Abstract
OBJECTIVE
To check the hypothesis that myo-inositol supplementation may reduce gestational diabetes mellitus (GDM) onset in pregnant women with a family history of type 2 diabetes.
RESEARCH DESIGN AND METHODS
A 2-year, prospective, randomized, open-label, placebo-controlled study was carried out in pregnant outpatients with a parent with type 2 diabetes who were treated from the end of the first trimester with 2 g myo-inositol plus 200 µg folic acid twice a day (n = 110) and in the placebo group (n = 110), who were only treated with 200 µg folic acid twice a day. The main outcome measure was the incidence of GDM in both groups. Secondary outcome measures were as follows: the incidence of fetal macrosomia (>4,000 g), gestational hypertension, preterm delivery, caesarean section, shoulder dystocia, neonatal hypoglycemia, and neonatal distress respiratory syndrome. GDM diagnosis was performed according to the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommendations.
RESULTS
Incidence of GDM was significantly reduced in the myo-inositol group compared with the placebo group: 6 vs. 15.3%, respectively (P = 0.04). In the myo-inositol group, a reduction of GDM risk occurrence was highlighted (odds ratio 0.35). A statistically significant reduction of fetal macrosomia in the myo-inositol group was also highlighted together with a significant reduction in mean fetal weight at delivery. In the other secondary outcome measures, there were no differences between groups.
CONCLUSIONS
myo-Inositol supplementation in pregnant women with a family history of type 2 diabetes may reduce GDM incidence and the delivery of macrosomia fetuses.
Gestational diabetes mellitus (GDM) is defined as carbohydrate intolerance that begins or is first recognized during pregnancy (1). It is associated with an increased risk for the fetus, including macrosomia and birth injuries for shoulder dystocia, and also for the newborn, such as neonatal hypoglycemia, respiratory distress syndrome, and childhood obesity. Maternal risks include caesarean delivery, hypertensive disorders, and an increased risk of developing type 2 diabetes later in life. Recently, the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study, a multicenter, observational study, evaluated the relationship between maternal hyperglycemia and adverse pregnancy outcomes (2). The study demonstrated a clear and continuous relationship between maternal hyperglycemia and increasing rates of large-for-gestational-age infants, fetal hyperinsulinemia, neonatal hypoglycemia, and caesarean delivery. After, the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) published recommendations for the diagnosis and classification of hyperglycemia during pregnancy (3). In accordance with a very recent report (4), these new criteria could increase the number of GDM diagnoses to more than double, particularly in women at risk. Among strategies to reduce the occurrence of GDM in high-risk pregnancies, insulin-sensitizing substances, such as metformin, have been used throughout the pregnancy with contrasting results (5,6). Another substance primarily used in polycystic ovary syndrome (PCOS), with the aim of lowering hyperinsulinemia and restoring ovarian function, was inositol; it was given either in the isomer d-chiro-inositol (7) or in the myo-inositol isomer (8). Inositol is normally present in cereals, corn, legumes, and meat, and the principal organ in which it is synthesized is the liver; consequently, it is considered a supplement. Recently, our group has shown that myo-inositol may reduce insulin resistance markers in women affected by GDM (9); thus, in this pilot study the first aim was to reduce GDM onset by giving myo-inositol from the first trimester in women at risk, in particular to those who have a parent affected by type 2 diabetes.
