Abstract
OBJECTIVE
The influence of diabetes on cardiac resynchronization therapy (CRT) remains unclear. The aims of the current study were to 1) assess the changes in left ventricular (LV) systolic and diastolic function and 2) evaluate long-term prognosis in CRT recipients with diabetes.
RESEARCH DESIGN AND METHODS
A total of 710 CRT recipients (171 with diabetes) were included from an ongoing registry. Echocardiographic evaluation, including LV systolic and diastolic function assessment, was performed at baseline and 6-month follow-up. Response to CRT was defined as a reduction of ≥15% in LV end-systolic volume (LVESV) at the 6-month follow-up. During long-term follow-up (median = 38 months), all-cause mortality (primary end point) and cardiac death or heart failure hospitalization (secondary end point) were recorded.
RESULTS
At the 6-month follow-up, significant LV reverse remodeling was observed both in diabetic and non-diabetic patients. However, the response to CRT occurred more frequently in non-diabetic patients than in diabetic patients (57 vs. 45%, P < 0.05). Furthermore, a significant improvement in LV diastolic function was observed both in diabetic and non-diabetic patients, but was more pronounced in non-diabetic patients. The determinants of the response to CRT among diabetic patients were LV dyssynchrony, ischemic cardiomyopathy, and insulin use. Both primary and secondary end points were more frequent in diabetic patients (P < 0.001). Particularly, diabetes was independently associated with all-cause mortality together with ischemic cardiomyopathy, renal function, LVESV, LV dyssynchrony, and LV diastolic dysfunction.
CONCLUSIONS
Heart failure patients with diabetes exhibit significant improvements in LV systolic and diastolic function after CRT, although they are less pronounced than in non-diabetic patients. Diabetes was independently associated with all-cause mortality.
Diabetes and heart failure (HF) are two major health care problems with worldwide growing prevalence and incidence (1,2). Their interrelationship is largely established (3–5), with diabetes being a well-known risk factor for the development of HF and an important prognostic factor among HF patients (6). The pathophysiological mechanisms underlying the association between diabetes and HF are still unclear but may include a higher risk of atherosclerosis and microvascular dysfunction (7), deposition of interstitial myocardial fibrosis (8), and specific neurohumoral deregulations (9). Any of these alterations may in fact lead to an impaired cardiac contractility (systolic dysfunction) and/or compliance (diastolic dysfunction), which are both independently associated with a significantly higher risk of HF development and long-term mortality (10,11).
Cardiac resynchronization therapy (CRT) is an established therapy in patients with drug-refractory HF and wide QRS duration, providing significant improvement of symptoms, left ventricular (LV) function, and long-term morbidity and mortality. The precise impact of diabetes on the efficacy of CRT still remains controversial (12–16). Therefore, the aim of the current study was to evaluate the potential differences in LV systolic and diastolic function improvement after CRT between diabetic and non-diabetic patients. Furthermore, the influence of diabetes on long-term outcome after CRT was assessed.
RESEARCH DESIGN AND METHODS
Patient population and protocol
A total of 710 consecutive CRT recipients from an ongoing, single-center registry were included in the present analysis (17). Patient data were prospectively collected in the departmental Cardiology Information System (EPD-Vision, Leiden University Medical Center) and retrospectively analyzed. Patients were selected for CRT according to the presence of LV ejection fraction (LVEF) ≤35%, HF symptoms despite optimal medical therapy, and a QRS duration ≥120 ms (18). The etiology of HF was considered ischemic in the presence of significant coronary artery disease (>50% stenosis in one or more major epicardial coronary arteries) on coronary angiography and/or a history of myocardial infarction or previous revascularization. Patients with recent myocardial infarction (<3 months) or decompensated HF were excluded. During the implantation procedure, LV lead position was assessed on fluoroscopy, as previously described (19).
All patients underwent extensive clinical evaluation and transthoracic two-dimensional echocardiography assessment at baseline and 6 months after CRT. The relation between the presence of diabetes and the effect of CRT on LV systolic and diastolic function at the 6-month follow-up, as well as the clinical outcome during long-term follow-up after CRT, was evaluated.
Definition of diabetes
Diabetes was defined as treated or presently diagnosed glucose intolerance, according to the World Health Organization criteria (fasting blood glucose ≥7.0 mmol/L, or 2-h oral glucose tolerance test glucose ≥11.1 mmol/L) (20). According to the American Diabetes Association criteria, diabetic patients were stratified as having type 1 or type 2 diabetes (21). Patients with exogenous insulin use as the cornerstone of their glycemic control regimen were classified as insulin dependent. HbA1c, a marker of glycemic control level, was measured and expressed according to the International Federation of Clinical Chemistry and Laboratory Medicine in mmol/mol units.
