Abstract
OBJECTIVE
Age at menopause is an important determinant of future health outcomes, but little is known about its relationship with type 2 diabetes. We examined the associations of menopausal age and reproductive life span (menopausal age minus menarcheal age) with diabetes risk.
RESEARCH DESIGN AND METHODS
Data were obtained from the InterAct study, a prospective case-cohort study nested within the European Prospective Investigation into Cancer and Nutrition. A total of 3,691 postmenopausal type 2 diabetic case subjects and 4,408 subcohort members were included in the analysis, with a median follow-up of 11 years. Prentice weighted Cox proportional hazards models were adjusted for age, known risk factors for diabetes, and reproductive factors, and effect modification by BMI, waist circumference, and smoking was studied.
RESULTS
Mean (SD) age of the subcohort was 59.2 (5.8) years. After multivariable adjustment, hazard ratios (HRs) of type 2 diabetes were 1.32 (95% CI 1.04–1.69), 1.09 (0.90–1.31), 0.97 (0.86–1.10), and 0.85 (0.70–1.03) for women with menopause at ages <40, 40–44, 45–49, and ≥55 years, respectively, relative to those with menopause at age 50–54 years. The HR per SD younger age at menopause was 1.08 (1.02–1.14). Similarly, a shorter reproductive life span was associated with a higher diabetes risk (HR per SD lower reproductive life span 1.06 [1.01–1.12]). No effect modification by BMI, waist circumference, or smoking was observed (P interaction all > 0.05).
CONCLUSIONS
Early menopause is associated with a greater risk of type 2 diabetes.
Menopause is an important transition in women’s reproductive life, as it signals the end of fertility. Timing of menopause is also an important determinant of future disease risk. For example, an early age at menopause is associated with an increased risk of cardiovascular disease (CVD) (1) and bone fractures (2,3). Conversely, an early menopause protects against breast (4,5), endometrial (6), and ovarian cancer (7). Changes associated with the menopause transition, in particular loss of ovarian function and subsequent decline in endogenous estrogens, have been postulated as mediators of these differences in risk (8,9).
While the relationship between menopausal age and CVD risk is well established, its association with type 2 diabetes, one of the major risk factors for CVD, remains unclear. Oophorectomized women seem to have less favorable glucose and insulin levels (10,11), which is suggestive of a link between premature menopause and diabetes risk. However, the few epidemiological studies that have investigated the relationship between menopausal age and diabetes yielded conflicting results with either an inverse (12) or no (13,14) association. Thus far, no prospective studies have been performed, and no studies have examined the relationship with reproductive life span (defined as menopausal age minus menarcheal age), which is a marker of total duration of endogenous estrogen exposure.
In the current study, we investigated the associations of menopausal age and reproductive life span with incident type 2 diabetes in InterAct. This large prospective case-cohort study with contributions of eight European countries is part of the European Prospective Investigation into Cancer and Nutrition (EPIC) and provides a unique opportunity to study these associations.
RESEARCH DESIGN AND METHODS
The InterAct study is a case-cohort study nested within EPIC. The participants, methods, study design, and measurements have previously been described (15). Briefly, the InterAct consortium was initiated to investigate how genetic and lifestyle factors interact in their influence on the risk of type 2 diabetes. A case-cohort study was established based on incident type 2 diabetes cases occurring between 1991 and 2007 in 26 centers from 8 of 10 EPIC countries (Italy, Spain, U.K., the Netherlands, France, Germany, Sweden, and Denmark) participating in InterAct. All participants gave written informed consent, and the study was approved by the local ethics committee in the participating countries and the internal review board of the International Agency for Research on Cancer.
Case ascertainment and verification
Ascertainment and verification of incident diabetes have previously been described in detail (15). New cases occurring up until 31 December 2007 were ascertained by multiple data sources including self-report, linkage to primary or secondary care registers, linkage to pharmacy databases, medication use, hospital admissions, and mortality data. Verification of incident diabetes was undertaken for participants with fewer than two independent sources of information by individual medical record checking or confirmation from another independent source of information.
Follow-up was censored at the date of diagnosis, 31 December 2007, or the date of death—whichever occurred first. In total, 12,403 verified incident cases were identified.
Subcohort
A subcohort of 16,835 individuals was randomly selected from those with available stored blood samples stratified by center. We oversampled the number of individuals in the subcohort for the proportion of prevalent diabetes cases in each center to account for later exclusion of individuals with prevalent diabetes from InterAct analyses. After exclusion of 548 individuals with prevalent diabetes and 133 individuals with unknown diabetes status, 16,154 subcohort individuals remained, of whom 778 had developed type 2 diabetes during follow-up.
