Abstract
OBJECTIVE
Fibroblast growth factor (FGF)-21 is an endocrine factor with potent metabolic effects. Its day–night patterns of secretion and/or its physiological response to energy deprivation and relationship to free fatty acids (FFAs) and/or leptin remain to be fully elucidated. We aim to elucidate day–night pattern of FGF-21 levels and its relationship to FFA, to assess whether energy deprivation alters its circulating patterns, and to examine whether leptin may mediate these changes.
RESEARCH DESIGN AND METHODS
Six healthy lean females were studied for 72 h in a cross-over interventional study under three different conditions: on isocaloric diet and in a fasting state with administration of either placebo or metreleptin in physiological replacement doses. Blood samples were obtained hourly from 8:00 a.m. on day 4 until 8:00 a.m. on day 5.
RESULTS
FGF-21 exhibited day–night variation pattern during the isocaloric fed state. Fasting significantly increased FGF-21 levels (P < 0.01) via a leptin-independent pathway. Day–night variation pattern in the fed state was lost on fasting. Leptin replacement in the hypoleptinemic state restored approximate entropy of FGF-21 time series but did not alter circulating levels. FGF-21 levels were closely cross-correlated with FFA levels in all three states.
CONCLUSIONS
A day–night variation in the levels of FGF-21 exists in young lean females in the fed state. Energy deprivation increases FGF-21 levels via a leptin-independent pathway. The interaction between FGF-21 and starvation-induced lipolysis, as indicated by its close cross-correlations with FFA in both fed state and energy deprivation, needs to be studied further.
Fibroblast growth factor (FGF)-21 is an endocrine factor predominantly expressed in the liver (1) that acts as a potent regulator of glucose and lipid metabolism (2). FGF receptors are expressed in pancreatic β cells of adult mice, and dominant-negative mutations of the FGF receptors lead to decreased number of β cells and development of diabetes (3). Administration of FGF-21 in rodents reduces plasma glucose and triglycerides to near-normal levels and improves insulin sensitivity independent of reduction in body weight and adiposity (2,4). In humans, FGF-21 is positively correlated with glycemia, adiposity, fasting insulin, and triglycerides, and is significantly higher in obese than in lean subjects (5,6). The higher FGF-21 levels suggest the possibility of an FGF-21–resistant state in obesity (7). Recently, high plasma FGF-21 levels were found to be an independent predictor of diabetes (8), highlighting its metabolic role in humans.
Despite significant roles in metabolic regulation and energy homeostasis, the physiology of FGF-21 in humans, including its biological rhythm in states of energy deprivation, remains unclear. A previous study reported the absence of any diurnal variation in FGF-21 in healthy subjects (9). Other studies in contrast reported the presence of a circadian rhythm, with varying response to fasting (10–13). Furthermore, although free fatty acids (FFAs) have been shown to be a positive regulator of FGF-21 production through the activation of peroxisome proliferator-activated receptor-α (14), the relationship between FFA and FGF-21 in both physiological conditions of energy-repleted and energy-deficient states in humans remain unclear. The lack of consistent data for its biological characteristics and its potential interaction with lipolysis hampers a clearer understanding of its biological role in humans. Importantly, despite the substantial interest in FGF-21 as a therapeutic target in diabetes, there is lack of knowledge of potential day–night variation pattern and responses thereof in the energy-repleted and energy-deprived states, and it remains unknown whether any effects of the energy-deprived condition could be mediated by energy deprivation–induced changes of leptin levels. Such findings could have significant ramifications on how various clinical studies of FGF-21, each using varying sampling time and conditions, can be robustly interpreted.
Leptin is an adipocytokine with a pivotal role in signaling energy availability in the energy-deficient state (15). Energy-deficient states, leading to hypoleptinemia, induce several neuroendocrine adaptations facilitating the mobilization of alternative energy sources via processes such as lipolysis (15,16). FGF-21 levels have been previously shown to be elevated in subjects with anorexia nervosa, a condition characterized by hypoleptinemia (17). Moreover, circulating levels of FGF-21 have been shown to be strongly related to leptin levels in both anorectic and normal weight women (18). We have previously shown that decreasing leptin levels mediate some of these neuroendocrine adaptations to starvation in mice (19) and humans (15,20,21). However, it is unknown if an interaction exists between leptin and FGF-21.
In this study, we aimed to examine the relationship between FFA and FGF-21, and to elucidate the biological rhythm of FGF-21 in both energy-replete and energy-deficient states. We also aimed to determine whether an interaction exists between FGF-21 and leptin in view of previous studies highlighting a relationship between FGF-21 and energy homeostasis. These studies present a comprehensive examination of the biological characteristics of FGF-21 and clarify the relationship between lipolysis and FGF-21 in humans, thereby shedding light on the role of FGF-21 in energy homeostasis and diabetes in humans, and thus paving the way on how future clinical studies of FGF-21 can be interpreted.