RESEARCH DESIGN AND METHODS
A prospective, randomized, open-label, placebo-controlled study was performed with the pregnant outpatients attending the Department of Gynecology and Obstetrics, University of Messina. The enrollment started at the beginning of 2010 and lasted 2 years; this period included 220 pregnant Caucasian women with the following inclusion criteria: 1) first-degree relatives (mother, father, or both) affected by type 2 diabetes, 2) prepregnancy BMI <30 kg/m2, 3) fasting plasma glucose <126 mg/dL and random glycemia <200 mg/dL, 4) single pregnancy, and 5) Caucasian race. Exclusion criteria were as followings: 1) prepregnancy BMI ≥30 kg/m2, 2) previous GDM, 3) pregestational diabetes, 4) first-trimester glicosuria, 5) first-degree relative(s) (mother or father) not affected by type 2 diabetes, 6) fasting plasma glucose ≥126 mg/dL or random glycemia ≥200 mg/dL, 7) twin pregnancies, 8) associated therapy with corticosteroids, 9) not Caucasian race, and 10) PCOS women. The main outcome measure was the occurrence of GDM in both groups; secondary outcomes were a prevalence of fetal macrosomia (fetal weight >4,000 g at delivery), caesarean section, gestational hypertension, preterm delivery, shoulder dystocia, respiratory distress syndrome, and neonatal hypoglycemia (<45 mg/dL). According to IADPSG recommendations (3), a diagnosis of GDM was performed with a 75-g, 2-h glucose tolerance test, with cutoff values of ≥92 mg/dL for time 0, ≥180 mg/dL after 1 h, and ≥153 mg/dL after 2 h; at least one of the three values over or equal to the cutoff was enough for diagnosis of GDM. At the time of the recruitment, at 12–13 weeks’ gestation, a computer randomization was used with an allocation of 1:1 in each group. It was an open-label trial: in the treated group, 2 g myo-inositol was given twice a day plus 200 µg folic acid, whereas in the placebo group only 200 µg folic acid was given twice a day. Insulin resistance was calculated by homeostasis model assessment of insulin resistance (HOMA-IR) (10). The protocol was consistent with the principles of the Declaration of Helsinki, and all participants gave written informed consent.
Statistical analysis was carried out with SPSS statistical package version 17 (SPSS, Chicago, IL). Data are expressed as means ± SD for categorical variables. The means of independent groups were compared using Student t test after checking for normal distribution. For analysis of paired data, Student t test was used. For comparison of frequencies, Pearson χ2 test was used or, in the case of small frequencies, Fisher exact test. Odds ratios (ORs) and adjusted ORs (95% CI) (adjusted for maternal age, parity, and prepregnancy BMI) were also calculated. Multiple logistic regression analysis was used to assess the ORs of the independent variables, and 95% CI was calculated as well. A value of P < 0.05 was considered significant.
RESULTS
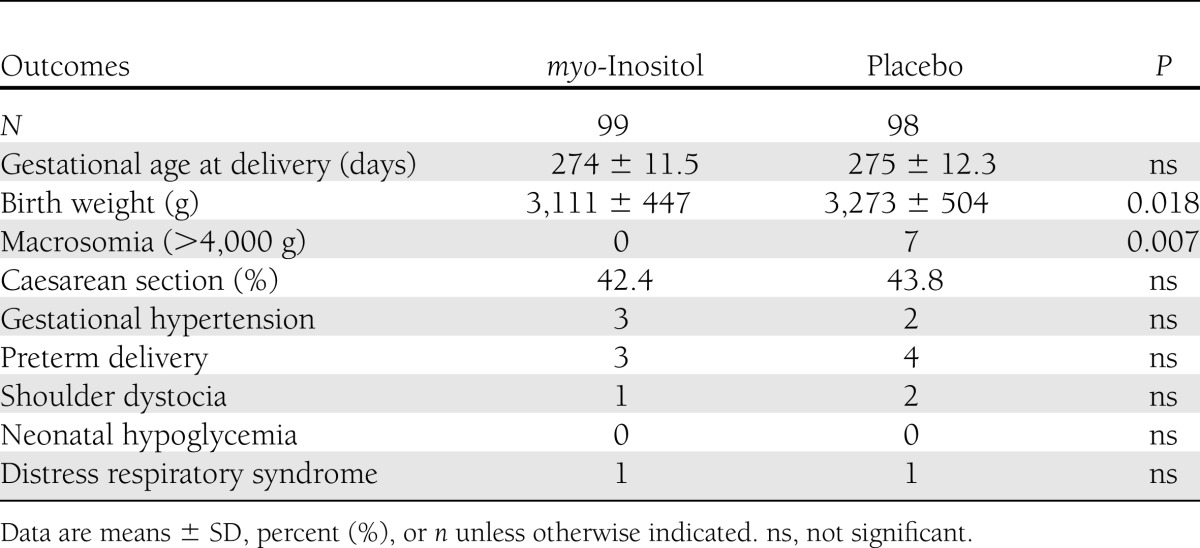
