Abstract
OBJECTIVE
Emotional distress is common in outpatients with diabetes, affecting ∼20–40% of the patients. The aim of this study was to determine the effectiveness of group therapy with Mindfulness-Based Cognitive Therapy (MBCT), relative to usual care, for patients with diabetes with regard to reducing emotional distress and improving health-related quality of life and glycemic control.
RESEARCH DESIGN AND METHODS
In the present randomized controlled trial, 139 outpatients with diabetes (type 1 or type 2) and low levels of emotional well-being were randomized to MBCT (n = 70) or a waiting list group (n = 69). Primary outcomes were perceived stress (Perceived Stress Scale), anxiety and depressive symptoms (Hospital Anxiety and Depression Scale), mood (Profiles of Mood States), and diabetes-specific distress (Problem Areas In Diabetes). Secondary outcomes were health-related quality of life (12-Item Short-Form Health Survey), and glycemic control (HbA1c). Assessments were conducted at baseline and at 4 and 8 weeks of follow-up.
RESULTS
Compared with control, MBCT was more effective in reducing stress (P < 0.001, Cohen d = 0.70), depressive symptoms (P = 0.006, d = 0.59), and anxiety (P = 0.019, d = 0.44). In addition, MBCT was more effective in improving quality of life (mental: P = 0.003, d = 0.55; physical: P = 0.032, d = 0.40). We found no significant effect on HbA1c or diabetes-specific distress, although patients with elevated diabetes distress in the MBCT group tended to show a decrease in diabetes distress (P = 0.07, d = 0.70) compared with the control group.
CONCLUSIONS
Compared with usual care, MBCT resulted in a reduction of emotional distress and an increase in health-related quality of life in diabetic patients who had lower levels of emotional well-being.
Emotional distress, which can consist of symptoms of depression, anxiety, and diabetes-specific distress affects ∼20 to 40% of outpatients with type 1 or type 2 diabetes (1–3), making it a common comorbid health problem in these patients. Emotional distress results in lower quality of life (4) and more negative appraisals of insulin therapy (5). In addition, depression is associated with suboptimal self-care behaviors (6), suboptimal glycemic control (7), adverse cardiovascular outcomes, and higher mortality rates (8,9). Although the emotional problems in diabetic patients have received increasing attention in the last decade, they are still often not recognized in clinical practice and remain untreated (10).
Previous research has shown that antidepressant medication and cognitive behavioral therapy are effective treatments for major depression in diabetic patients (11,12). However, the use of antidepressant medication is often accompanied by serious side effects, and a substantial percentage of the patients (∼30–50%) still do not respond to treatment or they relapse (13). Hence, we need to conduct new studies testing new treatments for emotional distress in diabetes. Because the number of diabetic patients is rapidly increasing, we need to develop interventions that are not only effective but also affordable. Web-based therapies and group therapies are good candidates.
One easily accessible group intervention that proved successful in reducing emotional distress and improving quality of life in nonpatients and in diverse patient groups (14–16) is Mindfulness-Based Cognitive Therapy (MBCT) (17). MBCT is an 8-week protocolized group therapy program that combines meditation exercises with elements of cognitive therapy. The central component of this intervention is the cultivation of mindfulness. This can be defined as the self-regulation of one’s attention focusing on direct experience, while adopting a curious, open, and accepting attitude toward these experiences, especially one’s psychological processes, such as thoughts and feelings (18). A recent meta-analysis has shown medium- to large-effect sizes for mindfulness-based interventions in reducing symptoms of anxiety and depression (19).
Two other studies examined the effect of a mindfulness-based intervention on emotional distress in people with diabetes (20,21). In one uncontrolled study, the mindfulness group showed a significant decrease in depressive symptoms at postintervention and in HbA1c at the 1-month follow-up (20). The other study found no significant effects directly after the intervention, but significant improvements in depressive symptoms (Cohen d = 0.71) and mental health–related quality of life (d = 0.54) were reported at the 1-year follow-up (21). The results of these studies are in line with the notion that mindfulness-based interventions could be adequate in reducing emotional problems in people with diabetes. However, the presence of emotional distress was not an inclusion criterion in either study, and only the latter study was a randomized controlled trial (21).
Studies testing the effectiveness of a mindfulness-based intervention in outpatients with type 1 diabetes are still lacking. Therefore, the purpose of the current study was to test the effectiveness of MBCT for people with type 1 or type 2 diabetes and comorbid emotional distress. The primary outcome was the effect on emotional distress, including symptoms of depression, anxiety, diabetes-specific distress, and general perceived stress. Secondary outcomes were health-related quality of life and glycemic control. From the results of two systematic reviews (19,22), we hypothesized that after MBCT, diabetic patients would experience significant greater reductions in emotional distress compared with a waiting list control group. We also hypothesized that MBCT would lead to better health-related quality of life and lower HbA1c.
RESEARCH DESIGN AND METHODS
The Diabetes and Mindfulness (DiaMind) study is a randomized controlled trial. The study protocol has been approved by the medical ethics committee of the St. Elisabeth Hospital in Tilburg, the Netherlands (P0948), and the study is performed according to the 2000 revised version of the Helsinki Declaration. An extensive overview of the methods of the DiaMind study has recently been published elsewhere (23).