Clinical evaluation
Clinical status was evaluated at baseline and 6-month follow-up, including the New York Heart Association (NYHA) functional class, quality-of-life score according to the Minnesota Living with Heart Failure Questionnaire (higher scores indicate poorer quality of life), and exercise capacity by 6-min walk test (22,23). The renal function was evaluated with the glomerular filtration rate (GFR) according to an equation from Cockcroft and Gault (24).
Echocardiographic evaluation
Echocardiographic studies were performed with patients in the left lateral decubitus position, using a commercially available ultrasound system (Vivid-7 and Vivid-E9; GE Vingmed Ultrasound, Horten, Norway) equipped with a 3.5-MHz transducer. Complete two-dimensional, color, pulsed, and continuous wave Doppler images were acquired according to standard techniques and digitally stored for offline analysis in cine-loop format (EchoPac 110.0.0; GE Vingmed Ultrasound). LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and LVEF were measured from the apical two- and four-chamber views according to the biplane Simpson rule (25). LV volumes were indexed by body surface area and noted as LVEDVi and LVESVi, respectively. Patients with a reduction of ≥15% in LVESV at the 6-month follow-up were considered responders to CRT, whereas patients who died before the 6-month follow-up, or who did not show a reduction of ≥15%, were classified as nonresponders (26).
Mitral regurgitation severity was determined semiquantitatively from color Doppler images obtained from the conventional parasternal long-axis and apical views (27). LV dyssynchrony was quantified using color-coded tissue Doppler imaging (TDI) as the maximum delay between peak systolic velocities among the four basal segments (septal, lateral, anterior, and inferior). A delay of ≥65 ms was defined as substantial LV dyssynchrony (28).
The presence of an impaired LV relaxation (LV diastolic dysfunction) was evaluated according to current recommendations, using transmitral flow Doppler velocities and TDI-derived mitral annular velocities (29,30). In particular, transmitral early (E) and late (A) diastolic velocities and the E-wave deceleration time were measured using the apical four-chamber view with a 2-mm sample volume at the tips of the mitral leaflets. An E/A ratio ≥2 and a shorter E-wave deceleration time identify an LV-restrictive filling pattern. Using TDI, the peak early diastolic myocardial velocities at septal and lateral borders of the mitral annulus were measured and averaged to calculate the mean early diastolic myocardial velocities (E′). The E/E′ ratio was therefore derived as a measure of LV filling pressures; a higher E/E′ ratio is associated with increased LV filling pressures. LV diastolic dysfunction was therefore graded (grade I, II, and III) according to a multiparametric approach, including E/A ratio, E-wave deceleration time, and average E/E′ based on current guidelines (29).
Long-term follow-up and definition of end points
The long-term follow-up was performed by medical chart review, outpatient clinical visits, and telephone contact. The primary end point was all-cause mortality, and the secondary end point was HF hospitalization, heart transplantation, or cardiac death (whichever came first). Cardiac deaths were classified as sudden cardiac death or death due to decompensated HF or other cardiac causes.
Statistical analysis
Results are presented as mean ± SD for continuous variables and as numbers and percentages for dichotomous data. Independent Student t tests were used to compare continuous variables, and χ2 tests were used to compare categorical variables. The differences at the 6-month follow-up within and between the patient groups were compared by repeated-measures ANOVA, including the interaction between group and time. The Wilcoxon signed rank test was used to test the change in nonparametric paired samples. In diabetic patients, univariable (binary) logistic regression identified the variables that are associated with the response to CRT. A multivariable (binary) logistic regression was performed with relevant or statistically significant (P < 0.05) clinical, echocardiographic, and diabetes-related variables to identify the independent predictors of response to CRT in diabetic patients. Survival was evaluated by the Kaplan-Meier method, and the effect of diabetes on survival was evaluated with the Cox proportional hazards model. All relevant clinical and echocardiographic variables were included, and the variables that showed a statistically significant effect (P < 0.05) in the univariable analysis were entered in the multivariable Cox proportional hazards model. In the case of colinearity of the variables, only one of these variables was entered in the multivariable model. All statistical tests were two sided, and for all tests, a P value <0.05 was considered statistically significant. Windows PASW Statistics software (SPSS version 18.0; PASW Statistics, Chicago, IL) was used for data analyses.
RESULTS
Patient population
The study population consisted of 710 consecutive patients (536 men, mean age = 66 ± 10 years). All patients received optimal medical treatment, and echocardiography showed dilated LV (LVEDVi = 113 ± 40 mL/m2; LVESVi = 86 ± 37 mL/m2) with depressed LVEF (25 ± 8%) (Table 1).
Table 1.
