Abstract
OBJECTIVE
Moderately elevated iron stores below the levels commonly associated with hemochromatosis have been implicated in the etiology of diabetes. Studies suggest that iron status (measured by serum ferritin) differs significantly according to sex, but inconsistent findings have been reported. Our aim is to test the association between serum ferritin and the prevalence of type 2 diabetes and fasting glucose concentrations in a population-based, multiethnic, cross-sectional study including men and women of African Surinamese, South Asian Surinamese, and ethnic Dutch origin.
RESEARCH DESIGN AND METHODS
We analyzed data on 508 ethnic Dutch, 597 African Surinamese, and 339 South Asian Surinamese aged 35–60 years. Type 2 diabetes was defined as a fasting plasma glucose level ≥7.0 mmol/L or a self-reported diagnosis.
RESULTS
Serum ferritin was positively associated with type 2 diabetes and fasting glucose, but differences in the associations according to sex were observed. Serum ferritin concentration was positively associated with type 2 diabetes among women in all ethnic groups (odds ratio [OR] ethnic Dutch: 1.07 [95% CI 1.01–1.13]; OR South Asian Surinamese: 1.05 [1.00–1.10]; OR African Surinamese: 1.05 [1.01–1.10]), but not among men. Serum ferritin was also more strongly associated with fasting glucose in women than in men. Moreover, the magnitude of sex differences in the association between serum ferritin and fasting glucose, but not type 2 diabetes, was more pronounced in the African Surinamese group than in the other ethnic groups (P for interaction ≤0.0001).
CONCLUSIONS
We found a positive association between serum ferritin and type 2 diabetes and fasting glucose in our multiethnic population, which appeared stronger among women than men. Further evaluation of the variation in sex differences between ethnic groups is warranted, particularly among the African Surinamese, to understand the mechanisms behind these sex differences.
Moderately elevated iron stores below the levels commonly associated with hemochromatosis have been implicated in the etiology of type 2 diabetes (1–3). Although a mechanism linking iron concentrations and diabetes is yet to be established, it is known that iron is a catalyst in the formation of hydroxyl radicals (4), which may contribute initially to insulin resistance, subsequently to decreased insulin secretion, and ultimately to the development of type 2 diabetes (5). Animal models suggest that iron excess may result in β-cell oxidative stress and decreased insulin secretion (6). Levels of serum ferritin, a predominant iron-storage protein and a biomarker of iron stores, are elevated in persons with prevalent diabetes as compared with nondiabetic controls (7) and correlate with impaired fasting glucose levels (8), an early marker of type 2 diabetes. In addition, several cross-sectional or case-control studies and two prospective studies have identified an independent association between baseline elevations in iron stores and the occurrence of type 2 diabetes (2,5,9–12). However, several questions remain unanswered.
It is yet unclear whether the association between serum ferritin and diabetes differs among men and women. Some have found that differences in iron status exist according to sex (8,13–16), which might have implications for the association with the etiology of diabetes (13). Others have suggested that sex differences might exist due to differences in iron accumulation in the peripheral muscles, which may cause derangement of muscle glucose uptake because of muscle damage (17,18). However, robust studies on the influence of sex on the association between serum ferritin and diabetes are rather inconsistent. Some have reported sex differences in the association (9,18), whereas others have not demonstrated this (19–21). Moreover, in those studies in which different associations between serum ferritin and type 2 diabetes were found for men and women, it appeared that the direction of the differences varied across studies (7–9).
These discrepancies might be the result of differences in the ethnic composition of study populations. A first argument to support this is a study reporting variations in the serum ferritin and type 2 diabetes associations across men and women from different ethnic populations, including white, black, Hispanic, Asian, and Pacific Islander populations (9). This study reported that the association between serum ferritin and type 2 diabetes differed significantly between the ethnic groups among women but not among men. Unfortunately, other multiethnic studies have not considered differential sex effects across ethnic groups (10,19,20). Another argument for the potential role of ethnicity is a difference in body composition that may occur between ethnic groups. For instance, in the Netherlands, differences in waist circumference or waist-to-height ratio have been reported among ethnic Dutch, South Asian Surinamese, and African Surinamese (22,23). This is relevant, as body composition is suggested to affect the association between serum ferritin and the insulin resistance syndrome (17,18).
The aim of this exploratory study is: 1) to test whether the association between serum ferritin and diabetes and fasting glucose differs between men and women of African Surinamese, South Asian Surinamese, and ethnic Dutch origin, and 2) to test whether the differences in the association between serum ferritin between men and women varies between these populations.