RESEARCH DESIGN AND METHODS
Six young, healthy, and lean women (age, 22.8 ± 3.4 years; BMI, 21.7 ± 2.2 kg/m2) who were eumenorrheic were enrolled in a clinical research center–based, randomized, cross-over interventional study involving three separate 5-day-long inpatient admissions (22). Six subjects with a cross-over design, enabling paired comparisons, would provide 80% power to detect a difference of 1.4 SD between different conditions at the conventional α=0.05 level. In the first admission, the subjects were studied in the isocaloric fed state, whereas in the following two admissions the subjects were studied in the prolonged fasting state for 72 h and were randomized to receive either placebo or metreleptin at replacement doses. A cross-over to the opposite arm took place in the later admission so that all six subjects received both placebo and metreleptin. The subjects were admitted on day 1 at 9:00 p.m. the night before the commencement of the study on day 2. The study concluded after the 3-day intervention and ended on day 5, when they were discharged after the last blood-taking at 8:00 a.m.; the study admissions were separated by at least 8 weeks to enable adequate washout and recovery of metabolic status. All subjects had a regular menstrual cycle and were not using any medications, including oral contraceptive pills. Study visits were standardized to occur between the days 6 and 11 of their menstrual cycles.
In the fed-state study, subjects were given a standardized isocaloric diet with breakfast at 8:00 a.m., lunch at 1:00 p.m., dinner at 6:00 p.m., and a snack at 10:00 p.m. daily. Caloric intake was distributed with 20% of calories from breakfast, 35% from lunch, 35% from dinner, and 10% from the evening snack.
In the prolonged fasting studies, subjects received only caffeine-free and calorie-free liquids for 3 days, which included NaCl (500 mg), KCl (40 mEq), and a standard multivitamin daily. Starting at 8:00 a.m. on day 1 of the fasting/leptin admission, metreleptin was administered as a subcutaneous injection every 6 h for 3 days, at a dose of 0.08 mg/kg per day on day 1 and 0.2 mg/kg per day on days 2 and 3, on the basis of previous pharmacokinetic studies (23–25). During the fasting/placebo admission, a buffer solution of similar volume was administered subcutaneously every 6 h, similar to the leptin arm.
All physical activities, light–dark intervals, and blood sampling schedule were standardized for all three studies. On day 3, blood was drawn through an indwelling intravenous catheter every 15 min from 8:00 a.m. on day 3 until 8:00 a.m. on day 4, and then was pooled every hour to meet the assays’ sample volume requirements.
The study protocols were approved by the Institutional Review Board of Beth Israel Deaconess Medical Center, and written informed consent was obtained from all the subjects. Clinical-quality metreleptin (formerly known as r-metHuLeptin) was supplied by Amgen Inc. (Thousand Oaks, CA) and administered under an investigational new drug application submitted to the Food and Drug Administration.
Assays
Serum FGF-21 was measured by ELISA (R&D Systems, Minneapolis, MN), with a sensitivity of 4.67 pg/mL, intra-assay coefficient of variation of 2.9–3.9%, and interassay coefficient of variation of 5.2–10.9%, in accordance with the manufacturer’s instructions. All serum samples were stored at −80°C until analysis and were analyzed in duplicate. Leptin and insulin levels were measured as previously reported (22). FFA levels were measured by an enzymatic colorimetric assay (Wako Diagnostic USA). Glucose was measured by an automated analyzer (Hitachi cobas c311; Roche Diagnostics).
Statistical analysis
Statistical analysis was performed using Stata version 12 (Stata Corp, College Station, TX). Descriptive statistics are presented as means ± SD. Normality of the variables was evaluated using the Shapiro-Wilkes test. Variables that were not normally distributed were normalized using the appropriate, for each time, transformation. Analysis for the existence of any potential day–night variation pattern in FGF-21 and FFA levels and cross-correlation analysis between FGF-21 and FFA circulating levels were performed, at the level of each individual, using the COSINE and CORRELATION algorithms of the Pulse XP software accordingly (UVA Pulse Analysis Software, Charlottesville, VA). In addition, we performed trigonometric ordinal least-squares (OLS) regression analysis on the data from all subjects, estimating the parameters of FGF-21 oscillations and the adjusted coefficient of determination (R2). Comparisons between mean FGF-21 and FFA levels across the three states were performed with hierarchical mixed-effects linear models. The 24-h trajectories of the analytes were expressed as a linear function of time at the level 1 of the model, and “state” was introduced as a level 2 predictor using dummy encoding. The optimal model was selected based on Akaike information criterion and Bayesian information criterion. Normality and homoscedasticity of residuals and random effects were verified through frequency histograms and box plots. Area under the curve (AUC) of FGF-21 was estimated using the trapezoid method and correlations of the change of FGF-21 AUC and the change at the levels of different hormones were analyzed using simple linear regression. Comparisons between baseline levels and AUC across the three groups were performed with repeated-measures ANOVA because of the cross-over design of the study. Significant comparisons were further analyzed with post hoc paired comparisons using the least significant difference correction for multiple comparisons. All P values are two-tailed and the α criterion was set to 0.05.