Participants
Dutch-speaking adult patients with diabetes (type 1 or type 2) with low levels of emotional well-being [as evidenced by a score of <13 on the World Health Organization-5 Well-Being Index (24)] were recruited from outpatient diabetes clinics between May 2010 and November 2011. Exclusion criteria are reported in the DiaMind study protocol (23). Eligible patients (n = 139) were randomly allocated to the MBCT group (n = 70) or the waiting list (usual care) control group (TAU; n = 69). The TAU group received the program 6 months after the intervention of the MBCT group. The first MBCT group started in September 2010 and the last TAU group in October 2012. All participants provided written informed consent.
Intervention
The protocolized mindfulness intervention was based on the Mindfulness-Based Stress Reduction (MBSR) and MBCT programs as described by Kabat-Zinn (25) and Segal et al. (17), consisting of eight weekly 2-hour sessions in groups of 4 to 10 participants. Because the program is closest to the protocol as described by Segal et al. (17), we decided to call it MBCT. The central component of the program was the development of mindfulness, which was done by practicing several meditation exercises. A specific theme was also discussed in each session (e.g., “how to cope with thoughts”). At the end of the sessions, the participants received homework assignments that took about 30 min, 5 days/week. Instead of one whole-day session, which is part of the original program, a 2-hour booster session was added 3 months after the end of the intervention as a means to boost mindfulness practice. All sessions were supervised by certified psychologists who had at least 4 years of personal experience with mindfulness practice and also completed at least one certified mindfulness instructors training of 8 days in the Netherlands.
Randomization
After completion of the baseline assessment, participants were randomized according to a 1:1 ratio within blocks of 4 to receive MBCT or TAU. A random list was prepared by an independent statistician using PASW Statistics 17 software with a random number generator.
Outcome measures
The primary outcome assessment for MBCT and TAU took place at pre- (T1), mid- (at 4 weeks: T2), and postintervention (at 8 weeks: T3). The secondary outcome, health-related quality of life, was only assessed at T1 and T3. HbA1c values were looked up pre- and postintervention, but within a wider period of time (see below).
Demographic and clinical variables.
Demographic and clinical variables, such as existing diabetes complications and comorbid conditions, were collected by means of a questionnaire, which the participants completed during the baseline assessment. HbA1c was retrieved from the hospitals’ computerized patient records. The policy in the outpatient diabetes clinics is to measure HbA1c every 3 months. Because HbA1c reflects the state of the preceding ∼2 to 3 months, the value for the preintervention assessment was obtained between 24 weeks before and 1 week after the start of the intervention, and this period for the postintervention measures was between 6 and 24 weeks after the intervention.
Emotional distress.
We defined emotional distress as symptoms of anxiety, depression, and/or diabetes-specific distress and operationalized the concept by means of four questionnaires. We included the Dutch version of the Perceived Stress Scale to measure general perceived stress, defined as the degree to which situations in one’s life are appraised as stressful (e.g., “lately, how often have you felt nervous and stressed?”). The items of the present 10-item version of the Perceived Stress Scale are answered on a 5-point Likert scale, ranging from “never” (0) to “very often” (4) (26). The Cronbach α was 0.81 in this sample.
The Hospital Anxiety and Depression Scale (HADS) was included to measure symptoms of anxiety (e.g., “Worrying thoughts go through my mind”) and depression (e.g., “I feel as if I am slowed down”) (27). Both subscales comprise seven items that are answered on a 4-point Likert scale of 0 to 3. The score range for the anxiety and the depressive symptoms subscales is 0 to 21. The Cronbach α in this sample was 0.75 for the anxiety and 0.81 for the depression subscale.
In addition, we used the short Dutch version of the Profile of Mood States (POMS) (28) to assess transient, fluctuating mood states. In this scale, 32 adjectives about positive and negative mood states are rated on a 5-point Likert scale (0 “not at all”; 4 “very much”) according to how well each item describes one’s mood during the last couple of weeks. We selected the most relevant subscales: Tension-anxiety (six items), Depression-dejection (eight items), and Fatigue-inertia (six items). These subscales in the present sample had a Cronbach α of 0.77–0.93. There is an overlap between the HADS and POMS. We decided to include both scales in the study because they have complementary qualities: whereas the HADS is a more used and well-known instrument in medical settings, the POMS has three additional subscales and appears to be more sensitive for change (29).
The Dutch version of the Problem Areas In Diabetes (PAID) survey was included to measure diabetes-specific distress. This scale consists of 20 statements about common negative feelings related to living with diabetes (e.g., “Feeling depressed when you think about living with diabetes,” “feeling discouraged with your diabetes regimen”) (30). The items are rated on a 6-point Likert scale (1 “not a problem”; 6 “a serious problem”). To facilitate interpretation, the PAID scores were transformed to a 0–100 scale (31). A higher score indicates more distress, with a cutoff score of 40 indicating seriously elevated diabetes distress (32). The Cronbach α in this sample was 0.91.
Health-related quality of life.
The Dutch version of the 12-item Short-Form Health Survey was included to assess health-related quality of life. The 12 items of this self-report scale are grouped into two component summary scores: a physical and a mental component score. Both component scores are measured on a scale from 0 to 100, with a high score indicating good health-related quality of life. The Dutch 12-item Short-Form Health Survey has established reliability and validity (33).
Data analyses
The χ2 test or the Fisher exact test, as appropriate, were used to examine differences on discrete variables. Possible differences on continuous variables were examined with the Student t test for independent samples. Mixed-models analyses (SPSS 18 software) were used to test the differences between groups on the dependent variables (time × group interaction effect). We used mixed-models analysis instead of repeated-measures ANOVA to make more efficient use of our data with likely occasional missing values. In sensitivity analyses, linear regression analyses on change scores were conducted after multiple imputation was used to address missing data. In instances when the groups differed on pretreatment variables, these variables would be included as covariates. Age, sex, and comorbidity were regarded as important variables to be included as covariates at all occasions (23). All analyses were based on the intention-to-treat approach.
