnas.86.11.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W., Satola S., Sanchez C. H., Amaldi I., Lisowska-Grospierre B., Griscelli C., Hadam M. R., Mach B. Congenital immunodeficiency with a regulatory defect in MHC class II gene expression lacks a specific HLA-DR promoter binding protein, RF-X. Cell. 1988 Jun 17;53(6):897–906. doi: 10.1016/s0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Sakurai M., Strominger J. L. B-cell-specific enhancer activity of conserved upstream elements of the class II major histocompatibility complex DQB gene. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6909–6913. doi: 10.1073/pnas.85.18.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servenius B., Rask L., Peterson P. A. Class II genes of the human major histocompatibility complex. The DO beta gene is a divergent member of the class II beta gene family. J Biol Chem. 1987 Jun 25;262(18):8759–8766. [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Sherman P. A., Basta P. V., Ting J. P. Upstream DNA sequences required for tissue-specific expression of the HLA-DR alpha gene. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4254–4258. doi: 10.1073/pnas.84.12.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J., Young J. A., Kelly A. P., Austin P. J., Carson S., Meunier H., So A., Erlich H. A., Spielman R. S., Bodmer J. Structure, sequence and polymorphism in the HLA-D region. Immunol Rev. 1985 Jul;85:5–43. doi: 10.1111/j.1600-065x.1985.tb01129.x. [DOI] [PubMed] [Google Scholar]
- Tsang S. Y., Nakanishi M., Peterlin B. M. B-cell-specific and interferon-gamma-inducible regulation of the HLA-DR alpha gene. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8598–8602. doi: 10.1073/pnas.85.22.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- de Préval C., Hadam M. R., Mach B. Regulation of genes for HLA class II antigens in cell lines from patients with severe combined immunodeficiency. N Engl J Med. 1988 May 19;318(20):1295–1300. doi: 10.1056/NEJM198805193182003. [DOI] [PubMed] [Google Scholar]
- de Préval C., Lisowska-Grospierre B., Loche M., Griscelli C., Mach B. A trans-acting class II regulatory gene unlinked to the MHC controls expression of HLA class II genes. Nature. 1985 Nov 21;318(6043):291–293. doi: 10.1038/318291a0. [DOI] [PubMed] [Google Scholar]