Gestational diabetes mellitus (GDM), glucose intolerance with onset or first recognition during pregnancy, has been increasing (1) and will increase further with widespread adoption of new diagnostic criteria recommended by the American Diabetes Association (ADA) (2). GDM, even at the milder end of the diagnostic spectrum, is associated with fetal macrosomia, neonatal adiposity, preeclampsia, and cesarean section (3), which can be reduced by diagnosis and treatment (4,5). Such treatment is not without cost (6), and an effective, relatively simple, inexpensive approach to prevention could result in significant savings to the health care system, not to mention decreasing morbidity. In this issue of Diabetes Care, D’Anna et al. (7) describe a randomized controlled trial (RCT) of such a potential prevention strategy.
Insulin resistance is characteristic of human pregnancy and may have evolved to ensure the fetus a continued supply of nutrients even in times of famine. Most gravidas increase insulin release and maintain euglycemia, while those with GDM are unable to do so adequately. While metformin, an insulin-sensitizing drug, initially appeared to prevent GDM in nonrandomized cohort studies, a double-blind RCT did not demonstrate efficacy (8). Inositol, present in many foods, is a component of inositolphosphoglycans, a second messenger for insulin action (9) (Fig. 1), and two of its nine isoforms, myo-inositol and chiro-inositol, have been used as insulin-sensitizing agents to treat insulin-resistant states such as polycystic ovary syndrome (PCOS) in doses ranging from 200 mg/day to 4 g/day (10–12). The authors of the current study have also demonstrated in an RCT a beneficial effect of myo-inositol in treating the metabolic syndrome in postmenopausal women (13). These same authors (14) reported a lower incidence of GDM (17 vs. 54%) among 46 PCOS patients who conceived on myo-inositol (4 g/day) and continued this regimen throughout pregnancy compared with 37 PCOS patients who conceived on metformin and discontinued it once pregnancy was diagnosed. In a randomized trial they demonstrated a greater reduction in insulin resistance in women with GDM treated with myo-inositol and folic acid than in control GDM subjects treated with folic acid alone (15).
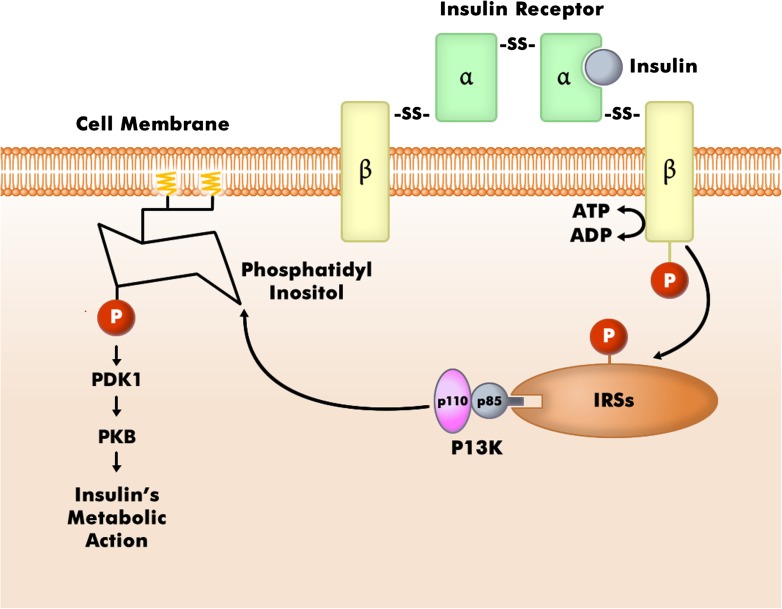
Figure 1.
Relation of the insulin pathway to phosphatidylinositols. The insulin receptor resides in the cell membrane. When its β subunit is phosphorylated by the presence of insulin, insulin receptor substrates (IRSs) are activated. P13K docks to the IRSs, which then leads to phosphorylation of phosphatidylinositol, eventuating in inulin action. It is postulated that myo-inositol may increase insulin sensitivity by making more phosphatidylinositol available. PI3K, phosphatidyl inositol 3-kinase; PDK1, phosphoinositide-dependent kinase 1; PKB, protein kinase B. (Adapted with permission from Agarwal AK, Garg A. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol Metab 2003;14:214–221.)