Study population
The present analysis was restricted to postmenopausal women (Supplementary Fig. 1). Women were considered postmenopausal when they reported not having had any menses over the past 12 months or when they reported bilateral oophorectomy. Women with missing or incomplete questionnaire data or with reported previous hysterectomy were considered postmenopausal only if they were older than 55 years. Age at menopause was defined as the self-reported age at the last menstrual period. Among the postmenopausal women (N = 9,054), we excluded those who had not reported their age at last menses (N = 1,190), leaving 3,691 incident type 2 diabetes cases and a subcohort of 4,408 postmenopausal women (including 235 incident diabetes cases) for the analyses.
Reproductive factors
At the baseline visit between 1991 and 2000, information on reproductive factors was collected using self-administered questionnaires. In all centers, participants were asked to report their age at first and last menstrual period, current and past use of oral contraceptives, and hormone replacement therapy (HRT) and whether they had undergone a hysterectomy and/or oophorectomy. Except for the Bilthoven cohort (the Netherlands), all centers provided information on the number of full-term pregnancies (the sum of live births and stillbirths). In the Bilthoven cohort, the number of children was used as a proxy for the number of full-term pregnancies.
Age at menarche was defined as the age at the first menstrual period and was missing for 96 women. Reproductive life span was calculated by subtracting the age at menarche from the age at menopause.
Measurement of other baseline characteristics
Baseline questionnaires included questions on education level, smoking status, alcohol consumption, and physical activity. Presence of hypertension and hyperlipidemia at baseline was based on self-reported diagnosis and/or use of medication. Information on hyperlipidemia was not collected in the Swedish cohorts. In most centers, trained health professionals measured weight, height, and waist circumference during a visit to the study center. In part of the French cohort and Oxford cohort (U.K.), height, weight, and waist circumference were self-reported, and in Umea (Sweden) only weight and height was measured. BMI was calculated as weight divided by the square of height in meters.
Data analysis
Twenty percent of the women (N = 1,576) had missing values on one or more covariates. Because the missing values were likely to be missing at random and for avoidance of loss in efficiency, missing values for BMI, smoking status, alcohol consumption, physical activity, education level, number of full-term pregnancies, ever oral contraceptive and HRT use, and hysterectomy/oophorectomy status were imputed within countries using a multiple imputation technique (10 imputation sets) (16).
We used Cox proportional hazards models adapted to the case-cohort design (Prentice weighted method [17]) to estimate hazard ratios (HRs) and 95% CIs of type 2 diabetes for menopausal age and reproductive life span. In all analyses, age was used as the underlying time variable (with entry and exit time defined as the participant’s age at recruitment and age at type 2 diabetes diagnosis, death, loss-to follow-up, or censoring at the end of the follow-up), and all models were stratified by center to account for study center effects such as follow-up procedures and covariate measurement.
In the analyses, age at menopause was entered as a categorical variable (<40, 40–44, 45–49, 50–54, and >55 years) with menopausal age between 50 and 54 years taken as a reference. Reproductive life span was divided into quartiles based on its distribution in the subcohort, and HRs were calculated using the highest quartile as a reference. We also modeled menopausal age and reproductive life span as continuous variables to investigate the linear association with type 2 diabetes risk. For this purpose, we estimated HRs of type 2 diabetes per SD younger age at menopause and per SD lower reproductive life span.
Analyses were adjusted for potential confounders in three consecutive models. The first model was adjusted for age. Next, we added known diabetes risk factors to the model, including BMI (continuous), smoking status (current, former, and never), alcohol consumption (≤10, 11–24, and ≥25 g/day), education level (none, primary school completed, technical or professional school, secondary school, and longer education), and physical activity (inactive, moderately inactive, moderately active, and active). In the final multivariable model, we further adjusted the analyses for reproductive factors including number of full-term pregnancies (continuous) and ever oral contraceptive and HRT use (yes versus no).
We also performed a series of sensitivity analyses. To investigate whether associations were independent of other risk factors, we additionally adjusted the multivariable model for 1) waist circumference (continuous), 2) hypertension (yes versus no), and 3) hyperlipidemia (yes versus no). Participants from, respectively, Umea and Sweden (Umea and Malmö) were excluded from these analyses because of missing values for these variables. Next, we excluded women who ever used HRT, as age at menopause may be difficult to determine under hormone use. Finally, we restricted the analysis to women who had not undergone a hysterectomy and/or oophorectomy in the past. In this sensitivity analysis, we were able to examine the potential influence of these surgical procedures as well as the effect of a reduction of circulating androgens that occurs with hysterectomy and oophorectomy.