Baseline characteristics of the study population, comparing patients with diabetes (DM) and without diabetes (non-DM)
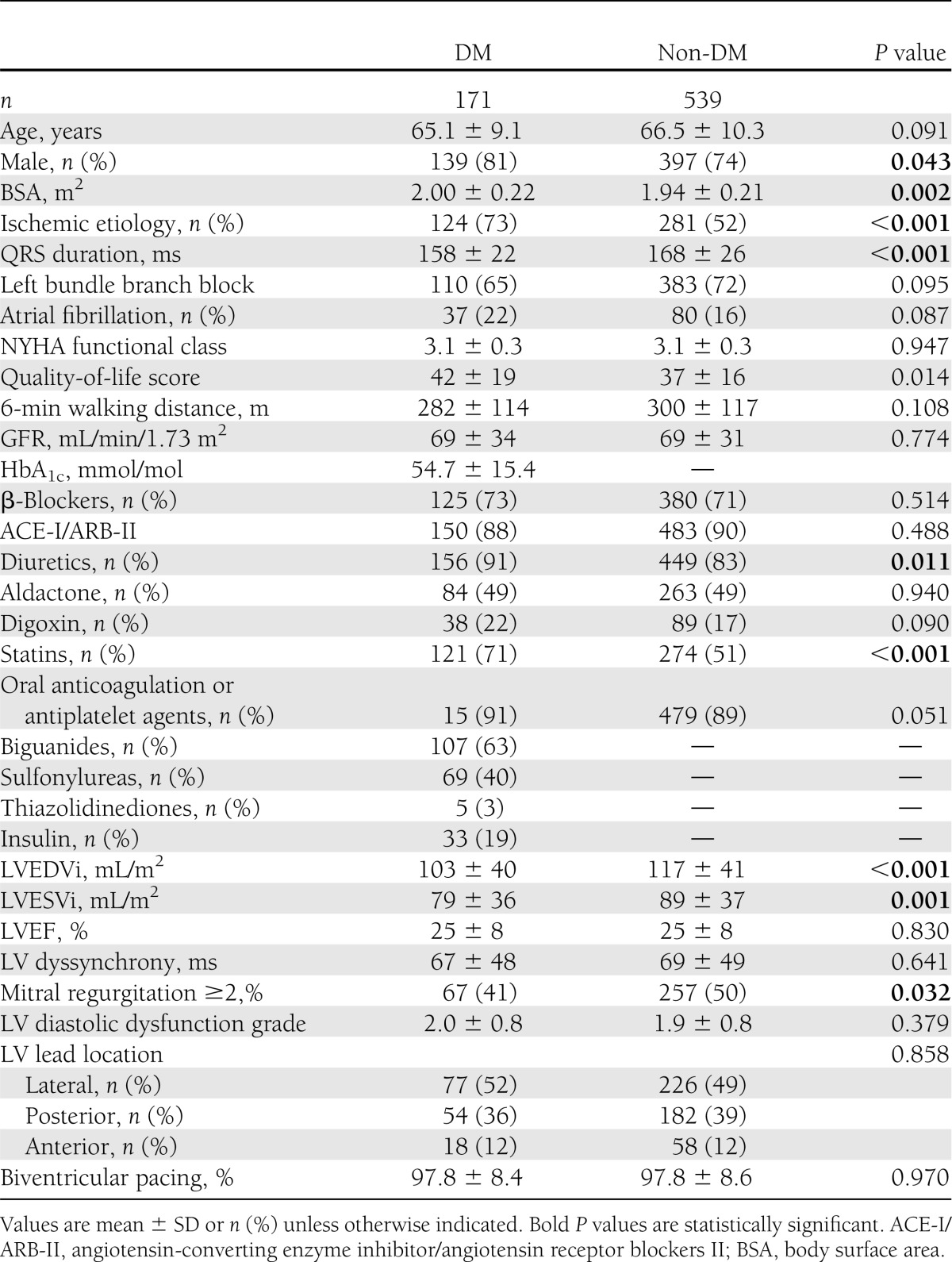
Diabetes was present in 171 (24%) patients, with the majority having type 2 diabetes (158 patients, 93%). Exogenous insulin use was present in 33 (19%) patients, and dietary restrictions or oral antidiabetics were recorded in 138 (81%) patients. Baseline characteristics of diabetic and non-diabetic patients were compared and summarized in Table 1. Patients with diabetes were more likely to have lower quality of life, have ischemic etiology of cardiomyopathy, and use diuretics and statins. In addition, diabetic patients had shorter mean QRS durations, smaller LV volumes (indexed), and lower degrees of mitral regurgitation, as compared with non-diabetic patients. The degree of LV diastolic dysfunction was comparable between the two groups; in particular, LV diastolic dysfunction grade II was observed in 43% of diabetic patients and 39% of non-diabetic patients, and LV diastolic dysfunction grade III was present in 28% of diabetic and 27% of non-diabetic patients (P = 0.514). Of interest, the distribution of the LV lead position was similar among the two groups.