To determine clinically significant change, we followed the definition of Jacobson et al. (34). The first step was to identify participants who had moved outside the range of the “dysfunctional population” at postintervention assessment (the “recovered” participants) by using a cutoff score of ≥8 on both subscales of the HADS (35). The second step was to identify individuals who showed a significant improvement at postintervention. Therefore, for each individual, we calculated the Reliable Change Index (RCI = x2 − x1/Sdiff) on the HADS (34). The participants who both “recovered” and showed a “significant improvement” were considered as being “clinically significantly improved.”
RESULTS
Recruitment and attrition
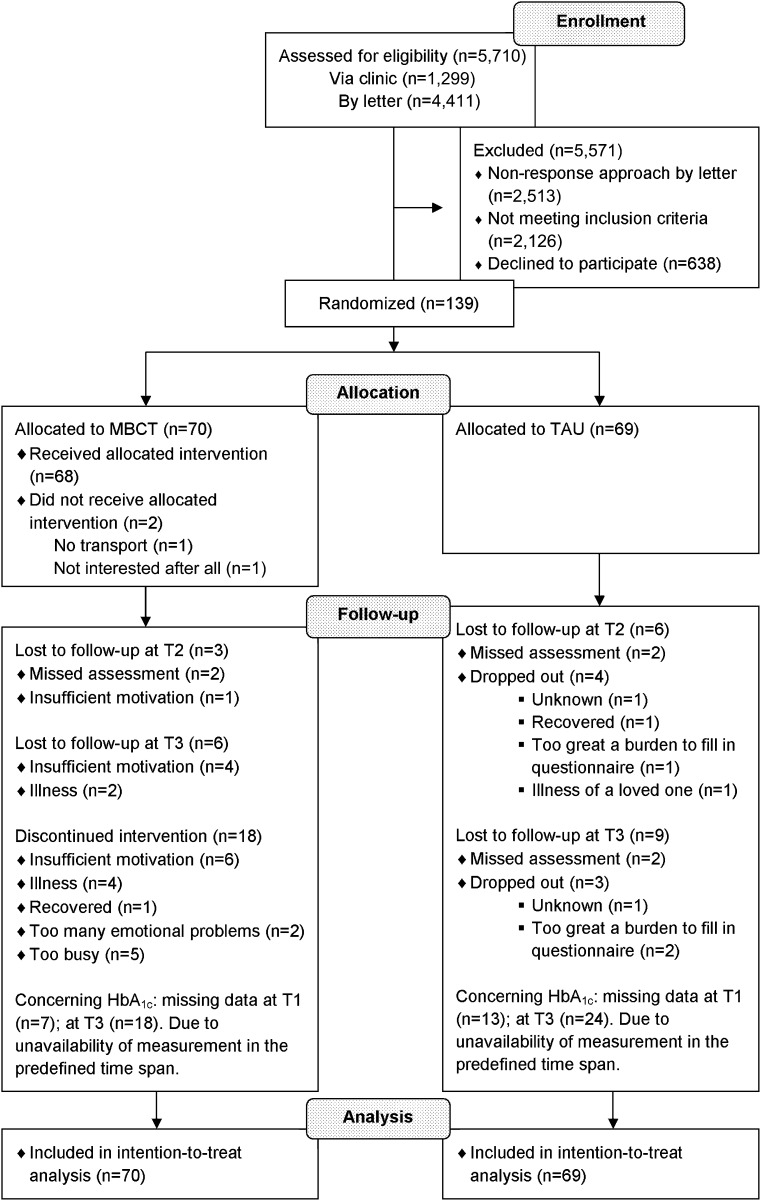
Figure 1 displays the participants’ flowchart. Of 5,710 diabetic patients who were assessed for eligibility, 1,299 (23%) were directly invited by the researcher, diabetes nurse, or secretary in the outpatient diabetes clinic during a regular appointment, and 4,411 (77%) were invited by an invitational letter. Of the latter group, the response rate was 43% (n = 1,898). Of the remaining 3,197 patients, 2,126 (67%) did not meet inclusion criteria (e.g., they had good to optimal emotional well-being), 638 (20%) declined to participate, and 294 (9%) were excluded because of other reasons, including insufficient patient information or inability to contact the patient. The two main reasons for decline were no interest or no need for an intervention, and practical problems, such as being too busy to follow the intervention or not being able to attend the meetings.
Figure 1.
Flow diagram of patient enrollment, allocation, and attrition.
In the MBCT group, 2 patients (3%) dropped out before the start of the intervention, 13 (19%) before the fourth session, and 5 (7%) between the fourth and the eighth session. Of the remaining 50 participants, 41 (82%; 59% of total MBCT group) attended at least six of the eight sessions. The overall average attendance was 5.5 (SD, 2.5) sessions. Seven patients (10%) in the TAU group dropped out of the study. Ten dropouts in the MBCT group continued to fill in the questionnaires. Eventually, we missed data of 9 participants (MBCT: n = 3; TAU: n = 6) at T2, and of 16 participants (MBCT: n = 7; TAU: n = 9; Fig. 1) at T3. Participants who prematurely stopped with the intervention were less likely to have a partner (55% vs. 80%, P = 0.034) and to have prior experience with meditation (5% vs. 31%, P = 0.022). The MBCT and TAU participants who did not complete the T3 assessment were younger (P = 0.028) and had a higher score on the POMS fatigue subscale at baseline (P = 0.007). They were also less likely to have a partner (53% vs. 77%, P = 0.042) and more likely to use psychotropic medication (47% vs. 18%, P = 0.010) and to smoke (40% vs. 14%, P = 0.010).