In our center for the screening of GDM, in the last 2 years the prevalence of GDM diagnosis following the IADPSG recommendations (3) was 10.9%. In the myo-inositol group, there was one midtrimester miscarriage, three women delivered in other hospitals (thus, it was impossible to obtain their records), and there were seven dropouts who stopped the treatment for various reasons: four stopped the treatment following their family doctor’s advice, and three were negatively influenced by their relatives. However, no women complained about the side effects of the drug. In the placebo group, there were two midtrimester miscarriages; four women abandoned the trial, not attending the oral glucose tolerance test (OGTT) evaluation; and six delivered in other hospitals. An intention-to-treat analysis was performed, which did not show results different from those of “per protocol analysis”; moreover, we evaluated the clinical characteristics of the patients who abandoned the trial, but no significant differences from those who concluded the trial were found. Therefore, 99 women in the myo-inositol group and 98 in the placebo group completed the trial. The two groups were comparable for maternal age, BMI, percentage of nulliparous women, and insulin resistance calculated with the HOMA-IR in the first trimester (Table 1). First-trimester fasting plasma glucose was <100 mg/dL in all of the participants in the study. Furthermore, the two groups were also comparable for weight increase and gestational age at OGTT (Table 2). A prevalence of GDM, the main outcome measure, was significantly reduced in the myo-inositol group (6 case subjects) compared with the placebo group (15 case subjects). This difference was significant (P = 0.04) even after adjustment for previously reported confounding factors: maternal age, prepregnancy BMI, and parity [β = 0.37 (95% CI 0.32–0.93), P = 0.03]. Assessing OR in the myo-inositol group, we registered a reduction of the risk for GDM of ~65% [OR 0.35 (0.13–0.96)]. In the myo-inositol group, all of the GDM case subjects were treated only by diet; in the placebo group, all were treated by diet but there was one who needed insulin. A significant difference was also highlighted in glycemia at the OGTT either at basal values (P = 0.001) or in the first hour (P = 0.02); instead, no difference after a 2-h glycemia OGTT was shown (Table 2). For the other outcome measures, there was a significant difference in mean birth weight between groups in comparison of gestational age at delivery (Table 3). Seven fetuses weighed >4,000 g, and they were all in the placebo group, whereas there were none in the myo-inositol group (Table 3). There was no difference between groups in cases of gestational hypertension, preterm deliveries, caesarean section, neonatal hypoglycemia, and percentage of neonatal distress respiratory syndrome (Table 3). Of the two cases of shoulder dystocia in the placebo group, one occurred with a macrosomia; however, all the cases were without consequence for the neonates. A logistic regression analysis was performed, with GDM diagnosis as dependent variable, myo-inositol supplementation as an independent variable, and maternal age, prepregnancy BMI, and parity as covariates. This model showed that both myo-inositol supplementation and prepregnancy BMI independently affected the onset of GDM [β = 0.37 (0.116–0.935) and 0.289 (0.11–0.758), respectively].
Table 1.
Characteristics of both groups at the beginning of the study
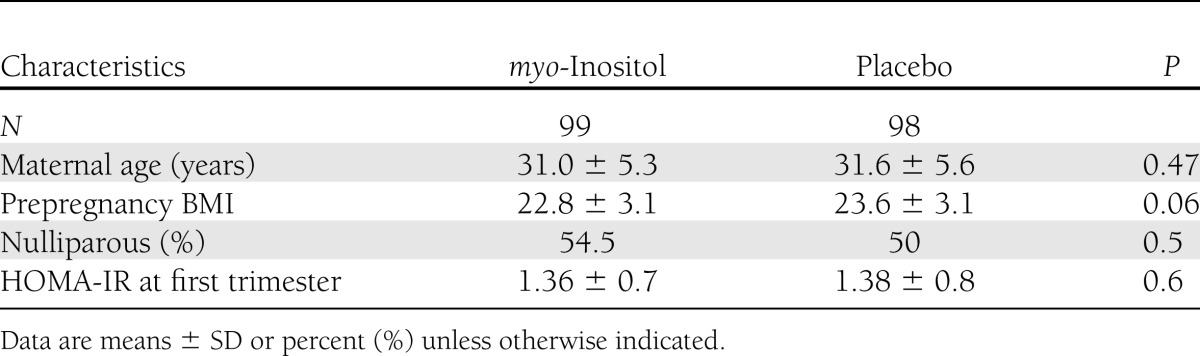
Table 2.