Diabetes and clinical and echocardiographic changes after CRT
At 6 months follow-up, all evaluated parameters improved significantly in the entire population. NYHA functional class improved from 3.1 ± 0.3 to 2.1 ± 0.7 (P < 0.001), 6-min walk test distance increased from 301 ± 113 to 374 ± 129 m (P < 0.001), and quality-of-life score decreased from 38 ± 17 to 25 ± 18 (P < 0.001). In addition, LVEDVi decreased from 113 ± 40 to 102 ± 39 mL/m2 (P < 0.001), LVESVi decreased from 86 ± 37 to 72 ± 34 mL/m2 (P < 0.001), and consequently, LVEF increased from 25 ± 8 to 31 ± 9% (P < 0.001). In particular, a total of 371 patients (53%) were classified as responders to CRT. Furthermore, the percentage of mitral regurgitation ≥2 in the studied population decreased significantly as compared with the baseline, from 47 to 32% (P < 0.001). Also, LV diastolic function improved at 6 months follow-up in the entire patient population. The E/E′ ratio decreased from 21 ± 13 to 17 ± 12 cm/s (P < 0.001), and the diastolic dysfunction grade decreased from 1.9 ± 0.8 to 1.4 ± 0.9 (P < 0.001) among all CRT recipients.
Comparisons of clinical and echocardiographic data between diabetic and non-diabetic patients, during follow-up after CRT, are displayed in Table 2. At 6 months follow-up, similar improvements in clinical characteristics were observed among diabetic and non-diabetic patients. Furthermore, significant LV reverse remodeling was observed in both groups, but the reduction in LVEDVi and LVESVi was more pronounced in non-diabetic patients as compared with diabetic patients (interaction group and time P values in Table 2). In particular, the percentage of response to CRT was lower in the diabetic patients as compared with non-diabetic patients (45 vs. 57%, P = 0.017). LV dyssynchrony before implantation was comparable between the two groups, and the degree of resynchronization (reduction in LV dyssynchrony) was similar between diabetic and non-diabetic patients (67 ± 48 to 37 ± 36 ms in diabetes vs. 69 ± 49 to 38 ± 34 ms, interaction group and time P = 0.656) (Table 2).
Table 2.
Changes in clinical and echocardiographic variables after 6 months' CRT in HF patients with diabetes (DM) and without diabetes (non-DM)

Furthermore, the improvement in LV diastolic function at 6 months follow-up was more pronounced among non-diabetic patients compared with diabetic patients, including the measure of E′, E/E′, E-wave deceleration time, and overall LV diastolic dysfunction grade (interaction group and time P value in Table 2). In particular, the percentage of patients with LV diastolic dysfunction grade III decreased from 28 to 16% among diabetic patients (P < 0.001) and from 27 to 13% among non-diabetic patients (P < 0.001), and the percentage of patients with LV diastolic dysfunction grade II decreased from 43 to 38% among diabetic patients (P < 0.001) and from 39 to 28% among non-diabetic patients (P < 0.001). Of interest, 20% of non-diabetic patients normalized LV diastolic function, compared with 10% of diabetic patients (P < 0.001).
In order to identify potential characteristics that might have an impact on LV reverse remodeling after CRT among diabetic patients, a logistic regression analysis was performed to predict response to CRT. LV dyssynchrony (odds ratio 3.950 [95% CI 1.851–8.430], P < 0.001), ischemic etiology (0.409 [0.172–0.970], P = 0.043), and insulin use (0.388 [0.123–0.931], P = 0.036) were independent predictors of echocardiographic response to CRT in diabetic patients. Furthermore, none of the other tested parameters in the logistic regression analysis (age, sex, atrial fibrillation, QRS duration, percentage of biventricular pacing, GFR, HbA1c, biguanide use, sulfonylurea use, thiazolidinedione use, LVEDVi, LVESVi, LVEF, and LV diastolic dysfunction) independently predicted the response to CRT (see online appendix for univariable and multivariable analysis of predictors of response to CRT among diabetic patients).
Diabetes and long-term prognosis after CRT
During a median follow-up of 38 months (interquartile range 22–64 months), the primary end point of all-cause mortality was recorded in 255 (36%) patients. Cardiac death occurred in 160 (63%) patients, including decompensated HF in 132 (83%), sudden cardiac death in 15 (9%), and other cardiac causes in 13 (8%). Cardiac death was more frequently observed among diabetic patients (75 vs. 56%, P = 0.004) compared with non-diabetic patients and was mainly caused by decompensated HF (89 vs. 78%, P < 0.001). Additionally, six patients (2%) underwent heart transplantation, and HF hospitalizations were recorded in 100 (14%) patients.