In the open-label RCT published in this issue of Diabetes Care (7), 110 nonobese gravidas, whose only risk factor for GDM was a first-degree relative with diabetes, were given myo-inositol 2 g twice daily along with folic acid 400 µg twice daily from the end of the first trimester throughout the remainder of the pregnancy. Control subjects were 110 similar gravidas randomized to receive only the 400 µg of folic acid twice daily. GDM (ADA criteria [2]) occurred in 6% of myo-inositol treated subjects versus 15% of control subjects (P = 0.04). Fetal macrosomia was also reduced (0 vs. 7%, P = 0.007) and average birth weight was 162 g lower in the treated group. The authors conclude that this preliminary report is good news, although larger confirmatory studies are needed.
RCTs are the strongest level of evidence, although it would have been preferable if the study were double-blinded rather than open-label. While GDM and macrosomia were reduced by the intervention, some less frequent outcomes such as hypertensive disorders, cesarean section, and shoulder dystocia were not different between myo-inositol–treated subjects and control subjects. While the study was underpowered to assess an effect on these outcomes, larger trials should answer these open questions. In this study, as in the authors’ previous report on gravidas with GDM (15), fasting plasma glucose was reduced with myo-inositol treatment, as was the 1-h value on the 75-g oral glucose tolerance test compared with control subjects. Much of the increase in GDM is thought to be attributable to population increases in obesity. Because obese subjects were excluded from this study—and even overweight subjects were probably not common since the average prepregnancy BMI was around 23 kg/m2—it remains to be seen whether myo-inositol would be similarly effective in overweight and obese subjects. In the analysis reported, BMI had an effect on the development of GDM that was independent of myo-inositol supplementation.
When a treatment is proposed for use in pregnancy, special consideration must be given to safety for the mother and the fetus. myo-Inositol is present in many foods, particularly fresh fruits and vegetables, beans, grains, and nuts. It is not considered a drug but rather a dietary supplement and is thus not subject to the jurisdiction of the U.S. Food and Drug Administration. It is widely available online and in health food stores, but the advertised composition of such supplements must be interpreted with caution given the lack of regulation and monitoring. When the myo-inositol content of various foods was analyzed, an average 2,500 kcal American diet was estimated to contain approximately 900 mg of inositol (16). A review of data from 12 clinical trials in which myo-inositol was used for treatment of PCOS, erectile dysfunction, depression, and other psychiatric disorders found that mild gastrointestinal side effects were reported only with doses of 12 g/day or more (17). The dosage used in the RCT reported herein was 4 g/day. Fetal effects, if any, should be proportional to the ease with which a substance crosses the placenta. Metformin, for example, is concentrated on the fetal side of the placenta (18), and it is unclear whether fetal effects are harmful, beneficial, or neutral. Measurements of fetal levels of maternally infused stable isotope-labeled myo-inositol in normal pregnancies at term demonstrated that less than 10% of fetal inositol was maternally derived, suggesting little placental transport in late pregnancy (19).
A review of exogenous use of inositol (20) recommended caution in its use during pregnancy, citing two studies suggesting that inositol may stimulate uterine contractions. The cited studies demonstrated that oxytocin induced the formation of inositol triphosphate in cultured myometrial cells, suggesting that inositol triphosphate may act as a second messenger for oxytocin (21), and that inositol triphosphate can stimulate isolated rat uterine muscle segment contractions (22). Inositol triphosphate is formed in situ and is not the same as dietary myo-inositol. The fetus produces most of its own inositol. Nevertheless, it is reassuring that in the RCTs involving gravidas with PCOS (14), GDM (15) and in the current study (7) preterm birth were not increased with myo-inositol supplementation.
If inositol supplementation is indeed effective in preventing GDM, the most appropriate dose needs to be determined. Could dietary enhancement be as effective as powder or capsules? Studies of its use in other conditions have used doses ranging from 200 mg/day (11) to 1,200 mg/day (11) to 18 g/day (17). myo-Inositol has been found in higher concentrations in the urine of subjects with intrauterine growth restriction compared with normally grown neonates (23), and inositolphosphoglycans have been reported in higher concentrations in the urine of GDM subjects compared with control subjects (24). The meaning of these findings is unclear. Because inositol is ubiquitous in its potential role as a component of a second messenger, care must be taken to avoid unintended consequences.
This study by D’Anna et al. (7), along with earlier investigations of the effect of inositol supplementation on insulin resistance in GDM subjects and in preventing GDM in women with PCOS, lays the groundwork for more and larger studies to test the hypothesis that inositol supplementation can prevent GDM in the general pregnant population, including overweight and obese gravidas. myo-Inositol is inexpensive, particularly compared with most prescribed medications. If this intervention turns out to be safe and effective it could have a profound impact on improving pregnancy outcomes and lowering health care costs. If GDM diagnosed by the new ADA recommended criteria (2) is preventable by an intervention such as this, the anticipated onslaught of new cases may be dampened considerably!