OGTT evaluation in both groups
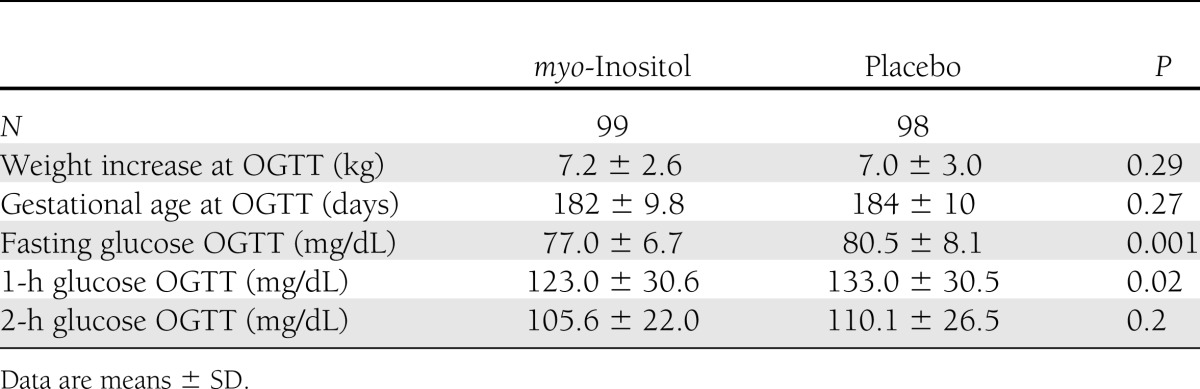
Table 3.
Secondary outcomes in both groups
CONCLUSIONS
Recent reports have supported the involvement of inositol in the mechanisms of glycemic control; in particular, Scioscia et al. (11) showed an increased urinary excretion of inositolphosphoglycan in women affected by GDM, which was positively correlated with blood glucose levels. The authors concluded that inositolphosphoglycan may play a role not only in glycemic control but also in the fetal growth of GDM women. Furthermore, our previous experiences with myo-inositol justified the rationale of this study. In fact, we have demonstrated that myo-inositol may reduce insulin resistance by ~70% in postmenopausal women affected by the metabolic syndrome (12,13), and in pregnant women we have shown how insulin resistance may be significantly reduced in GDM women (9). Recently, in a retrospective small study carried out in pregnant women affected by PCOS, we highlighted how myo-inositol intake, through the whole pregnancy, may reduce the prevalence of GDM (14). Similar studies have been carried out with metformin in pregnant women with a history of PCOS, but the results were conflicting between those who reported a significant reduction of GDM incidence (5,15) and those who failed (6). However, it is worth noting that only the multicenter study by Vanky et al. (6) was a prospective, randomized, controlled trial, in which no metformin effect in preventing GDM was found. Thus, we designed this pilot study in which myo-inositol was used from the end of the first trimester through the whole pregnancy in women at risk for GDM because of a family history of type 2 diabetes, with the aim of reducing its incidence. Family history has been recognized as a significant risk factor, with an OR of 7 in an Australian study (16), even if in a recent trial its importance was considered questionable (4). We chose not to include other important risk factors such as obesity and a previous GDM because they may heavily affect the results, introducing other variables that would not have allowed us a clear interpretation of the data. In fact, in our study prepregnancy BMI was the only maternal variable that independently affected the onset of GDM.
As previously reported, in our center for the screening of GDM in the last 2 years the prevalence of GDM diagnosis following the IADPSG recommendations (3) was 10.9%. Thus, it is not strange that in a group of women at risk, like those with parents affected by type 2 diabetes, GDM prevalence was ~15.3%, which was the value reported in the placebo group; this value is almost 30% more than that in the unselected population. On the other hand, myo-inositol seems to reduce by 40% the incidence of GDM in this group at risk, to a level also under the mean level of the unselected population, and worth noting is the risk of GDM occurrence, which decreased by 65% in the myo-inositol group. According to the multivariate analysis, this result is not a case; it was shown that myo-inositol supplementation independently and significantly affected the incidence of GDM. Furthermore, myo-inositol partially achieved secondary outcomes, too. The most important results were the significant reduction in mean birth weight and the incidence of macrosomia, which was absent in the myo-inositol group. Significant differences in mean glycemia values at OGTT were reported only for fasting and 1-h glycemia, which, for GDM diagnosis, are the most significant ones. In fact, according to the IADPSG panel criteria (3), in the HAPO study the majority of the women were diagnosed on the basis of fasting and 1-h glycemia. This is the most important proof concerning the insulin-like effect of a substance that is considered a supplement; the reduced birth weight in the myo-inositol group compared with the placebo group is only the consequence of glycemia myo-inositol reduction. Insulin resistance in pregnancy is the means by which the placenta protects the fetus growth and development from famine and hunger, which have been so frequent in human history. But in our Western countries, the problem now is an excess of food; thus, what should have protected the fetus is now becoming an insidious boomerang for the mother, with the result of a growing number of GDM diagnoses and overweight fetuses. As for the other secondary outcomes, we failed to demonstrate a positive effect of myo-inositol on the incidence of gestational hypertension, preterm delivery, caesarean section, and neonatal distress respiratory syndrome, but probably the number of people enrolled was too small to achieve significant differences. Furthermore, it is worth noting that no cases of hypoglycemia occurred in any of the participants of the study.