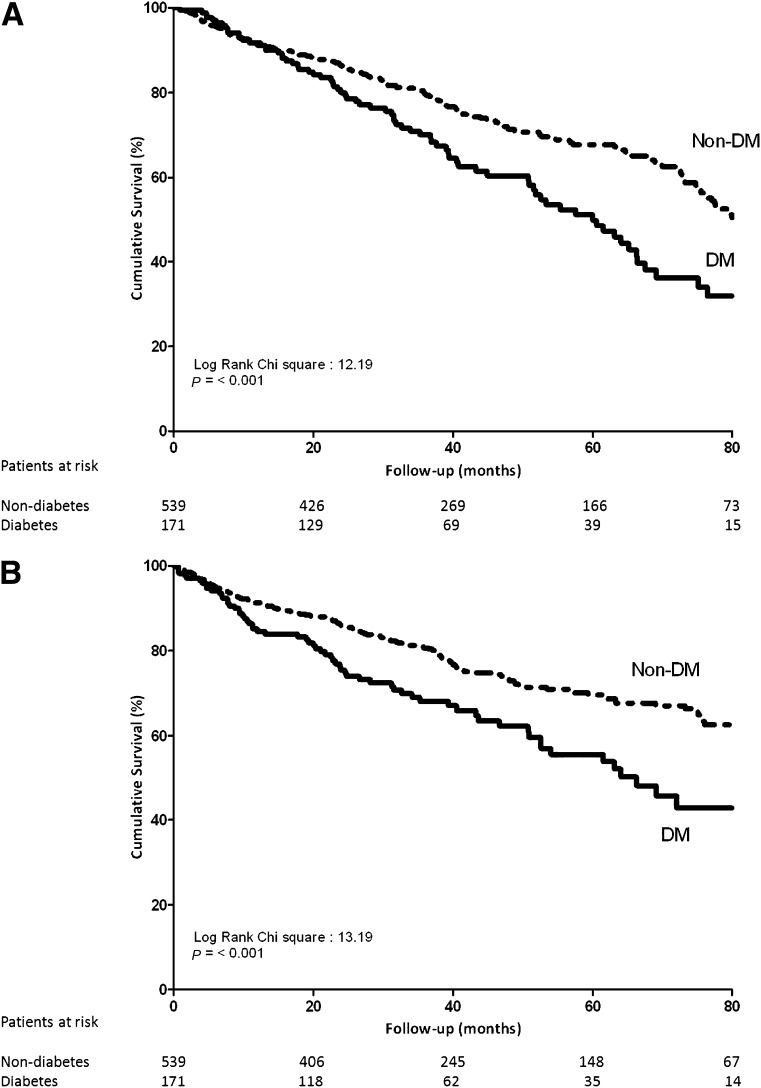
The overall survival (primary end point) was worse in diabetic versus non-diabetic patients. The Kaplan-Meier curves show a significant survival difference between the two groups from the third year after implantation (log rank P = 0.001) (Fig. 1A). In particular, respective 3- and 5-year survival rates were 79% (95% CI 76–83%) and 68% (63–73%) in non-diabetic patients compared with 70% (63–78%) and 50% (40–59%) in diabetic patients.
Figure 1.
A: Kaplan-Meier survival curves for the time to all-cause mortality (primary end point) in diabetic versus non-diabetic patients. B: Kaplan-Meier survival curves for the time to cardiac death and heart failure hospitalization (secondary end point) in diabetic (DM) versus non-diabetic (non-DM) patients.
Similarly, as shown in Fig. 1B, the secondary end point of HF hospitalizations and cardiac death was more frequent in diabetic patients when compared with non-diabetic patients (χ2 = 13.19, log rank P < 0.001).