The HbA1c data were missing for 20 participants at T1 (MBCT: n = 7; TAU: n = 13) and for 42 participants at T3 (MBCT: n = 18; TAU: n = 24). There were no significant differences regarding our outcomes between participants of which the HbA1c measurement was or was not available.
Characteristics of study participants
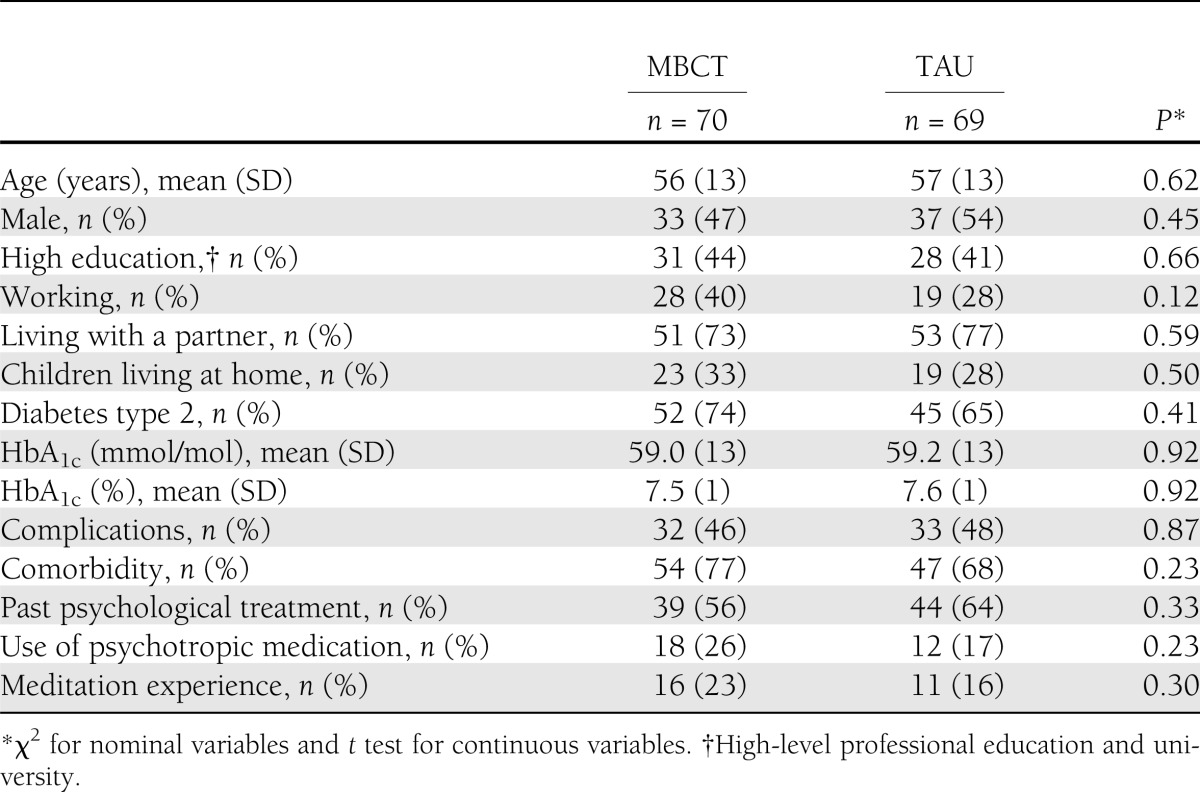
The baseline characteristics of the sample, stratified by group (MBCT or TAU), are presented in Table 1. At baseline, there were no statistically significant differences between the two groups on demographic and clinical variables.
Table 1.
Demographic and clinical characteristics of MBCT and TAU groups
Effect on primary outcome emotional distress
The mean scores of MBCT and TAU on the emotional distress measures are presented in Table 2. Mixed-models analyses showed that the individuals in the MBCT group had a significantly larger decrease in levels of perceived stress over time compared with TAU (P < 0.001). The effect size of the difference from pre- to postintervention between the two conditions was medium to large (Cohen d = 0.70). Post hoc between-subject analyses indicated that the groups differed at postintervention (P < 0.001) but not at T2 (P = 0.204).
Table 2.