RESEARCH DESIGN AND METHODS
Study population and data collection
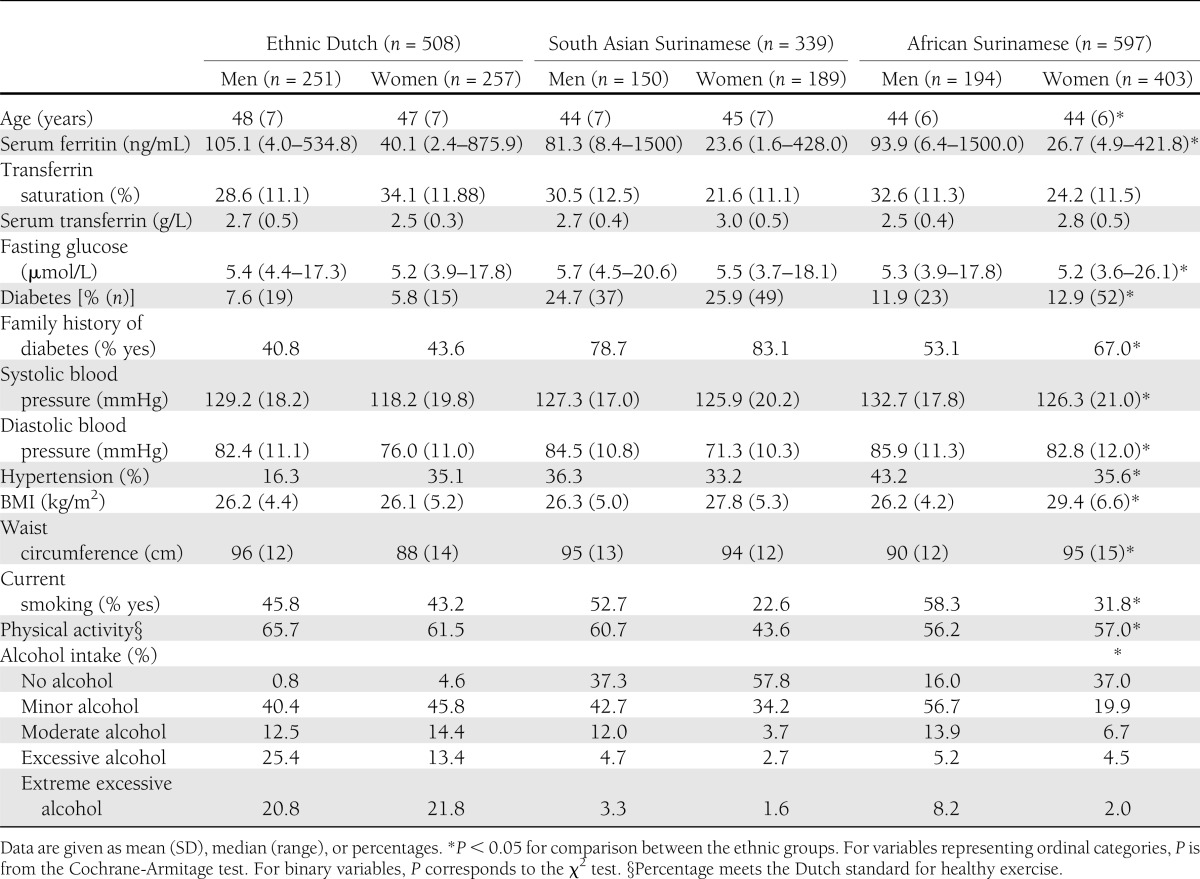
The study population consisted of participants in the population-based Surinamese in the Netherlands Study on Health and Ethnicity. The study was based on a random sample of 35–60-year-old, noninstitutionalized people in Amsterdam. Ethnicity was defined by self-identification of the participant as being Dutch, South Asian Surinamese (Hindustani Surinamese), or African Surinamese (predominantly of Creole origin). When ethnicity was not clear by self-identification, the parent’s country of birth and origin of ancestors defined the participant’s ethnicity. The recruitment, participation rates and design of the study are described more in detail elsewhere (24). In brief, potential participants were randomly sampled (n = 2,975) from the population register of Amsterdam, the Netherlands. Participants were approached at home for a structured face-to-face interview with a trained interviewer between 2001 and 2003. The overall participation in the interview was 60%. Subsequent participation in the medical examination was 84% among the Surinamese and 90% among the Dutch. More detailed information on the participation rate can be found in the flow chart of inclusion (Supplementary Fig. 1). The interview contained questions about lifestyle, migration history, demographic variables, and general health status. After the interview, the participants were invited for a physical examination at a local health center. In the current study, we included 339 South Asian Surinamese, 596 African Surinamese, and 508 ethnic Dutch who completed an interview, underwent a medical examination, and donated a fasting blood sample in which serum ferritin was measured.