Previously, it has been suggested that the association with menopausal age may vary depending on smoking (18) and obesity status (4,19). Therefore, we stratified the analyses according to BMI (<25, 25–29, and ≥30 kg/m2), waist circumference (<88 and ≥88 cm), and smoking status (current, former, never) and tested for effect modification by including continuous interaction terms in the multivariable-adjusted model.
To verify whether pooling of the data was justified, we calculated country-specific HRs using random-effects meta-analysis (20) and assessed the amount of heterogeneity (I2) between countries. For these analyses, we used the HRs of type 2 diabetes per SD younger age at menopause and per SD lower reproductive life span, using the multivariable-adjusted model including center, age, diabetes risk factors, and reproductive factors. All statistical analyses were performed using STATA, version 11.0 (Stata, College Station, TX).
RESULTS
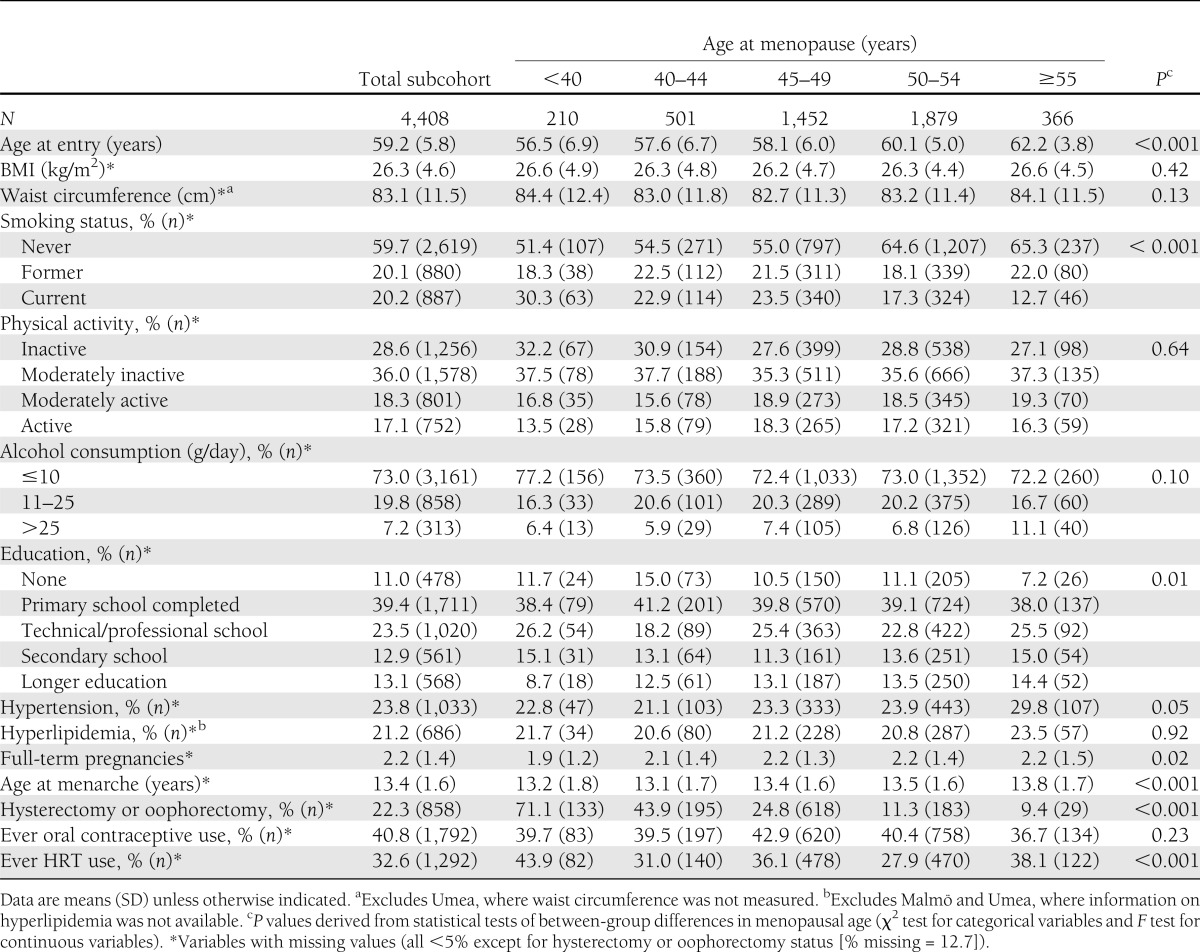
Table 1 shows the baseline characteristics of the subcohort across categories of menopausal age. The mean (SD) age at entry in the subcohort was 59.2 (5.8) years. The mean age at menopause was 48.6 (4.9) years, and 4.8% of the women had their menopause before 40 years of age. As expected, women who went through menopause at a younger age had given birth to fewer children, were more likely to be smokers, and more often reported having had a hysterectomy and/or oophorectomy. Earlier menopause was associated with a younger age at baseline and a younger age at menarche. Ever HRT users and a low education level were also more common among these women.