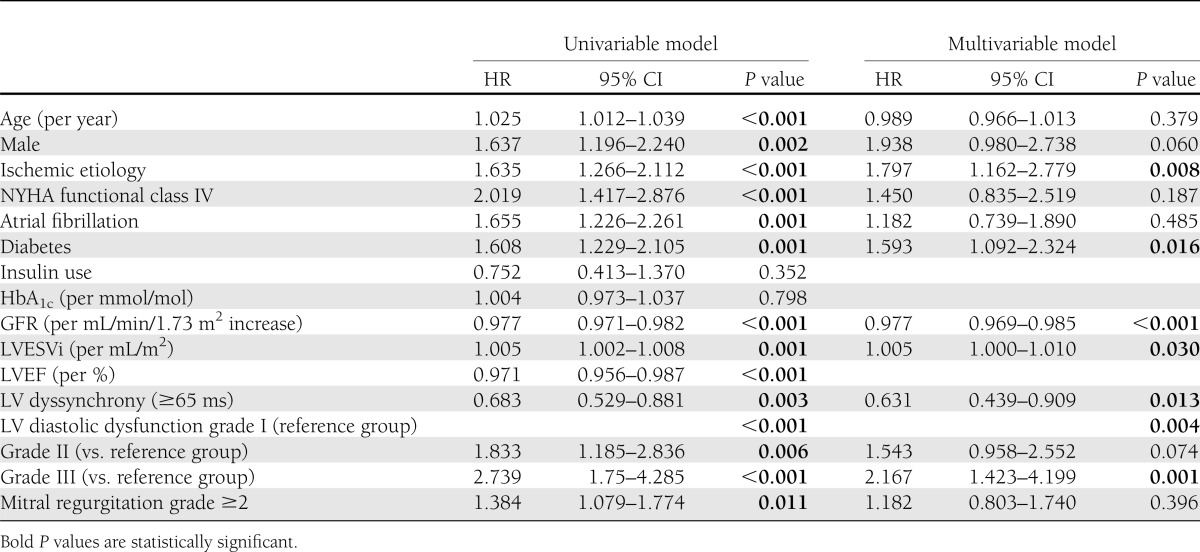
Finally, diabetes was tested as independent predictor of mortality using the Cox proportional hazards model. After adjusting for age, sex, ischemic etiology, NYHA functional class, presence of atrial fibrillation, GFR, LVESVi, significant LV dyssynchrony, LV diastolic dysfunction grade, and mitral regurgitation ≥2, diabetes remained as a strong independent predictor of all-cause mortality (hazard ratio [HR] 1.593 [95% CI 1.092–2.324], P = 0.016) (Table 3) together with ischemic etiology, renal dysfunction, LVESVi, lack of significant LV dyssynchrony, and LV diastolic dysfunction grade.
Table 3.
Univariable and multivariable Cox proportional hazards model for all-cause mortality in the overall population
CONCLUSIONS
The key findings of this study are as follows: 1) A significant improvement in LV systolic function was observed both in diabetic and non-diabetic patients after CRT, but was more pronounced in non-diabetic patients; 2) the improvement in LV diastolic dysfunction after CRT was more pronounced among non-diabetic patients than in diabetic patients; and 3) the long-term outcome after CRT was superior in non-diabetic patients when compared with diabetic patients, with diabetic as independent predictor of all-cause mortality in CRT recipients.
Impact of diabetes on response to CRT: LV dimensions and systolic function
The beneficial effect of CRT on LV remodeling and function has been widely reported (31,32). Specific analyses exploring the influence of diabetes on LV reverse remodeling and improvement of function after CRT have also been performed but provided contradictory results. Initial single-center studies, with small patient populations, reported more pronounced improvement in LV function after CRT among non-diabetic versus diabetic patients (12,16). Conversely, subanalyses from CRT clinical trials showed similar improvements in LV performance in diabetic and non-diabetic patients (13–15). However, these results are difficult to translate outside the setting of the clinical trials, i.e., when patients are not selected according to specific inclusion criteria and are less closely monitored. In the current tertiary referral hospital registry, CRT resulted in significant LV function improvement both in diabetic and non-diabetic patients. However, LV reverse remodeling was more pronounced in non-diabetic patients. This finding could not be related to the degree of resynchronization, since the reduction in LV dyssynchrony was similar between the two groups. However, several potential diabetes-related pathophysiological mechanisms might contribute to the relatively limited LV reverse remodeling in diabetic patients: 1) higher incidence of coronary artery disease and therefore a larger myocardial scar burden and recurrence of ischemia, 2) reduced microvascular blood flow (33), 3) increased myocardial fat and interstitial fibrotic tissue content (8,34), 4) advanced glycation end product deposition (11), and 5) neurohumoral and autonomic functional changes (9).
To explore which baseline characteristics might help to predict the response to CRT specifically among diabetic patients, the association of glycemic control level and insulin use with LV reverse remodeling after CRT was evaluated, together with other important clinical and echocardiographic variables. Previous studies suggested that exogenous insulin use, a well-known predictor of HF (5,13), may play an important role in the myocardial compensatory capacity. Decreased insulin availability can impair the energy-independent transport of glucose across the cell membrane, resulting in a shift toward fatty acid metabolism and increased myocardial oxygen utilization (34). Results from the multivariable analysis in the current study suggested that insulin use, LV dyssynchrony, and ischemic etiology were independent predictors of the response to CRT in diabetic patients, whereas the level of glycemic control prior to CRT implantation influenced the response to CRT. In line with this observation, data from the Cardiac Resynchronization in Heart Failure (CARE-HF) trial revealed that insulin use was predictive of all-cause mortality in CRT recipients (13). Furthermore, insulin use in a population with a high prevalence of type 2 diabetes probably reflects a long history of diabetes and/or poorly controlled diabetes under oral antidiabetic medication.