Mean (SD) scores and results of mixed-models analyses for primary outcomes
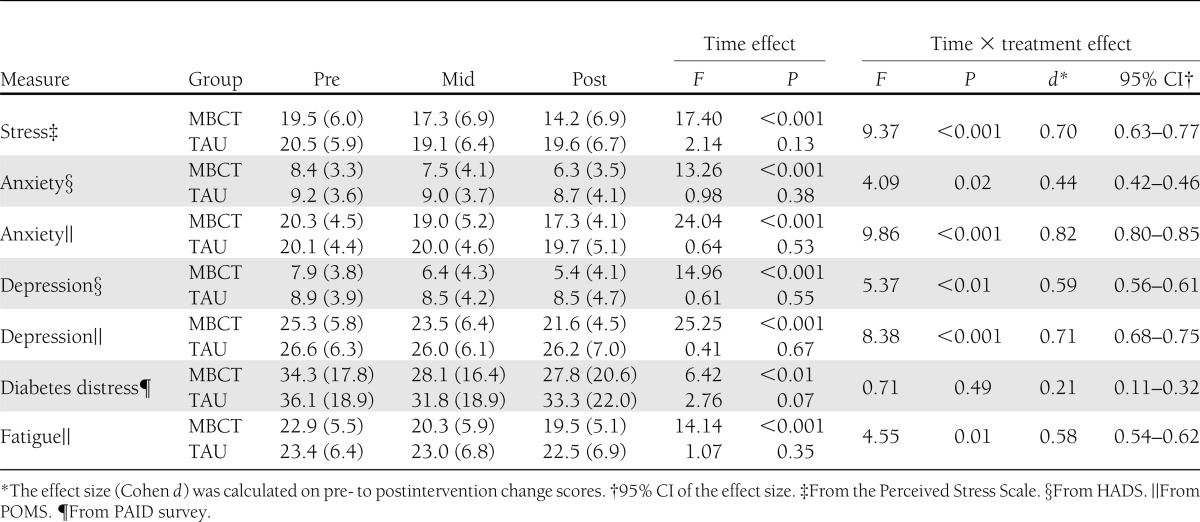
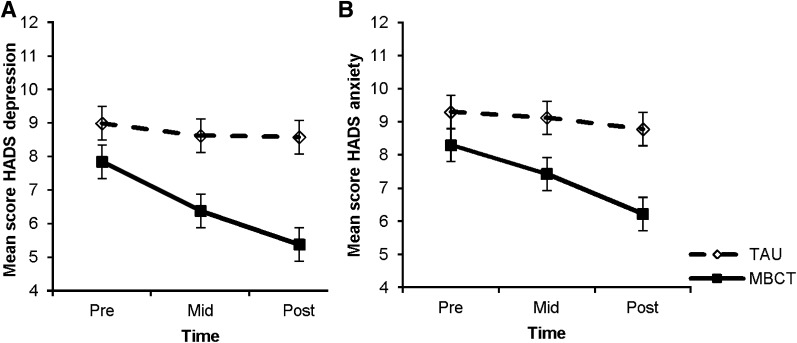
The analyses also showed a significant effect of MBCT on depressive symptoms (HADS) compared with TAU (P = 0.006), with a medium effect size (Cohen d = 0.59; Fig. 2A). This difference between groups was already significant at T2 (P = 0.011), as post hoc analyses revealed, but was increased at postintervention (P < 0.001). The results on the depression subscale of the POMS were comparable, but with a larger effect size (Cohen d = 0.71; Table 2).
Figure 2.
A: Effect of MBCT on depressive symptoms. Data are presented as means and SE for MBCT (solid line) and TAU (dashed line) groups. Results of mixed-models analyses: P < 0.01, Cohen d = 0.52. B: Effect of MBCT on anxiety symptoms. Data are presented as means and SE for MBCT (solid line) and TAU (dashed line) groups. Results of mixed-models analyses: P = 0.02, Cohen d = 0.44.
Concerning symptoms of anxiety (HADS), there was a significant improvement in the MBCT group compared with TAU (P = 0.019). The effect size was small to medium (Cohen d = 0.44; Fig. 2B). Post hoc analyses showed a trend for significance at T2 (P = 0.064) and a significant difference at postintervention (P = 0.001). The results on the anxiety subscale of the POMS were comparable, but the effect size was larger (Cohen d = 0.82; Table 2). Similar results were also obtained for the POMS subscale of fatigue (Table 2).
In contrast, there was no significant difference between MBCT and TAU on diabetes-specific distress (P = 0.488, Cohen d = 0.21). Post hoc analyses revealed that there was a significant decrease in diabetes-specific distress over time in the MBCT group (P = 0.003), whereas the TAU group showed a trend for a significant decrease over time (P = 0.072). Because the participants were selected on general emotional distress, a considerable percentage (52%) did not have elevated diabetes distress at baseline. Therefore, we conducted an ad hoc subgroup analysis in participants with elevated diabetes distress levels (PAID ≥40). This analysis revealed a trend for a significant mean (SD) reduction in the MBCT group [35 (20)] compared with the TAU group [49 (17); P = 0.066], with a moderate to large effect size (Cohen d = 0.70).
Clinical significance
At baseline, 81 participants (MBCT: n = 40; TAU: n = 41) had a score above the anxiety cutoff at baseline and 71 (MBCT: n = 33; TAU: n = 38) had a score above the depression cutoff. In the MBCT group, 37% of these participants showed a clinically significant improvement on symptoms of anxiety compared with 5% in the TAU group (P = 0.001, ϕ = 0.39) and 27% on symptoms of depression versus 8% in the TAU group (P = 0.064, ϕ = 0.26).
Effect on secondary outcomes health-related quality of life and HbA1c
Mixed-models analyses showed that the MBCT group had a significantly more strongly improved mental quality of life (P = 0.003; Cohen d = 0.55) as well as physical quality of life (P = 0.032; Cohen d = 0.40) compared with the TAU group (Table 3).
Table 3.