Approximate entropy
Approximate entropy (ApEn) is a model-independent regularity statistic developed to quantify the orderliness of sequential measures (26), such as hormonal time series. Values close to zero denote a high degree of orderliness in the data, whereas higher values nearer to 1.0 indicate a great degree of disorderliness. Two input parameters, window length (m) and tolerance (r), were specified to compute ApEn. For this study, we calculated ApEn values for each leptin profile with window length of m = 1 and tolerance parameter r = 0.20 of the average SD of the individual subject’s FGF-21 time series. We used 10,000 Monte Carlo simulations to calculate the ApEn in our FGF-21 time series data. All calculations were performed with ApEn algorithm of Pulse XP software.
RESULTS
Fed state
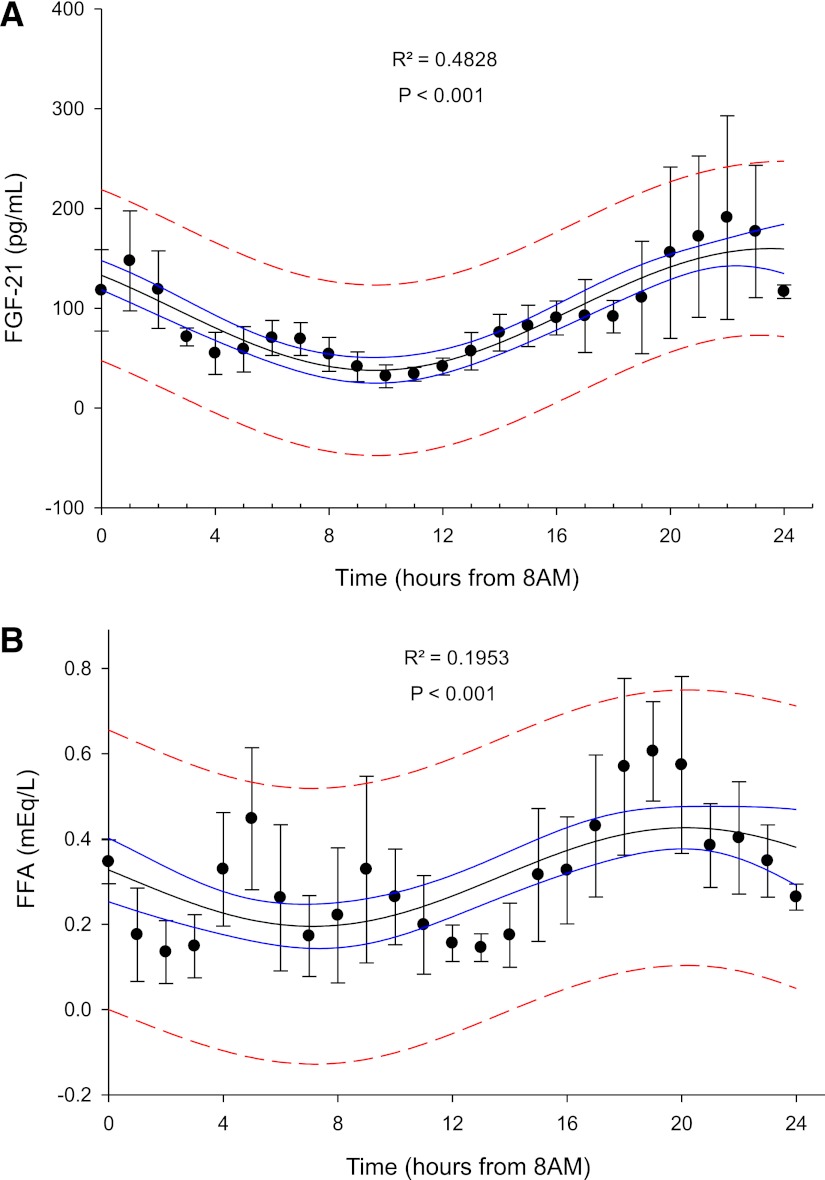
Our four-parameter, sine, OLS regression analysis revealed a significant day–night variation pattern of circulating FGF-21 levels during the isocaloric fed state. The estimated oscillation of FGF-21 levels had a period of 27.86 ± 2.76 h (P < 0.001), amplitude of 60.988 ± 5.866 pg/mL (P < 0.001), and mean hormonal levels of 98.75 ± 6.67 pg/mL (P < 0.001). The adjusted coefficient of determination (R2) of our final model was 48.28% (P < 0.001), suggesting that a significant proportion of circulating FGF-21 levels variability could be explained by an underlying day–night variation pattern (Fig. 1A). Linear regression analysis revealed no association between FGF-21 levels at 8:00 a.m. and FGF-21 AUC in the fed state (β = 0.603; P = 0.204). FFA 24-h pattern exhibited a pattern of day–night variation similar to FGF-21 in the fed state with OLS regression analysis (Fig. 1B). We have further analyzed FGF-21 and FFA levels of each individual alone using the COSINE algorithm of the PulseXP software (as described in the statistical section analysis), in which the day–night variability of each subject was compared against the error of the assay. This analysis, on an individual basis, also revealed the presence of a day–night variability pattern in both FGF-21 and FFA during the fed state, with parameters, similar to the ones summarized in Figs. 1A and B.