Because no a priori knowledge was available regarding our research question, a solid priori power analysis could not be performed. This fits the explorative nature of the study. However, to provide insight into the uncertainty of our results, we have performed a post hoc analysis to calculate the power of demonstrating the observed differences in fasting plasma glucose. Among South Asian men, the smallest subgroup in our analyses, we found an increase in R2 of ∼0.04 due to serum ferritin while adjusting for all other variables. The power to demonstrate such a difference given the number of participants is ∼74%. In the female African Surinamese group, the largest subgroup in our analyses, the power was 98% to demonstrate this difference. The power for dichotomous variables was slightly lower. Additionally, the uncertainty about the true value of the parameter of interest is reflected by the 95% CIs.
All participants signed an informed consent form. The Medical Ethical Committee of the Academic Medical Centre in Amsterdam approved the study protocol.
Measurements
Serum ferritin.
During the physical examination, the study participants donated a fasting blood sample. Serum samples were stored at −80°C until serum ferritin was determined in 2010 using an Access-2 immunianalyzer (Beckman Coulter, Woerden, the Netherlands). The interassay variation coefficient was 6.7% based on two quality-control samples (25).
Type 2 diabetes and fasting glucose.
Fasting glucose concentrations were measured in plasma in 2003 (mmol/L; HK/Glucose-6-P dehydrogenase test; P800 analyzer; Roche Diagnostics, Indianapolis, IN). Type 2 diabetes was defined as fasting glucose ≥7.0 mmol/L. Persons with a self-reported diagnosis of type 2 diabetes were also considered to have diabetes mellitus (n = 146).
Additional measurements.
Body weight was measured in light clothing on a SECA mechanical scale (SECA, Hamburg, Germany) to the nearest 0.2 kg, and height was measured without shoes using a wall tape measure to the nearest 0.01 m. BMI was calculated as weight in kilograms divided by height in meters squared. Physical activity was measured using the Short Questionnaire to Assess Health—Enhancing Physical Activity questionnaire, which has been validated for the Dutch population (26). Smoking status was assessed by questionnaire, and participants were classified into nonsmokers, ex-smokers, or current smokers. Self-reported alcohol consumption was categorized in five groups of alcohol intake: no alcohol, minor alcohol, moderate alcohol, excessive alcohol, and extreme excessive alcohol intake. Blood pressure was measured with a validated oscillometric automated digital blood pressure device (OMRON-M4; Omron Healthcare Europe, Hoofddorp, the Netherlands) by trained staff. Using appropriate cuff sizes, two readings were taken on the right arm in a seated position after the subject had emptied the bladder and had been seated for at least 5 min. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, and/or using antihypertensive medication.
Data analysis
Baseline characteristics of the study population were reported according to sex and ethnicity. Differences between groups were assessed using χ2 tests for categorical data, ANOVA for continuous measures, and the Cochrane-Armitage test for ordinal variables. The characteristics were expressed as percentages, means and SD, or medians and ranges. Although serum ferritin was not normally distributed, we did not transform this variable in our analysis. Our models assume linearity between the independent variable (serum ferritin) and the dependent variables type 2 diabetes and fasting glucose and a normal distribution of the log odds (logistic model) or the error term (linear model). Both assumptions were true for both models.
To confirm the previously reported association of serum ferritin with the prevalence of type 2 diabetes and fasting glucose, logistic and linear regression analyses were performed while adjusting for sex, age, BMI, hypertension, waist circumference, and family history of type 2 diabetes, as these factors were previously associated with type 2 diabetes in the Surinamese in the Netherlands Study on Health and Ethnicity (22). We also took into account smoking and alcohol use (27,28). In unstratified analyses, we added interaction terms to the model to investigate whether the association was different in men and women. Moreover, to further test the consistency of differences in the odds of having type 2 diabetes or increased fasting glucose across ethnic and sex groups, we considered interaction based on the combined effect of sex, ethnicity, and serum ferritin (second-order interaction). Additionally, in order to compare our results with previously published literature (5,7,8,10,19,20), we repeated the analyses with sex-specific quartiles of serum ferritin concentrations and tested for interaction by age. Finally, to verify whether the observed associations were not explained by differences in the use of diabetes medication (insulin/tablets) between groups, we added this variable to a final model. We used SPSS 18 (SPSS Inc., Chicago, IL) for all analyses; the figures were extracted using the R statistical program.
RESULTS
South Asian Surinamese and African Surinamese were younger, had a lower prevalence of smoking, and were less physically active than ethnic Dutch participants (Table 1). The prevalence of type 2 diabetes was highest in South Asian Surinamese (25.4%), followed by African Surinamese (12.6%) and ethnic Dutch (6.7%). Serum ferritin concentrations were lower in women (46.6 ng/mL) compared with men (128.3 ng/mL), particularly among those of African-Surinamese (48.6 ng/mL) and Asian-Surinamese origin (41.7 ng/mL).