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
See D'Anna et al., p. 854
References
- 1.Albrecht SS, Kuklina EV, Bansil P, et al. Diabetes trends among delivery hospitalizations in the U.S., 1994–2004. Diabetes Care 2010;33:768–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes–2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477–2486 [DOI] [PubMed] [Google Scholar]
- 5.Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moss JR, Crowther CA, Hiller JE, Willson KJ. Robinson JS; Australian Carbohydrate Intolerance Study in Pregnant Women Group. Costs and consequences of treatment for mild gestational diabetes mellitus–evaluation from the ACHOIS randomised trial. BMC Pregnancy Childbirth 2007;7:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Anna R, Scilipoti A, Giordano D, et al. myo-Inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: a prospective, randomized, placebo-controlled study. Diabetes Care 2013;36:854–857 [DOI] [PMC free article] [PubMed]
- 8.Vanky E, Stridsklev S, Heimstad R, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab 2010;95:E448–E455 [DOI] [PubMed] [Google Scholar]
- 9.Saltiel AR. Second messengers of insulin action. Diabetes Care 1990;13:244–256 [DOI] [PubMed] [Google Scholar]
- 10.Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, Allan G. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N Engl J Med 1999;340:1314–1320 [DOI] [PubMed] [Google Scholar]
- 11.Gerli S, Mignosa M, Di Renzo GC. Effects of inositol on ovarian function and metabolic factors in women with PCOS: a randomized double blind placebo-controlled trial. Eur Rev Med Pharmacol Sci 2003;7:151–159 [PubMed] [Google Scholar]
- 12.Unfer V, Carlomagno G, Dante G, Facchinetti F. Effects of myo-inositol in women with PCOS: a systematic review of randomized controlled trials. Gynecol Endocrinol 2012;28:509–515 [DOI] [PubMed] [Google Scholar]
- 13.Santamaria A, Giordano D, Corrado F, et al. One-year effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome. Climacteric 2012;15:490–495 [DOI] [PubMed] [Google Scholar]
- 14.D’Anna R, Di Benedetto V, Rizzo P, et al. Myo-inositol may prevent gestational diabetes in PCOS women. Gynecol Endocrinol 2012;28:440–442 [DOI] [PubMed] [Google Scholar]
- 15.Corrado F, D’Anna R, Di Vieste G, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med 2011;28:972–975 [DOI] [PubMed] [Google Scholar]
- 16.Clements RS, Jr, Darnell B. Myo-inositol content of common foods: development of a high-myo-inositol diet. Am J Clin Nutr 1980;33:1954–1967 [DOI] [PubMed] [Google Scholar]
- 17.Carlomagno G, Unfer V. Inositol safety: clinical evidences. Eur Rev Med Pharmacol Sci 2011;15:931–936 [PubMed] [Google Scholar]
- 18.Vanky E, Zahlsen K, Spigset O, Carlsen SM. Placental passage of metformin in women with polycystic ovary syndrome. Fertil Steril 2005;83:1575–1578 [DOI] [PubMed] [Google Scholar]
- 19.Staat BC, Galan HL, Harwood JEF, et al. Transplacental supply of mannose and inositol in uncomplicated pregnancies using stable isotopes. J Clin Endocrinol Metab 2012;97:2497–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colodny L, Hoffman RL. Inositol—clinical applications for exogenous use. Altern Med Rev 1998;3:432–447 [PubMed] [Google Scholar]
- 21.Phaneuf S, Europe-Finner GN, Carrasco MP, Hamilton CH, López Bernal A. Oxytocin signalling in human myometrium. Adv Exp Med Biol 1995;395:453–467 [PubMed] [Google Scholar]
- 22.Chien EK, Saunders T, Phillippe M. The mechanisms underlying Bay K 8644-stimulated phasic myometrial contractions. J Soc Gynecol Investig 1996;3:106–112 [PubMed] [Google Scholar]
- 23.Dessì A, Atzori L, Noto A, et al. Metabolomics in newborns with intrauterine growth retardation (IUGR): urine reveals markers of metabolic syndrome. J Matern Fetal Neonatal Med 2011;24(Suppl. 2):35–39 [DOI] [PubMed] [Google Scholar]
- 24.Scioscia M, Kunjara S, Gumaa K, McLean P, Rodeck CH, Rademacher TW. Urinary excretion of inositol phosphoglycan P-type in gestational diabetes mellitus. Diabet Med 2007;24:1300–1304 [DOI] [PubMed] [Google Scholar]