Figure 1.
A: Day–night variation of mean FGF-21 levels (pg/mL) in the fed state. Adjusted coefficient of determination (R2) is displayed at the top center. Solid line represents 95% confidence interval and interrupted line represents 95% prediction interval (n = 6). B: Day–night variation of mean FFA (mEq/L) levels in the fed state. Adjusted coefficient of determination (R2) is displayed at the top center. Solid line represents 95% confidence interval and interrupted line represents 95% prediction interval (n = 6). (A high-quality color representation of this figure is available in the online issue.)
The adjusted coefficient of determination (R2) that reflects the percent of the variability that is explained by an underlying day–night pattern is very high (R2 = 48.28%; P < 0.001). This enables us to report with great confidence that there is clinically important day–night variability. Clinically important day–night secretion patterns traditionally have been associated with adjusted coefficients of determination >15%. As an example, studies of cortisol, a hormone with well-established circadian patterns of secretion, have revealed an R2 of >30% in similar models with the same sample size (27).
Energy deprivation state with 72-h fast and placebo replacement
Serum leptin levels decreased to <20% of baseline in response to energy deprivation (14.66 to 2.78 ng/mL; P < 0.001). Normalized AUC of FGF-21 levels were significantly higher during energy deprivation compared with the fed state (P = 0.0066) (Fig. 2A). Linear regression analysis revealed no association between FGF-21 levels at 8:00 a.m. and FGF-21 AUC in the fasting state (β = 0.56; P = 0.25). OLS regression analysis revealed absence of any clinically significant day–night variation pattern of circulating FGF-21 levels during the energy deprivation state. Similar to FGF-21 levels, circulating FFA levels do not exhibit any clinically significant day–night variation pattern while in the fasted state (adjusted coefficient of determination 9.31%).
Figure 2.
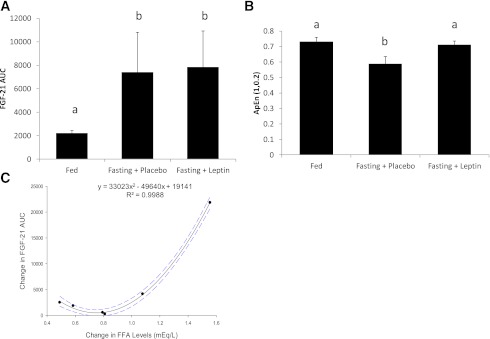
A: FGF-21 AUC in all three states demonstrating increase in levels in response to fasting; similar letters signify no statistical significant difference at the 0.05 level (n = 6). B: ApEn (m = 1, tolerance = 0.2, ApEn [1, 0.2]) of FGF-21 in all three states; similar letters signify no statistical significant difference at the 0.05 level (n = 6). C: Relationship between change of the AUC of FGF-21 between fed state and fasting state and FFA (mEq/L) levels before and after 72-h fast. Each point represents a single subject. Interrupted line represents 95% confidence interval (n = 6). (A high-quality color representation of this figure is available in the online issue.)
Energy deprivation state with 72-h fast and leptin replacement
Leptin replacement restored leptin levels to normal physiological range as previously reported (22), but had no effect on circulating FGF-21 levels. FGF-21 AUC during leptin replacement remained significantly higher compared with the isocaloric fed state (P = 0.0079) and was not statistically different from the AUC in the fasting state (P = 0.9165) (Fig. 2A). Similar to the fasting/placebo arm, OLS regression analysis revealed absence of any clinically significant day–night variation pattern of circulating FGF-21 and FFA levels.
ApEn
During fasting, the ApEn of the FGF-21 time series decreased from 0.731 ± 0.096 to 0.588 ± 0.114 [Tukey honestly significant difference (HSD) corrected for multiple comparisons, P < 0.05], whereas leptin replacement therapy restored ApEn back to 0.712 ± 0.102 (Tukey HSD corrected for multiple comparisons, P < 0.05) (Fig. 2B).