Table 1.
Baseline characteristics
Serum ferritin was associated with type 2 diabetes among women in the multivariate adjusted model, but not in men (Table 2). In addition, serum ferritin was positively associated with fasting glucose levels in all ethnic groups, except for Dutch men, after adjustment of relevant confounders (Table 2). The analyses with sex-specific quartiles of serum ferritin concentrations showed similar results (Table 3).
Table 2.
Association between serum ferritin (10 points in ferritin increase) and type 2 diabetes and fasting glucose (continuous variable) displayed for men and women of three different ethnic groups§
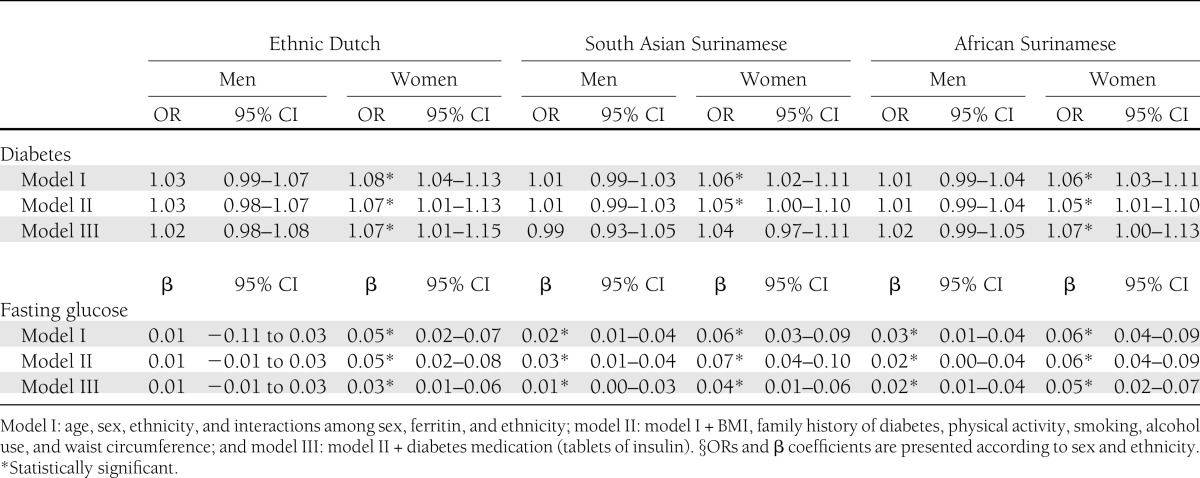
Table 3.
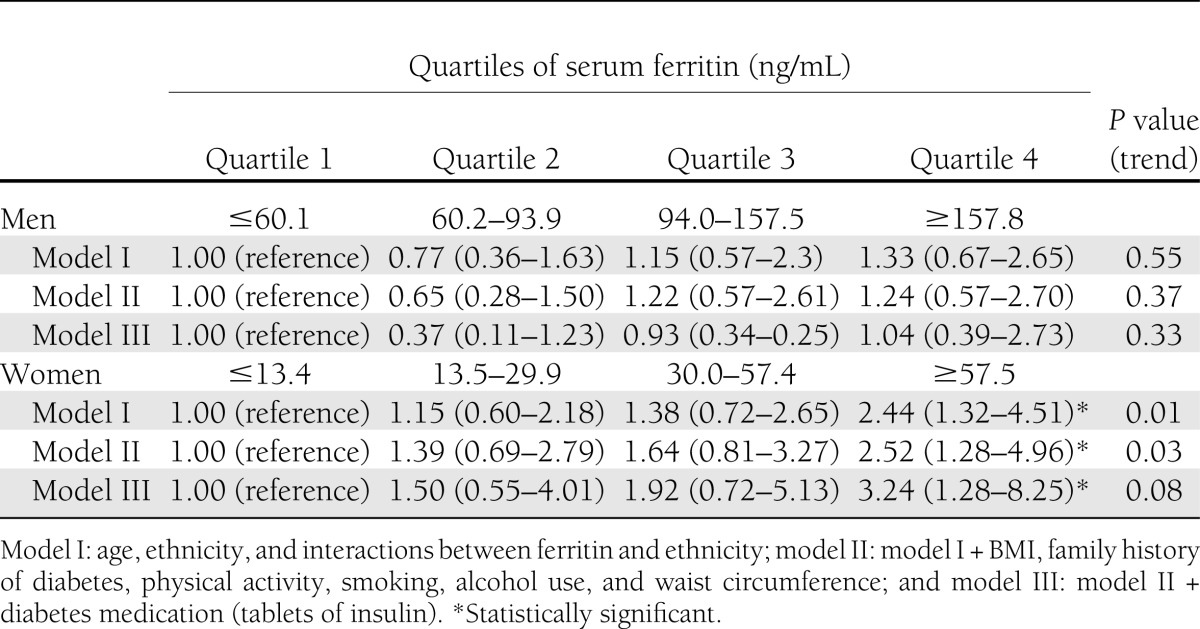
Additional analysis: ORs (95% CI) for type 2 diabetes according to quartile of serum ferritin concentration in men and women