Mean (SD) scores and results of mixed-models analyses for secondary outcomes
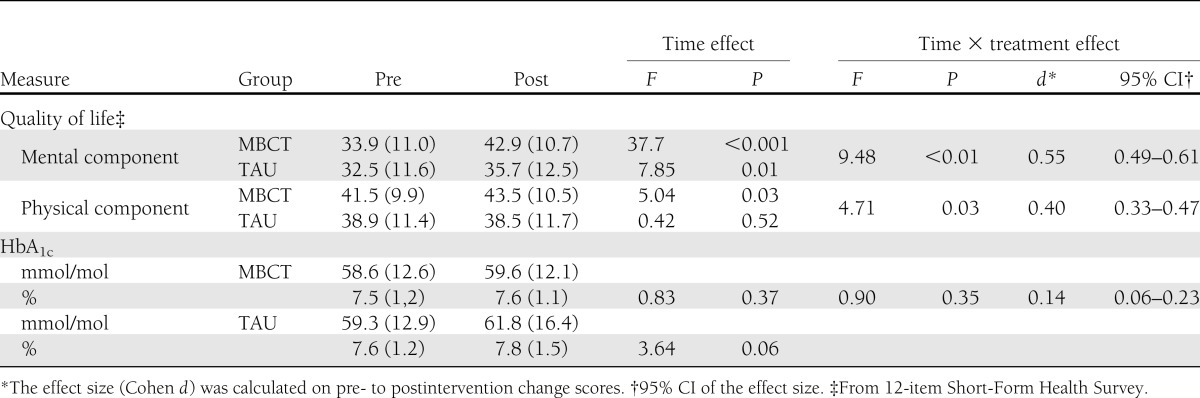
Mixed-model analyses showed no significant difference in HbA1c change in MBCT compared with TAU (P = 0.346; Cohen d = 0.14; Table 3). Post hoc analyses revealed that there was no significant difference in HbA1c over time in the MBCT group (P = 0.366), whereas the TAU group showed a trend for a significant increase in HbA1c over time (P = 0.064).
Sensitivity analyses
Sensitivity analyses based on multiple imputation data showed highly similar results; for example, all significant results reported above were also significant except for physical quality of life, which became marginally significant (P = 0.057). In addition, sensitivity analyses in which diabetes type and the diabetes type-by-time interaction were included as covariates revealed highly similar results. The diabetes type-by-time interaction was not significant in any analysis (all P > 0.05), showing that results were similar for both types.
CONCLUSIONS
The present report describes the results of the DiaMind study. This study’s main objective was to test the effectiveness of MBCT in improving the emotional well-being of distressed diabetic patients. The findings were largely in line with our a priori hypotheses regarding the effect of the intervention on emotional well-being and quality of life. Patients receiving MBCT showed significantly larger decreases in perceived stress, symptoms of depression and anxiety, and had significantly better improvements in health-related quality of life compared with those in the TAU group. The effect sizes were medium to large. To the best of our knowledge, this is the first randomized trial to find immediate effects of a mindfulness-based intervention on emotional well-being and quality of life in outpatients with type 1 and type 2 diabetes.
The participants in the MBCT group were approximately seven times more likely to show a clinically significant improvement at postintervention in symptoms of anxiety and three times more likely to show this improvement in symptoms of depression compared with the participants in the TAU group. Although the effect sizes were medium, the difference for depressive symptoms was almost significant. This can probably be considered as a power problem, because only approximately half of the participants scored above the HADS cutoff score of anxiety and depressive symptoms at baseline.
Interestingly, although the MBCT group also showed a significant reduction in diabetes-specific distress over time, we did not find a significant difference between MBCT and TAU at postintervention for this outcome. General emotional distress was an inclusion criterion, yet only a fraction of the participants (48%) experienced elevated diabetes distress (PAID ≥40). Hence, the nonsignificant finding could be caused by a floor effect. When we tested the effect of the intervention in the subgroup with elevated diabetes distress at baseline, the results revealed that MBCT reduced the diabetes distress with a moderate to large effect size compared with TAU. However, this finding was not statistically significant, probably due to a lack of statistical power, given the smaller size of this subsample.
No significant difference was observed between the groups regarding change in HbA1c. Although the MBCT group showed no significant change in levels of HbA1c from pre- to postintervention, the control group showed marginally significant increased values at postintervention. The nonsignificant difference between the MBCT and TAU groups is in line with the discrepancy in findings regarding the effect of psychological interventions on HbA1c in patients with diabetes, with one meta-analysis finding an effect (36) and one systematic review (37) and another meta-analysis finding no effect (38). One possible explanation for the absence of a decrease in the current study is that poor glycemic control was not an inclusion criterion, and so the mean (SD) HbA1c at baseline [59 mmol/mol (13) or 7.6% (1.2)] was slightly above target level. This fairly good baseline glycemic control contrasts with previous studies in the latter meta-analysis. Future research in a group with poor glycemic control should examine this possibility.
As mentioned before, only two other studies have tested the effectiveness of mindfulness therapy for patients with diabetes. Hartmann et al. (21) did not find significant reductions in depressive and stress symptoms or an increase in health-related quality of life compared with usual care directly after the intervention, whereas improvements for some of these outcomes were found at the 1-year follow-up. The main explanation for the difference in findings compared with the DiaMind study may be because the presence of emotional distress was an inclusion criterion in the current study. Rosenzweig et al. (20) did show a reduction in depressive symptoms and general psychological stress, but not in anxiety symptoms, in diabetic patients immediately after the intervention. However, they did not use a randomized controlled design, had a small sample size, and again, the presence of emotional distress was not an inclusion criterion.
The DiaMind study had a number of limitations. First, selection bias may have affected our results, because only a small portion of the patients we assessed for eligibility decided to participate in the trial. Therefore, generalizability of the findings is limited to diabetic patients who are open to participate in a psychological intervention. This effect applies for all psychological interventions but perhaps even more strongly for one based on mindfulness, although care was taken to use more neutral terms in communication to patients, such as “attention” or “attention exercises” instead of “mindfulness” or “meditation.”