Relationship between changes in FGF-21 AUC with other hormones/substrates during fasting
Other hormones and substrates of interest, including insulin, FFA, and glucose, were analyzed to elucidate possible association with the increase of FGF-21 during energy deprivation. There were no correlations between changes in insulin (P = 0.539), glucose (P = 0.12), and the increase in FGF-21 levels after fasting. However, FGF-21 AUC increased with a quadratic relationship to the increase in FFA AUC after fasting (standardized regression coefficients: linear β = −2.31; quadratic β = 3.22; adjusted R2 = 99.79%; P < 0.001) (Fig. 2D); individuals who exhibited the largest increase in the FFA levels from the fed to the fasting state also exhibited the largest increase in the FGF-21 levels.
Relationship of FGF-21 with FFA
FGF-21 levels were cross-correlated with FFA levels both in the fed and the fasting states (Fig. 3). Cross-correlation analysis demonstrated that the 24-h circulating pattern of FGF-21 was closely associated to the FFA 24-h pattern in both fasting and fed states. While the subjects were in the isocaloric fed state, four out of six subjects exhibited significant positive cross-correlation ranging from 0.35 to 0.81 between FGF-21 and FFA levels at 2- to 6-h lag, demonstrating that high levels of FFA were followed by high levels of FGF-21 with a 2- to 6-h lag (Fig. 3). Similarly, during the fasted state, in four out of six subjects FGF-21 and FFA levels were positively cross-correlated (0.25–0.52) at 5- to 8-h lag, demonstrating that high levels of FFA were followed by high levels of FGF-21 with a 5- to 8-h lag. At the high level of FGF-21, FFA was noted to momentarily decrease before recovering to high levels subsequently in both fed and fasting states. Regarding the subjects who did not exhibit significant cross-correlation, there were two subjects in the fed and two subjects in the fasting condition. One of these subjects was the same in the fed and fasting conditions. The other one subject who did not exhibit cross-correlation was different between the fed and fasting states.
Figure 3.
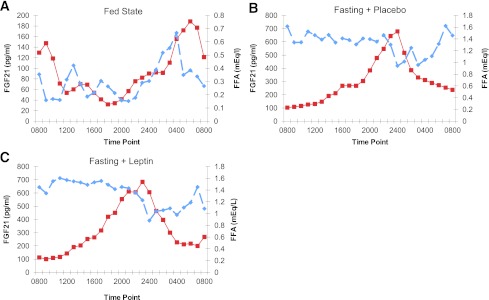
A: Mean FGF-21 and FFA for all six subjects in the fed state. Solid lines represent FGF-21 levels and interrupted lines represent FFA levels (n = 6). B: Mean FGF-21 and FFA for all six subjects in the fasting with placebo state. Solid lines represent FGF-21 levels and interrupted lines represent FFA levels (n = 6). C: Mean FGF-21 and FFA for all six subjects in the fasting with leptin replacement state. Solid lines represent FGF-21 levels and interrupted lines represent FFA levels (n = 6). (A high-quality color representation of this figure is available in the online issue.)
CONCLUSIONS
We report herein, using a controlled interventional study design, that FGF-21 levels display a day–night variation pattern in the fed state in young lean females. We also demonstrate that FGF-21 levels are increased in response to energy deprivation via a leptin-independent pathway and that leptin replacement in energy deprivation restores the ApEn of FGF-21 time series, although it has no significant effect in restoring circulating FGF-21 levels per se. Finally, FGF-21 is closely related to FFA levels in both fed and fasting states, indicating a relationship between the process of lipolysis and FGF-21 levels.
Studies describing day–night variation of FGF-21 are contradictory, with one study demonstrating the absence of any diurnal variation in healthy subjects (9), whereas other studies report its presence (11,12). In this study, we demonstrate the presence of a day–night variation pattern of FGF-21 with a peak in the early morning and a nadir in the early evening in young, lean female subjects in the fed study, suggesting a role of FGF-21 in maintaining energy homeostasis during the hours of sleep. In the fed state, FFA was similarly noted to exhibit a day–night variation pattern with higher levels in the early morning, signifying increased lipolysis in the early hours of the morning. During sleep, decreased food intake incites lipolysis and gluconeogenesis to maintain energy homeostasis. Although a causal relationship between FGF-21 and the process of lipolysis cannot be proven with the current study design, the cross-correlation between FFA and FGF-21 indicates an association between the nocturnal increase in FFA and FGF-21 levels. A recent study established a link between retinoic acid receptor–related orphan receptor-α, a nuclear hormone receptor that plays a critical role in lipid metabolism as well as in regulation of the circadian rhythm, and in FGF-21 expression (28). Another study showed that PGC-1α-mediated reduction of FGF-21 expression is dependent on Rev-Erbα, a transcriptional repressor important for maintenance of the circadian clock and lipid metabolism, suggesting that FGF-21 may be controlled by the same set of circadian clock regulators in humans (29).