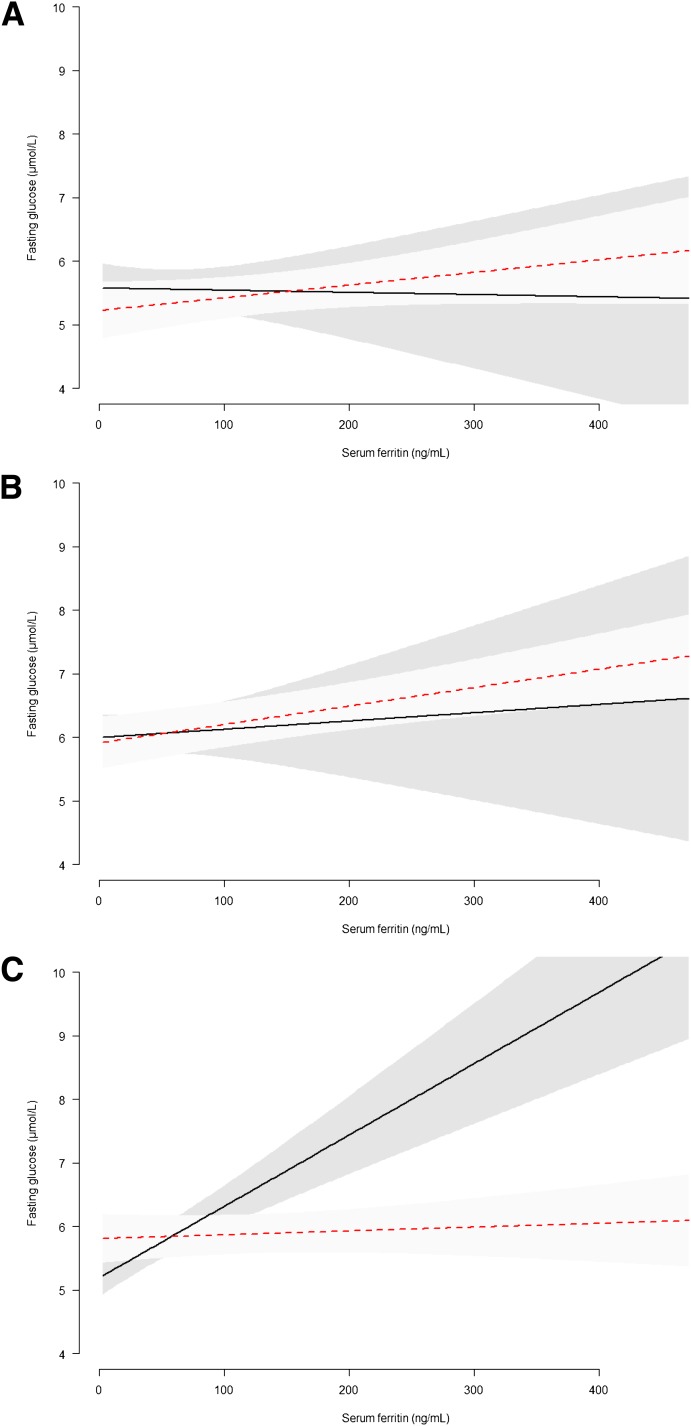
To test whether the sex differences in the association between serum ferritin and type 2 diabetes or fasting glucose were consistent across the different ethnicities, we added a second-order interaction term to our model. We found no evidence for ethnic differences in the association between serum ferritin and type 2 diabetes among men and women (P for interaction = 0.19). However, we did find evidence for a consistently different association between serum ferritin and fasting glucose between men and women across ethnic groups (P for interaction ≤0.0001). Further investigation, using linear regression modeling (model 2), showed that this significant second-order interaction was due to a greater difference in the association between serum ferritin and fasting glucose among African-Surinamese men and women (Fig. 1C).
Figure 1.