Second, the study had a significant dropout rate in the MBCT group: ∼26% (n = 18) stopped participating in the program. Although the dropout rate is comparable to some studies (14,15), it is relatively high compared with other studies (39,40). A possible explanation is that the participants were approached, instead of being the requesting party. Fortunately, efforts to collect the data of all randomized participants, even when treatment was prematurely terminated, were fruitful: 59% (n = 10) of the dropouts of the MBCT group were willing to continue to fill in the questionnaires.
Third, we did not investigate and control for changes in medication for diabetes or mental health.
Fourth, we used a nonactive control group design, which can lead to differences between the two conditions in attrition and in expectancy effects (placebo vs. nocebo) resulting in a risk of an overestimation of the treatment effect. However, we decided to use this design, because 1) we wanted to test the effectiveness of MBCT for patients with diabetes relative to usual care instead of comparing it with another psychological intervention, and 2) in this sample of patients with emotional problems, we felt it would be unethical to use a placebo intervention. Future studies should incorporate active control groups to examine to what extent our findings are mindfulness specific.
Finally, a substantial number of missing data were present for HbA1c due to unavailability of the measurements in the predefined time span in the patient information databases. The policy in the outpatient diabetes clinics is to measure HbA1c every 3 months. However, this is not always feasible in practice; therefore, we had lower statistical power to measure significant differences between the two conditions.
In conclusion, the DiaMind study demonstrated that MBCT could be used to treat comorbid emotional problems in patients with type 1 or type 2 diabetes. The emotional well-being and quality-of-life of these patients increased compared with the control group, whereas no significant effect was found for diabetes-specific distress and HbA1c, possibly due to a floor effect. MBCT may be offered as part of standard care to diabetic patients with emotional problems. However, the implementation of a group MBCT intervention may be more feasible if mixed chronic disease patient groups are formed. Although this should be tested in future research, such an approach is expected to be as effective as the present one focusing on patients with diabetes because 1) mindfulness-based interventions are broadly applicable, and 2) previous studies have found these interventions are effective in other chronic disease patient groups (41). In addition, given the prospected increase in people with diabetes and the increasing health care costs, it will be worthwhile to examine the effectiveness of Internet-based mindfulness therapy also.
Acknowledgments
The study is supported by grants from the Dutch Diabetes Research Foundation or “Diabetesfonds” (project number 2008.13.005, awarded to I.N.) and Tilburg University, Center of Research on Psychology in Somatic diseases.
No potential conflicts of interest relevant to this article were reported.
J.v.S. researched data and wrote the manuscript. I.N. researched data, contributed to discussion, and reviewed and edited the manuscript. V.J.P. and F.P. contributed to discussion and reviewed and edited the manuscript. M.C.B., R.J.E., P.F.S., and A.W.T. reviewed and edited the manuscript. J.v.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in poster form at the 70th Annual Scientific Meeting of the American Psychosomatic Society, Athens, Greece, 14–17 March 2012, and as oral presentation at the 42nd Annual Congress of the European Association for Behavioral and Cognitive Therapies, Geneva, Switzerland, 29 August–1 September 2012.
The authors thank all of the patients who participated in the study, the psychologists of Apanta-GGZ and Maria A. of Maria A. practice for the guidance of the MBCT sessions, and the secretaries and diabetes nurses of the Catharina Hospital, Máxima Medical Center, TweeSteden Hospital, and St. Anna Hospital for their effort.
Footnotes
Clinical trial reg. no. NTR2145, http://www.trialregister.nl.
References
- 1.Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. J Psychosom Res 2002;53:1053–1060 [DOI] [PubMed] [Google Scholar]
- 2.Pouwer F, Geelhoed-Duijvestijn PH, Tack CJ, et al. Prevalence of comorbid depression is high in out-patients with type 1 or type 2 diabetes mellitus. Results from three out-patient clinics in the Netherlands. Diabet Med 2010;27:217–224 [DOI] [PubMed] [Google Scholar]
- 3.Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med 2008;25:1096–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schram MT, Baan CA, Pouwer F. Depression and quality of life in patients with diabetes: a systematic review from the European Depression in Diabetes (EDID) research consortium. Curr Diabetes Rev 2009;5:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makine C, Karşidağ C, Kadioğlu P, et al. Symptoms of depression and diabetes-specific emotional distress are associated with a negative appraisal of insulin therapy in insulin-naïve patients with type 2 diabetes mellitus. A study from the European Depression in Diabetes [EDID] Research Consortium. Diabet Med 2009;26:28–33 [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care 2008;31:2398–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000;23:934–942 [DOI] [PubMed] [Google Scholar]
- 8.Egede LE, Nietert PJ, Zheng D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care 2005;28:1339–1345 [DOI] [PubMed] [Google Scholar]
- 9.Bogner HR, Morales KH, Post EP, Bruce ML. Diabetes, depression, and death: a randomized controlled trial of a depression treatment program for older adults based in primary care (PROSPECT). Diabetes Care 2007;30:3005–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pouwer F, Beekman AT, Lubach C, Snoek FJ. Nurses’ recognition and registration of depression, anxiety and diabetes-specific emotional problems in outpatients with diabetes mellitus. Patient Educ Couns 2006;60:235–240 [DOI] [PubMed] [Google Scholar]