Impact of diabetes on response to CRT: LV diastolic function
LV diastolic dysfunction is common in diabetic patients irrespective of the LV systolic function and is significantly associated with a poor prognosis and higher mortality rates (10,11). The mechanisms responsible for the increased myocardial stiffness among these patients might be relative myocardial hypertrophy and, more importantly, myocardial deposition of collagen and advanced glycation end products (11).
The contribution of LV diastolic dysfunction to the development of cardiomyopathy in diabetic patients is well known, but so far, no studies have specifically focused on the changes in LV diastolic function after CRT in patients with diabetes. In the current study, a significant improvement in LV diastolic function after CRT was observed both in diabetic and non-diabetic patients, but this improvement was more pronounced in non-diabetic patients. The magnitude of improvement in LV diastolic function might partially be related to the extent of LV reverse remodeling after CRT (35). In addition, significant LV diastolic dysfunction might persist despite an improvement in LV systolic function. Sustained LV diastolic dysfunction may have a significant impact on exercise capacity (15,16) and, most importantly, on long-term outcome.
Impact of diabetes on long-term prognosis after CRT
Conflicting results on the long-term survival benefit after CRT in diabetic patients have been reported. The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial reported similar survival in diabetic and non-diabetic patients (13,36). In contrast, the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) (14) demonstrated that diabetic patients had a worse outcome with higher all-cause mortality and more HF hospitalization rates (14). The results of the current study also suggest a worse prognosis in CRT recipients with diabetes than non-diabetic patients (17,37). Moreover, the current study extensively explored the potential predictors of long-term outcome, including clinical and echocardiographic characteristics. Diabetes was an independent determinant of all-cause mortality, together with renal function, LVESVi, LV dyssynchrony, and LV diastolic dysfunction grade III. Similarly, the recent data obtained in a large CRT registry demonstrated that HbA1c as an expression of poorly regulated glycemic control was predictive of a worse outcome at short-term follow-up after CRT (38). In contrast, at longer-term follow-up, the glycemic control may significantly change over time, and therefore, this variable may no longer influence the long-term outcome, as observed in the current study.
Study limitations
Data on the mean duration of diabetes prior to the implantation, mean duration of insulin therapy, or changes in antidiabetic regimen during follow-up were not systematically available. This information could have allowed a more accurate analysis of the impact of glycemic control on outcome after CRT in diabetic patients.
Conclusion
Diabetic patients experienced a significant benefit from CRT in terms of functional parameters and LV diastolic and systolic function. However, the magnitude of LV reverse remodeling and LV diastolic function improvement was less pronounced in diabetic patients as compared with non-diabetic patients. Furthermore, the presence of diabetes was independently associated with an increased risk of all-cause mortality.
Supplementary Material
Acknowledgments
The Department of Cardiology of Leiden University Medical Center received unrestricted research grants from Medtronic, Biotronik, Boston Scientific, Lantheus Medical Imaging, St. Jude Medical, Edwards Lifesciences, and GE Healthcare. V.D. received consulting fees from St. Jude Medical and Medtronic. No other potential conflicts of interest relevant to this article were reported.
U.H. collected, researched, and interpreted the data and drafted and edited the manuscript. J.T., R.J.v.B., and E.T.v.d.V. collected data and reviewed and edited the manuscript. L.v.E. and E.R.H. reviewed and edited the manuscript. M.J.S., J.J.B., V.D., and N.A.M. interpreted the data, contributed to discussion, and reviewed and edited the manuscript. N.A.M. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1116/-/DC1.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011;123:e18–e209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974;34:29–34 [DOI] [PubMed] [Google Scholar]
- 4.Soläng L, Malmberg K, Rydén L. Diabetes mellitus and congestive heart failure. Further knowledge needed. Eur Heart J 1999;20:789–795 [DOI] [PubMed] [Google Scholar]
- 5.Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care 2001;24:1614–1619 [DOI] [PubMed] [Google Scholar]
- 6.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 2001;161:996–1002 [DOI] [PubMed] [Google Scholar]
- 7.Shehadeh A, Regan TJ. Cardiac consequences of diabetes mellitus. Clin Cardiol 1995;18:301–305 [DOI] [PubMed] [Google Scholar]
- 8.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol 2006;47:693–700 [DOI] [PubMed] [Google Scholar]
- 9.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation 2007;116:434–448 [DOI] [PubMed] [Google Scholar]
- 10.From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol 2010;55:300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 2008;117:43–51 [DOI] [PubMed] [Google Scholar]