Sex differences across ethnic groups (A, ethnic Dutch; B, South Asian Surinamese; and C, African Surinamese) in the association of serum ferritin and fasting glucose. Linear regression of model II: age, sex, ethnicity, a second-order interaction term (sex/ferritin/ethnicity), BMI, family history of diabetes, physical activity, smoking, alcohol use, and waist circumference. Dotted line, males; straight line, females. (A high-quality color representation of this figure is available in the online issue.)
In an additional analysis (model 3), we adjusted for the use of diabetes medication; the odds ratios (ORs) were slightly attenuated for both men and women in all ethnic groups. Nonetheless, the sex differences across the ethnic groups remained.
CONCLUSIONS
The association between elevated serum ferritin and diabetes or increased fasting glucose differed between men and women. Although women in all ethnic groups had lower mean serum ferritin concentrations, serum ferritin was more strongly associated with diabetes in women compared with men. Interestingly, we found that these sex differences were not consistent across ethnic groups. Specifically, we found that sex differences in the association between serum ferritin and fasting glucose were stronger in the African-Surinamese population than in the other ethnic groups.
The finding that mean serum ferritin concentrations were markedly lower in women compared with men is consistent with previous studies (7,13,19). It is suggested that this lower iron status in women is likely attributable to menstrual blood loss (13,29). The finding that the association between serum ferritin and fasting glucose and type 2 diabetes is stronger in women than in men is partly in contrast with a study of Kim et al. (8) among Korean men and women, but is in line with three other studies (9,18,30) among Chinese, whites, blacks, Hispanics, Asians, Pacific Islanders, and Native Americans. The discrepancy in patterns observed by different studies may be due to ethnic differences in the association between serum ferritin and fasting glucose according to sex, as shown in the current study. Also in line with previous studies (9,10,21), analyses of sex-specific quartiles of serum ferritin concentrations (Table 3) revealed a clear positive linear trend of sex-specific serum ferritin concentrations in the association with type 2 diabetes, with a greater increase in the odds of type 2 diabetes among women. Nevertheless, the mechanisms underlying these sex differences merit further study. Our finding suggests that different optimal levels of serum ferritin according to sex might exist and that further study on the influence of muscle mass, sex hormone levels, and possibly body fat distribution may be required to clarify this issue (18).
Importantly, this is the first study to show differences in the degree of the sex differences, particularly in the association between serum ferritin and fasting glucose across ethnic groups. Although this pattern was not as apparent for type 2 diabetes, our results indicate that the strength of this association differs considerably more between men and women of African-Surinamese origin than between men and women in the other ethnic groups. This may be related to differences in iron storage, as one study has indicated that, after menopause, serum ferritin levels in black women show a steep rise, which is not seen in women of other ethnic groups (13). Unfortunately, we could not further investigate this, as data on menopause were not available.
Although our data provide relevant information on the association between serum ferritin and type 2 diabetes prevalence from a multiethnic population sample of middle-aged men and women in the Netherlands, the limitations of our study merit consideration. First, we only used serum ferritin as a marker of iron stores. Serum ferritin has been found to accurately reflect differences in body iron stores by age and sex (13). Nevertheless, it is important to recognize that although serum ferritin is widely used as a marker of iron status in epidemiological studies (31), the physiological role of serum ferritin is uncertain (32), and other biomarkers such as nontransferrin-bound iron may be needed to evaluate the impact of iron on disease development (33). Elevated serum ferritin concentrations, for example, may reflect systemic inflammation (34), which is thought to be involved in the pathophysiologic mechanisms underlying insulin resistance and diabetes (35). We were not able to investigate this in our study, but excluding participants with positive inflammation markers or by adjustment for inflammation markers (interleukin-6, fibrinogen, C-reactive protein) did not change the association between elevated serum ferritin and insulin resistance or type 2 diabetes in several studies (7,8). Second, we were unable to account for patterns of dietary intake in this study. Dietary iron intake, in particular haem iron from red meat, is one of the major determinants of body iron stores (36) but may be associated with the intake of other nutrients that influence the diabetes risk of individuals. However, adjusting for dietary variables did not change the positive association between serum ferritin and risk of type 2 diabetes among apparently healthy women as reported by Jiang et al. (5). Last, because this study is based on cross-sectional data, our results could have been biased when persons with diagnosed/self-reported type 2 diabetes changed their lifestyle as a part of treatment or by any interaction with antidiabetic drugs, which may interact with iron concentrations (37). The latter, however, does not seem likely, as inclusion of diabetes medication in the analysis did not affect the observed patterns of the association. Moreover, due to the cross-sectional nature, no conclusions can be drawn about causality. However, an important role of serum ferritin seems likely, as two prospective studies have shown an independent association between elevated serum ferritin and incident diabetes (5,12).