The response of FGF-21 levels to fasting remains unclear to date. Galman et al. (9) reported no change in FGF-21 levels after a 2-day fast or feeding of a ketogenic diet. Similarly, Christodoulides et al. (13) reported no significant variation in FGF-21 levels during 48-h fasting followed by a 24-h refeeding. Yu et al. (12) reported a significant increase in FGF-21 levels during overnight fasting, although the increase diminished at the end of a 24-h fast. If the postulation that the day–night variation of FGF-21 demonstrated herein is related to overnight energy deprivation-induced lipolysis, then we hypothesize that FGF-21 levels should be increased in association with adequate fasting-induced lipolysis. This study clearly demonstrates an increase in FGF-21 in response to 72 h of fasting, i.e., a period clearly longer than that in previous studies. Besides the increase in its levels with fasting, the day–night variation pattern of FGF-21 shown in the fed state was abolished. Similar responses in both the placebo and leptin arms in this study reinforce the reproducibility of the FGF-21 increase in response to fasting. The fasting-induced FGF-21 increase observed here is in agreement with data reported by Galman et al. (9) that demonstrated that prolonged fasting of 7 days with significant ketosis increased FGF-21 levels. The discrepancy in relation to other studies could be secondary to the shorter duration of fasting (11,13), different assays used (10–13,), different genders studied (13), and design of these studies. In particular, we found a poor correlation between a single early morning determination of FGF-21 and the total FGF-21 levels over the course of a 24-h period as determined by the AUC. Therefore, studies utilizing a single determination of FGF-21 level at a specific time point may not represent FGF-21 physiology fully (10,12,13). It is also possible that a sex dimorphism might exist in FGF-21 physiology, considering that a sex difference exists in lipids metabolism (30). These questions remain to be fully addressed by future studies in both genders in the fed and fasting states.
The increase of FGF-21 in response to fasting indicates a possible role of this molecule in mediating some of the metabolic adaptations in energy deprivation. Studies in rodents have demonstrated an increase in FGF-21 levels in fasting induced directly by peroxisome proliferator-activated receptor-α in liver (31,32). Peroxisome proliferator-activated receptor-α regulates the utilization of fat as an energy source during starvation and is the molecular target for drugs used to treat dyslipidemia. These studies in rodents identify hepatic FGF-21 as a regulator of lipid homeostasis and highlight a physiological role in adaptation to a low-energy state for this hepatic hormone. We herein demonstrate a positive relationship between the increase in FFA in response to fasting and the increase in FGF-21 levels, with subjects having higher FFA levels also having a greater increase in FGF-21 levels (Fig. 2C). Moreover, FFA levels were observed to precede the peaking of FGF-21 by a few hours, suggesting that the increase in FFA might have a role in inducing the peaking of FGF-21. This is supported by previous observations in which the physiologically elevated FFAs induced by lipid–heparin infusion were reported to increase circulating FGF-21 levels in humans (33). Interestingly, apart from the observed increase in FGF-21 levels that followed after the peaks of FFA, FFA levels were noted to decrease momentarily after the peak of FGF-21 levels in this study, suggesting a possible feedback interaction between FFA and FGF-21. This observation is consistent with recent studies demonstrating that FGF-21 may play a role in inhibiting hormone-stimulated lipolysis in human and murine adipocytes (34,35). Although causality remains to be proven by future interventional studies, the present findings highlight the possibility that FGF-21 may represent part of a feedback regulatory mechanism that serves to provide an alternate source of energy substrate during states of energy deprivation, and possibly the limitation of such a process that may result in excessive ketosis if left unchecked.
We then focused on leptin, the prototype adipokine, the levels of which herein decreased to ∼20% of baseline in response to 72 h of fasting in this study (22,36). Leptin plays a role in signaling some of the neuroendocrine adaptations in response to energy deprivation (21). To investigate whether the decrease in leptin levels during energy deprivation is associated with the increase in FGF-21 levels, we administered metreleptin to our subjects in doses calculated to restore physiological circulating levels as previously reported (23,24). Leptin administration did not change FGF-21 levels compared with fasting and placebo administration, concluding that the increase of FGF-21 levels in energy deprivation was mediated via a leptin-independent pathway. However, the ApEn of the FGF-21 time series was restored with leptin replacement. ApEn is a statistical test that has been introduced as a quantification of regularity of data (26), with higher values indicating a higher degree of disorderliness. ApEn correlates with “occult” or subclinical changes often undetected by classical time series and is predictive of subsequent clinical changes. Within endocrinology, ApEn has been used in multiple ways to determine subtle disruptions in pathophysiologic hormonal secretory patterns for many hormones, including insulin, growth hormone, and cortisol (37–39). The effect of leptin in restoring ApEn of the FGF-21 time series is intriguing and could possibly herald subsequent changes in FGF-21 metabolism, which may take longer to be detected than the current study duration. Future studies using a longer duration of time series examining physiology of FGF-21 with leptin replacement might shed light on the observation of ApEn restoration of FGF-21 with leptin.