In conclusion, this is the first report on myo-inositol supplementation preventing GDM occurrence in women with only a family history of type 2 diabetes; a reduced incidence of GDM and fetal macrosomia in this selected group of women at risk is good news, even if larger studies are needed to confirm this preliminary report.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
R.D. designed the study and wrote the manuscript. A.S. and D.G. contributed to the discussion. C.C., M.L.C., and M.L.I. researched data. F.C. edited the manuscript. A.D.B. performed statistical analysis and edited the manuscript. R.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Basilio Pintaudi (Department of Clinical Pharmacology and Epidemiology, Consorzio Mario Negri Sud, S. Maria Imbaro, Chieti, Italy) for critical revision of the statistics.
Footnotes
Clinical trial reg. no. NCT01047982, clinicaltrials.gov.
See accompanying commentary, p. 777.
References
- 1.American College of Obstetricians and Gynecologists Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol 2001;98:525–538 [PubMed] [Google Scholar]
- 2.Metzger BE, Lowe LP, Dyer AR, et al.; HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 3.Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy Study Groups Consensus Panel International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenum AK, Mørkrid K, Sletner L, et al. Impact of ethnicity on gestational diabetes identified with the WHO and the modified International Association of Diabetes and Pregnancy Study Groups criteria: a population-based cohort study. Eur J Endocrinol 2012;166:317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glueck CJ, Pranikoff J, Aregawi D, Wang P. Prevention of gestational diabetes by metformin plus diet in patients with polycystic ovary syndrome. Fertil Steril 2008;89:625–634 [DOI] [PubMed] [Google Scholar]
- 6.Vanky E, Stridsklev S, Heimstad R, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab 2010;95:E448–E455 [DOI] [PubMed] [Google Scholar]
- 7.Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, Allan G. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N Engl J Med 1999;340:1314–1320 [DOI] [PubMed] [Google Scholar]
- 8.Genazzani AD, Lanzoni C, Ricchieri F, Jasonni VM. Myo-inositol administration positively affects hyperinsulinemia and hormonal parameters in overweight patients with polycystic ovary syndrome. Gynecol Endocrinol 2008;24:139–144 [DOI] [PubMed] [Google Scholar]
- 9.Corrado F, D’Anna R, Di Vieste G, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med 2011;28:972–975 [DOI] [PubMed] [Google Scholar]
- 10.Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care 1997;20:1087–1092 [DOI] [PubMed] [Google Scholar]
- 11.Scioscia M, Kunjara S, Gumaa K, McLean P, Rodeck CH, Rademacher TW. Urinary excretion of inositol phosphoglycan P-type in gestational diabetes mellitus. Diabet Med 2007;24:1300–1304 [DOI] [PubMed] [Google Scholar]
- 12.Giordano D, Corrado F, Santamaria A, et al. Effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome: a perspective, randomized, placebo-controlled study. Menopause 2011;18:102–104 [DOI] [PubMed] [Google Scholar]
- 13.Santamaria A, Giordano D, Corrado F, et al. One-year effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome. Climacteric 2012;15:490–495 [DOI] [PubMed] [Google Scholar]
- 14.D’Anna R, Di Benedetto V, Rizzo P, et al. Myo-inositol may prevent gestational diabetes in PCOS women. Gynecol Endocrinol 2012;28:440–442 [DOI] [PubMed] [Google Scholar]
- 15.Khattab S, Mohsen IA, Aboul Foutouh I, et al. Can metformin reduce the incidence of gestational diabetes mellitus in pregnant women with polycystic ovary syndrome? Prospective cohort study. Gynecol Endocrinol 2011;27:789–793 [DOI] [PubMed] [Google Scholar]
- 16.Davey RX, Hamblin PS. Selective versus universal screening for gestational diabetes mellitus: an evaluation of predictive risk factors. Med J Aust 2001;174:118–121 [DOI] [PubMed] [Google Scholar]