In conclusion, we showed that serum ferritin levels were positively associated with type 2 diabetes prevalence and fasting glucose in all ethnic groups, but that this association varied by sex. Furthermore, we found that these sex differences may not have the same magnitude across ethnic groups. If future studies indeed confirm mechanism behind the sex differences in the association of serum ferritin with type 2 diabetes incidence and the differences across ethnic groups, incentive would exist to monitor iron stores often and to define a normal range for body iron stores based on disease correlates for men and women across different ethnic groups.
Supplementary Material
Acknowledgments
This study was conducted with support of The Netherlands Organization for Health Research and Development and the Academic Medical Centre.
No potential conflicts of interest relevant to this article were reported.
L.H.D., M.N., and I.G.M.v.V. designed the study. L.H.D. wrote the manuscript and analyzed the data. M.N. and I.G.M.v.V. oversaw all aspects of the study conduct. D.L.v.d.A., L.M.B., M.B.S., and K.S. reviewed and edited the manuscript. W.B.B. oversaw statistical analyses. All authors approved the final version. I.G.M.v.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1243/-/DC1.
References
- 1.Eshed I, Elis A, Lishner M. Plasma ferritin and type 2 diabetes mellitus: a critical review. Endocr Res 2001;27:91–97 [DOI] [PubMed] [Google Scholar]
- 2.Fernández-Real JM, López-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes 2002;51:2348–2354 [DOI] [PubMed] [Google Scholar]
- 3.Wilson JG, Lindquist JH, Grambow SC, Crook ED, Maher JF. Potential role of increased iron stores in diabetes. Am J Med Sci 2003;325:332–339 [DOI] [PubMed] [Google Scholar]
- 4.Andrews NC. Disorders of iron metabolism. N Engl J Med 1999;341:1986–1995 [DOI] [PubMed] [Google Scholar]
- 5.Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 2004;291:711–717 [DOI] [PubMed] [Google Scholar]
- 6.Cooksey RC, Jouihan HA, Ajioka RS, et al. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 2004;145:5305–5312 [DOI] [PubMed] [Google Scholar]
- 7.Forouhi NG, Harding AH, Allison M, et al. Elevated serum ferritin levels predict new-onset type 2 diabetes: results from the EPIC-Norfolk prospective study. Diabetologia 2007;50:949–956 [DOI] [PubMed] [Google Scholar]
- 8.Kim CH, Kim HK, Bae SJ, Park JY, Lee KU. Association of elevated serum ferritin concentration with insulin resistance and impaired glucose metabolism in Korean men and women. Metabolism 2011;60:414–420 [DOI] [PubMed] [Google Scholar]
- 9.Acton RT, Barton JC, Passmore LV, et al. Relationships of serum ferritin, transferrin saturation, and HFE mutations and self-reported diabetes in the Hemochromatosis and Iron Overload Screening (HEIRS) study. Diabetes Care 2006;29:2084–2089 [DOI] [PubMed] [Google Scholar]
- 10.Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care 1999;22:1978–1983 [DOI] [PubMed] [Google Scholar]
- 11.Tuomainen TP, Nyyssönen K, Salonen R, et al. Body iron stores are associated with serum insulin and blood glucose concentrations. Population study in 1,013 eastern Finnish men. Diabetes Care 1997;20:426–428 [DOI] [PubMed] [Google Scholar]
- 12.Salonen JT, Tuomainen TP, Nyyssönen K, Lakka HM, Punnonen K. Relation between iron stores and non-insulin dependent diabetes in men: case-control study. BMJ 1998;317:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J 2000;140:98–104 [DOI] [PubMed] [Google Scholar]
- 14.Harris EL, McLaren CE, Reboussin DM, et al. Serum ferritin and transferrin saturation in Asians and Pacific Islanders. Arch Intern Med 2007;167:722–726 [DOI] [PubMed] [Google Scholar]
- 15.Ramakrishnan U, Kuklina E, Stein AD. Iron stores and cardiovascular disease risk factors in women of reproductive age in the United States. Am J Clin Nutr 2002;76:1256–1260 [DOI] [PubMed] [Google Scholar]
- 16.van Dam RM, Nicolaou M, Stronks K. [Voedingspatroon van Surinaamse Amsterdammers in kaart gebracht. Groente- en fruitconsumptie lager dan verwacht]. Ned Tijdschr Diëtisten 2005;60:98–102 [Google Scholar]
- 17.Merkel PA, Simonson DC, Amiel SA, et al. Insulin resistance and hyperinsulinemia in patients with thalassemia major treated by hypertransfusion. N Engl J Med 1988;318:809–814 [DOI] [PubMed] [Google Scholar]