Our study has several strengths. The subjects and study conditions were highly standardized, with all subjects studied at the same period of their menstrual cycle and receiving similar isocaloric diet, activity level, and uniform light–dark interval. The long duration of interval between study visits allowed adequate washout and recovery of metabolic status. Blood sampling was frequent, resulting in a high temporal resolution in our time series, allowing us to conclude on the presence of a day–night pattern of FGF-21 secretion with great confidence. The cross-over design of the study maximizes standardization and eliminates any potential confounder. The FGF-21 assay used in the study has been thoroughly evaluated and all the assays were performed in duplicate by operators blinded to the study hypothesis, eliminating any measurement bias. The number of subjects provided adequate power, and the frequent blood sampling, cross-over design, and strict standardization of study conditions enhance the study quality.
Some limitations have to be addressed. Our study is confined to lean, healthy female subjects; therefore, our results should not be generalized to male, obese, or diabetic subjects. The current study design has allowed us to infer on possible interaction between FGF-21 and FFA, although causality remains to be proven.
In summary, this study contributes toward elucidation of FGF-21 physiology by demonstrating that FGF-21 displays day–night variation pattern in the fed state and is increased in energy deprivation via a leptin-independent mechanism. We also show for the first time that leptin replacement restored the approximate entropy of FGF-21 time series, but not the energy deprivation-induced changes of FGF-21 levels. Finally, we demonstrate that the day–night variation and the increase of FGF-21 production in response to fasting are closely related to FFA levels. The knowledge of these biological characteristics of FGF-21 is critical for future clinical studies to plan the timing and the situations in which samples are collected to evaluate FGF-21 levels across different individuals or groups with comparable results. Furthermore, given the significant role of lipolysis in insulin resistance and diabetes, the relationship between FGF-21 and lipolysis elucidated in this study paves the way on how future studies on FGF-21 and diabetes can be interpreted. Further studies are necessary to replicate these data in men, given the gender dimorphism in lipids metabolism (30), and to delineate the precise interactions between FGF-21 and all other hormones and substrates involved in energy homeostasis to clarify the metabolic role and clinical applications of FGF-21 in humans.
Acknowledgments
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants 58785, 79929, and 81913. The project described was also supported by Award Number 1I01CX000422-01A1 from the Clinical Science Research and Development Service of the VA Office of Research and Development. Funding was also received from the National Institutes of Health National Center for Research Resources grant M01-RR-01032 (Harvard Clinical and Translational Science Center). Amylin Pharmaceuticals, Inc. supplied metreleptin for this study but had no role in the study design, conduct of the study, collection, management, analysis, and interpretation of the data, or the preparation, review, or approval of the manuscript. No other potential conflicts of interest relevant to this article were reported.
J.-P.F. wrote the manuscript, researched data, and performed laboratory work. K.N.A. wrote the manuscript and analyzed data. J.P.C., J.P., and H.-S.M. performed laboratory work. C.S.M. is the principal investigator and reviewed and edited the manuscript. C.S.M. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Clinical trial reg. no. NCT00140231, clinicaltrials.gov.