- 11.van der Feltz-Cornelis CM, Nuyen J, Stoop C, et al. Effect of interventions for major depressive disorder and significant depressive symptoms in patients with diabetes mellitus: a systematic review and meta-analysis. Gen Hosp Psychiatry 2010;32:380–395 [DOI] [PubMed] [Google Scholar]
- 12.van Bastelaar KM, Pouwer F, Cuijpers P, Riper H, Snoek FJ. Web-based depression treatment for type 1 and type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2011;34:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pouwer F. Should we screen for emotional distress in type 2 diabetes mellitus? Nat Rev Endocrinol 2009;5:665–671 [DOI] [PubMed] [Google Scholar]
- 14.Bränström R, Kvillemo P, Brandberg Y, Moskowitz JT. Self-report mindfulness as a mediator of psychological well-being in a stress reduction intervention for cancer patients—a randomized study. Ann Behav Med 2010;39:151–161 [DOI] [PubMed] [Google Scholar]
- 15.Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain 2008;134:310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyklícek I, Kuijpers KF. Effects of mindfulness-based stress reduction intervention on psychological well-being and quality of life: is increased mindfulness indeed the mechanism? Ann Behav Med 2008;35:331–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York, Guilford Press, 2002 [Google Scholar]
- 18.Bishop SR, Lau M, Shapiro S, et al. Mindfulness: a proposed operational definition. Clin Psychol-Sci Pr 2004;11:230–241 [Google Scholar]
- 19.Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol 2010;78:169–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenzweig S, Reibel DK, Greeson JM, et al. Mindfulness-based stress reduction is associated with improved glycemic control in type 2 diabetes mellitus: a pilot study. Altern Ther Health Med 2007;13:36–38 [PubMed] [Google Scholar]
- 21.Hartmann M, Kopf S, Kircher C, et al. Sustained effects of a mindfulness-based stress-reduction intervention in type 2 diabetic patients: design and first results of a randomized controlled trial (the Heidelberger Diabetes and Stress-study). Diabetes Care 2012;35:945–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam R, Sturt J, Lall R, Winkley K. An updated meta-analysis to assess the effectiveness of psychological interventions delivered by psychological specialists and generalist clinicians on glycaemic control and on psychological status. Patient Educ Couns 2009;75:25–36 [DOI] [PubMed] [Google Scholar]
- 23.van Son J, Nyklíček I, Pop VJ, Pouwer F. Testing the effectiveness of a mindfulness-based intervention to reduce emotional distress in outpatients with diabetes (DiaMind): design of a randomized controlled trial. BMC Public Health 2011;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The WHO-Five Well-Being Index (WHO-5) [article online]. Available from http://www.who-5.org/ Accessed 5 Nov 2010
- 25.Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York, Delacorte, 1990 [Google Scholar]
- 26.Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. In: The Social Psychology of Health. Spacapan S, Oskamp S, Eds. Newbury Park, CA, Sage, 1988, p. 31–67 [Google Scholar]
- 27.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–370 [DOI] [PubMed] [Google Scholar]
- 28.Wald FDM, Mellenbergh GJ. The shortened version of the Dutch translation of the Profile of Mood States (POMS). Ned Tijdschr Psychol 1990;45:86–90 [In Dutch] [Google Scholar]
- 29.Bohlmeijer E, Prenger R, Taal E, Cuijpers P. The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: a meta-analysis. J Psychosom Res 2010;68:539–544 [DOI] [PubMed] [Google Scholar]
- 30.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754–760 [DOI] [PubMed] [Google Scholar]
- 31.Snoek FJ, Pouwer F, Welch GW, Polonsky WH. Diabetes-related emotional distress in Dutch and U.S. diabetic patients: cross-cultural validity of the problem areas in diabetes scale. Diabetes Care 2000;23:1305–1309 [DOI] [PubMed] [Google Scholar]
- 32.Hermanns N, Kulzer B, Krichbaum M, Kubiak T, Haak T. How to screen for depression and emotional problems in patients with diabetes: comparison of screening characteristics of depression questionnaires, measurement of diabetes-specific emotional problems and standard clinical assessment. Diabetologia 2006;49:469–477 [DOI] [PubMed] [Google Scholar]
- 33.Mols F, Pelle AJ, Kupper N. Normative data of the SF-12 health survey with validation using postmyocardial infarction patients in the Dutch population. Qual Life Res 2009;18:403–414 [DOI] [PubMed] [Google Scholar]
- 34.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12–19 [DOI] [PubMed] [Google Scholar]
- 35.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69–77 [DOI] [PubMed] [Google Scholar]
- 36.Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004;363:1589–1597 [DOI] [PubMed] [Google Scholar]
- 37.Wang MY, Tsai PS, Chou KR, Chen CM. A systematic review of the efficacy of non-pharmacological treatments for depression on glycaemic control in type 2 diabetics. J Clin Nurs 2008;17:2524–2530 [DOI] [PubMed] [Google Scholar]
- 38.Winkley K, Ismail K, Landau S, Eisler I. Psychological interventions to improve glycaemic control in patients with type 1 diabetes: systematic review and meta-analysis of randomised controlled trials. BMJ 2006;333:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foley E, Baillie A, Huxter M, Price M, Sinclair E. Mindfulness-based cognitive therapy for individuals whose lives have been affected by cancer: a randomized controlled trial. J Consult Clin Psychol 2010;78:72–79 [DOI] [PubMed] [Google Scholar]
- 40.Pradhan EK, Baumgarten M, Langenberg P, et al. Effect of Mindfulness-Based Stress Reduction in rheumatoid arthritis patients. Arthritis Rheum 2007;57:1134–1142 [DOI] [PubMed] [Google Scholar]
- 41.Fjorback LO, Arendt M, Ornbøl E, Fink P, Walach H. Mindfulness-based stress reduction and mindfulness-based cognitive therapy: a systematic review of randomized controlled trials. Acta Psychiatr Scand 2011;124:102–119 [DOI] [PubMed] [Google Scholar]