- 12.Soliman OI, van Dalen BM, Theuns DA, et al. The ischemic etiology of heart failure in diabetics limits reverse left ventricular remodeling after cardiac resynchronization therapy. J Diabetes Complications 2009;23:365–370 [DOI] [PubMed] [Google Scholar]
- 13.Hoppe UC, Freemantle N, Cleland JG, Marijianowski M, Erdmann E. Effect of cardiac resynchronization on morbidity and mortality of diabetic patients with severe heart failure. Diabetes Care 2007;30:722–724 [DOI] [PubMed] [Google Scholar]
- 14.Martin DT, McNitt S, Nesto RW, Rutter MK, Moss AJ. Cardiac resynchronization therapy reduces the risk of cardiac events in patients with diabetes enrolled in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT-CRT). Circ Heart Fail 2011;4:332–338 [DOI] [PubMed] [Google Scholar]
- 15.Fantoni C, Regoli F, Ghanem A, et al. Long-term outcome in diabetic heart failure patients treated with cardiac resynchronization therapy. Eur J Heart Fail 2008;10:298–307 [DOI] [PubMed] [Google Scholar]
- 16.Mangiavacchi M, Gasparini M, Genovese S, et al. Insulin-treated type 2 diabetes is associated with a decreased survival in heart failure patients after cardiac resynchronization therapy. Pacing Clin Electrophysiol 2008;31:1425–1432 [DOI] [PubMed] [Google Scholar]
- 17.van Bommel RJ, Borleffs CJ, Ypenburg C, et al. Morbidity and mortality in heart failure patients treated with cardiac resynchronization therapy: influence of pre-implantation characteristics on long-term outcome. Eur Heart J 2010;31:2783–2790 [DOI] [PubMed] [Google Scholar]
- 18.Dickstein K, Vardas PE, Auricchio A, et al. ESC Committee for Practice Guidelines (CPG) 2010 focused update of ESC guidelines on device therapy in heart failure: an update of the 2008 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur Heart J 2010;31:2677–2687 [DOI] [PubMed] [Google Scholar]
- 19.Albertsen AE, Nielsen JC, Pedersen AK, Hansen PS, Jensen HK, Mortensen PT. Left ventricular lead performance in cardiac resynchronization therapy: impact of lead localization and complications. Pacing Clin Electrophysiol 2005;28:483–488 [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications Geneva, World Health Org., 1999
- 21.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20(Suppl. 1):1183–1197 [DOI] [PubMed] [Google Scholar]
- 22.Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol 1993;71:1106–1107 [DOI] [PubMed] [Google Scholar]
- 23.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919–923 [PMC free article] [PubMed] [Google Scholar]
- 24.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41 [DOI] [PubMed] [Google Scholar]
- 25.Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group. American Society of Echocardiography’s Guidelines and Standards Committee. European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–1463 [DOI] [PubMed] [Google Scholar]
- 26.Bleeker GB, Bax JJ, Fung JW, et al. Clinical versus echocardiographic parameters to assess response to cardiac resynchronization therapy. Am J Cardiol 2006;97:260–263 [DOI] [PubMed] [Google Scholar]
- 27.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. American Society of Echocardiography Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777–802 [DOI] [PubMed] [Google Scholar]
- 28.Bax JJ, Bleeker GB, Marwick TH, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 2004;44:1834–1840 [DOI] [PubMed] [Google Scholar]
- 29.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–193 [DOI] [PubMed] [Google Scholar]
- 30.Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007;28:2539–2550 [DOI] [PubMed] [Google Scholar]
- 31.Cleland JG, Daubert JC, Erdmann E, et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549 [DOI] [PubMed] [Google Scholar]
- 32.St John Sutton MG, Plappert T, Abraham WT, et al. Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Study Group Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 2003;107:1985–1990 [DOI] [PubMed] [Google Scholar]
- 33.Galderisi M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J Am Coll Cardiol 2006;48:1548–1551 [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues B, Cam MC, McNeill JH. Myocardial substrate metabolism: implications for diabetic cardiomyopathy. J Mol Cell Cardiol 1995;27:169–179 [DOI] [PubMed] [Google Scholar]
- 35.Shanks M, Antoni ML, Hoke U, et al. The effect of cardiac resynchronization therapy on left ventricular diastolic function assessed with speckle-tracking echocardiography. Eur J Heart Fail 2011;13:1133–1139 [DOI] [PubMed] [Google Scholar]
- 36.Ghali JK, Boehmer J, Feldman AM, et al. Influence of diabetes on cardiac resynchronization therapy with or without defibrillator in patients with advanced heart failure. J Card Fail 2007;13:769–773 [DOI] [PubMed] [Google Scholar]
- 37.Bai R, Di Biase L, Elayi C, et al. Mortality of heart failure patients after cardiac resynchronization therapy: identification of predictors. J Cardiovasc Electrophysiol 2008;19:1259–1265 [DOI] [PubMed] [Google Scholar]
- 38.Shah RV, Altman RK, Park MY, et al. Usefulness of hemoglobin a(1c) to predict outcome after cardiac resynchronization therapy in patients with diabetes mellitus and heart failure. Am J Cardiol 2012;110:683–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.