References
- 1.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 2000;1492:203–206 [DOI] [PubMed] [Google Scholar]
- 2.Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005;115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart AW, Baeza N, Apelqvist A, Edlund H. Attenuation of FGF signalling in mouse beta-cells leads to diabetes. Nature 2000;408:864–868 [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009;58:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008;57:1246–1253 [DOI] [PubMed] [Google Scholar]
- 6.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009;32:1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher FM, Chui PC, Antonellis PJ, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010;59:2781–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Cheung BM, Tso AW, et al. High plasma level of fibroblast growth factor 21 is an Independent predictor of type 2 diabetes: a 5.4-year population-based prospective study in Chinese subjects. Diabetes Care 2011;34:2113–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gälman C, Lundåsen T, Kharitonenkov A, et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 2008;8:169–174 [DOI] [PubMed] [Google Scholar]
- 10.Dushay J, Chui PC, Gopalakrishnan GS, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010;139:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu H, Xia F, Lam KS, et al. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin Chem 2011;57:691–700 [DOI] [PubMed] [Google Scholar]
- 12.Andersen B, Beck-Nielsen H, Højlund K. Plasma FGF21 displays a circadian rhythm during a 72-h fast in healthy female volunteers. Clin Endocrinol (Oxf) 2011;75:514–519 [DOI] [PubMed] [Google Scholar]
- 13.Christodoulides C, Dyson P, Sprecher D, Tsintzas K, Karpe F. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J Clin Endocrinol Metab 2009;94:3594–3601 [DOI] [PubMed] [Google Scholar]
- 14.Mai K, Andres J, Biedasek K, et al. Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes 2009;58:1532–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 2005;366:74–85 [DOI] [PubMed] [Google Scholar]
- 16.Frühbeck G, Gómez-Ambrosi J, Salvador J. Leptin-induced lipolysis opposes the tonic inhibition of endogenous adenosine in white adipocytes. FASEB J 2001;15:333–340 [DOI] [PubMed] [Google Scholar]
- 17.Fazeli PK, Misra M, Goldstein M, Miller KK, Klibanski A. Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. J Clin Endocrinol Metab 2010;95:369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dostálová I, Kaválková P, Haluzíková D, et al. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab 2008;93:3627–3632 [DOI] [PubMed] [Google Scholar]
- 19.Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature 1996;382:250–252 [DOI] [PubMed] [Google Scholar]
- 20.Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 2004;351:987–997 [DOI] [PubMed] [Google Scholar]
- 21.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 2003;111:1409–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan JL, Matarese G, Shetty GK, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci USA 2006;103:8481–8486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan JL, Wong SL, Orlova C, Raciti P, Mantzoros CS. Pharmacokinetics of recombinant methionyl human leptin after subcutaneous administration: variation of concentration-dependent parameters according to assay. J Clin Endocrinol Metab 2007;92:2307–2311 [DOI] [PubMed] [Google Scholar]
- 24.Chan JL, Wong SL, Mantzoros CS. Pharmacokinetics of subcutaneous recombinant methionyl human leptin administration in healthy subjects in the fed and fasting states: regulation by gender and adiposity. Clin Pharmacokinet 2008;47:753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong SL, DePaoli AM, Lee JH, Mantzoros CS. Leptin hormonal kinetics in the fed state: effects of adiposity, age, and gender on endogenous leptin production and clearance rates. J Clin Endocrinol Metab 2004;89:2672–2677 [DOI] [PubMed] [Google Scholar]
- 26.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA 1991;88:2297–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vamvini MT, Aronis KN, Chamberland JP, Mantzoros CS. Energy deprivation alters in a leptin- and cortisol-independent manner circulating levels of activin A and follistatin but not myostatin in healthy males. J Clin Endocrinol Metab 2011;96:3416–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Solt LA, Burris TP. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor alpha. J Biol Chem 2010;285:15668–15673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estall JL, Ruas JL, Choi CS, et al. PGC-1alpha negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erb(alpha) axis. Proc Natl Acad Sci USA 2009;106:22510–22515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. J Clin Endocrinol Metab 2011;96:885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 2007;5:415–425 [DOI] [PubMed] [Google Scholar]
- 32.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007;5:426–437 [DOI] [PubMed] [Google Scholar]
- 33.Mai K, Bobbert T, Groth C, et al. Physiological modulation of circulating FGF21: relevance of free fatty acids and insulin. Am J Physiol Endocrinol Metab 2010;299:E126–E130 [DOI] [PubMed] [Google Scholar]
- 34.Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Rydén M. FGF21 attenuates lipolysis in human adipocytes: a possible link to improved insulin sensitivity. FEBS Lett 2008;582:1725–1730 [DOI] [PubMed] [Google Scholar]
- 35.Hotta Y, Nakamura H, Konishi M, et al. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology 2009;150:4625–4633 [DOI] [PubMed] [Google Scholar]
- 36.Wolfe BE, Jimerson DC, Orlova C, Mantzoros CS. Effect of dieting on plasma leptin, soluble leptin receptor, adiponectin and resistin levels in healthy volunteers. Clin Endocrinol (Oxf) 2004;61:332–338 [DOI] [PubMed] [Google Scholar]
- 37.Meneilly GS, Ryan AS, Veldhuis JD, Elahi D. Increased disorderliness of basal insulin release, attenuated insulin secretory burst mass, and reduced ultradian rhythmicity of insulin secretion in older individuals. J Clin Endocrinol Metab 1997;82:4088–4093 [DOI] [PubMed] [Google Scholar]
- 38.Veldhuis JD, Liem AY, South S, et al. Differential impact of age, sex steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab 1995;80:3209–3222 [DOI] [PubMed] [Google Scholar]
- 39.Roelfsema F, Pincus SM, Veldhuis JD. Patients with Cushing’s disease secrete adrenocorticotropin and cortisol jointly more asynchronously than healthy subjects. J Clin Endocrinol Metab 1998;83:688–692 [DOI] [PubMed] [Google Scholar]