- 18.Sheu WH, Chen YT, Lee WJ, Wang CW, Lin LY. A relationship between serum ferritin and the insulin resistance syndrome is present in non-diabetic women but not in non-diabetic men. Clin Endocrinol (Oxf) 2003;58:380–385 [DOI] [PubMed] [Google Scholar]
- 19.Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care 2004;27:2422–2428 [DOI] [PubMed] [Google Scholar]
- 20.Jehn ML, Guallar E, Clark JM, et al. A prospective study of plasma ferritin level and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol 2007;165:1047–1054 [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Franco OH, Hu FB, et al. Ferritin concentrations, metabolic syndrome, and type 2 diabetes in middle-aged and elderly chinese. J Clin Endocrinol Metab 2008;93:4690–4696 [DOI] [PubMed] [Google Scholar]
- 22.Bindraban NR, van Valkengoed IG, Mairuhu G, et al. Prevalence of diabetes mellitus and the performance of a risk score among Hindustani Surinamese, African Surinamese and ethnic Dutch: a cross-sectional population-based study. BMC Public Health 2008;8:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Valkengoed IG, Agyemang C, Krediet RT, Stronks K. Ethnic differences in the association between waist-to-height ratio and albumin-creatinine ratio: the observational SUNSET study. BMC Nephrol 2012;13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agyemang C, Bindraban N, Mairuhu G, Montfrans G, Koopmans R, Stronks K, SUNSET (Surinamese in The Netherlands: Study on Ethnicity and Health) Study Group Prevalence, awareness, treatment, and control of hypertension among Black Surinamese, South Asian Surinamese and White Dutch in Amsterdam, The Netherlands: the SUNSET study. J Hypertens 2005;23:1971–1977 [DOI] [PubMed] [Google Scholar]
- 25.Verkaik-Kloosterman J, van Valkengoed IG, Nicolaou M, van der A DL. [Nutritional status of Asian and African Surinamese and ethnic Dutch in the Netherlands—The SUNSET study] (Report No.: 350070003/2011). Bilthoven, the Netherlands, Rijksinstituut vor Volkegezondheid en Milieu (RIVM), 2011
- 26.Wendel-Vos GC, Schuit AJ, Saris WH, Kromhout D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol 2003; 56:1163–1169. [DOI] [PubMed] [Google Scholar]
- 27.Agardh EE, Ahlbom A, Andersson T, et al. Explanations of socioeconomic differences in excess risk of type 2 diabetes in Swedish men and women. Diabetes Care 2004;27:716–721 [DOI] [PubMed] [Google Scholar]
- 28.Haire-Joshu D, Glasgow RE, Tibbs TL, American Diabetes Association Smoking and diabetes. Diabetes Care 2004;27(Suppl. 1):S74–S75 [DOI] [PubMed] [Google Scholar]
- 29.Rushton DH, Dover R, Sainsbury AW, Norris MJ, Gilkes JJ, Ramsay ID. Why should women have lower reference limits for haemoglobin and ferritin concentrations than men? BMJ 2001;322:1355–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Z, Hu X, Yuan B, Pan X, Meyer HE, Holmboe-Ottesen G. Association between serum ferritin, hemoglobin, iron intake, and diabetes in adults in Jiangsu, China. Diabetes Care 2006;29:1878–1883 [DOI] [PubMed] [Google Scholar]
- 31.Witte DL, Crosby WH, Edwards CQ, Fairbanks VF, Mitros FA. Practice guideline development task force of the College of American Pathologists. Hereditary hemochromatosis. Clin Chim Acta 1996;245:139–200 [DOI] [PubMed] [Google Scholar]
- 32.Linder MC, Schaffer KJ, Hazegh-Azam M, Zhou CY, Tran TN, Nagel GM. Serum ferritin: does it differ from tissue ferritin? J Gastroenterol Hepatol 1996;11:1033–1036 [DOI] [PubMed] [Google Scholar]
- 33.Lee DH, Jacobs DR., Jr Serum markers of stored body iron are not appropriate markers of health effects of iron: a focus on serum ferritin. Med Hypotheses 2004;62:442–445 [DOI] [PubMed] [Google Scholar]
- 34.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–454 [DOI] [PubMed] [Google Scholar]
- 35.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004;25:4–7 [DOI] [PubMed] [Google Scholar]
- 36.Ascherio A, Willett WC, Rimm EB, Giovannucci EL, Stampfer MJ. Dietary iron intake and risk of coronary disease among men. Circulation 1994;89:969–974 [DOI] [PubMed] [Google Scholar]
- 37.Tajima S, Ikeda Y, Sawada K, et al. Iron reduction by deferoxamine leads to amelioration of adiposity via the regulation of oxidative stress and inflammation in obese and type 2 diabetes KKAy mice. Am J Physiol Endocrinol Metab 2012;302:E77–E86 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.