Table 1.
Baseline characteristics of the subcohort by categories of menopausal age: the EPIC-InterAct study
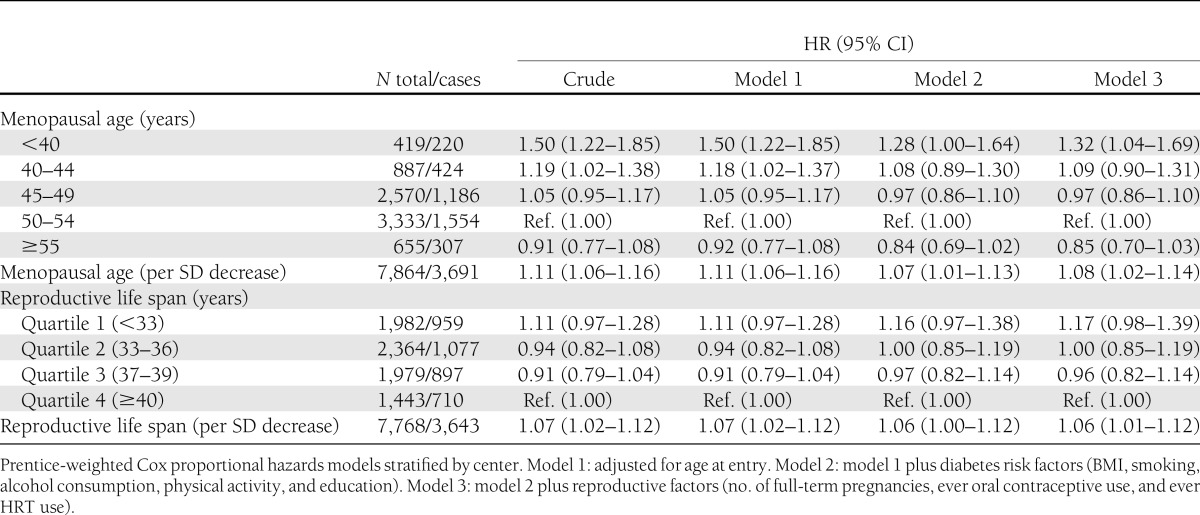
During a median follow-up of 10.7 years (interquartile range 6.6–12.6), 3,691 women had developed incident type 2 diabetes. An earlier age at menopause was associated with a higher risk of diabetes (Table 2). After adjustment for known risk factors for diabetes and reproductive factors, HRs of type 2 diabetes were 1.32 (95% CI 1.04–1.69), 1.09 (0.90–1.31), 0.97 (0.86–1.10), and 0.85 (0.70–1.03) for women with menopause at ages <40, 40–44, 45–49, and ≥55 years, respectively, relative to those with menopause at age 50–54 years. The HR per SD younger age at menopause was 1.08 (1.02–1.14). A shorter reproductive life span was also associated with a greater risk of type 2 diabetes (HRquartile 1 vs. quartile 4 1.17 [0.98–1.39], HRquartile 2 vs. quartile 4 1.00 [0.85–1.19], and HRquartile 3 vs. quartile 4 0.96 [0.82–1.14]). The HR per SD lower reproductive life span was 1.06 (1.01–1.12).
Table 2.
Hazard ratios of type 2 diabetes according to menopausal age and reproductive life span: the EPIC-InterAct study
Analysis with additional adjustment for waist circumference, hypertension, and hyperlipidemia yielded comparable results (Supplementary Table 1). When we restricted the analyses to women without a hysterectomy and/or oophorectomy and women not using HRT, effect estimates were also not materially different (Supplementary Table 1).
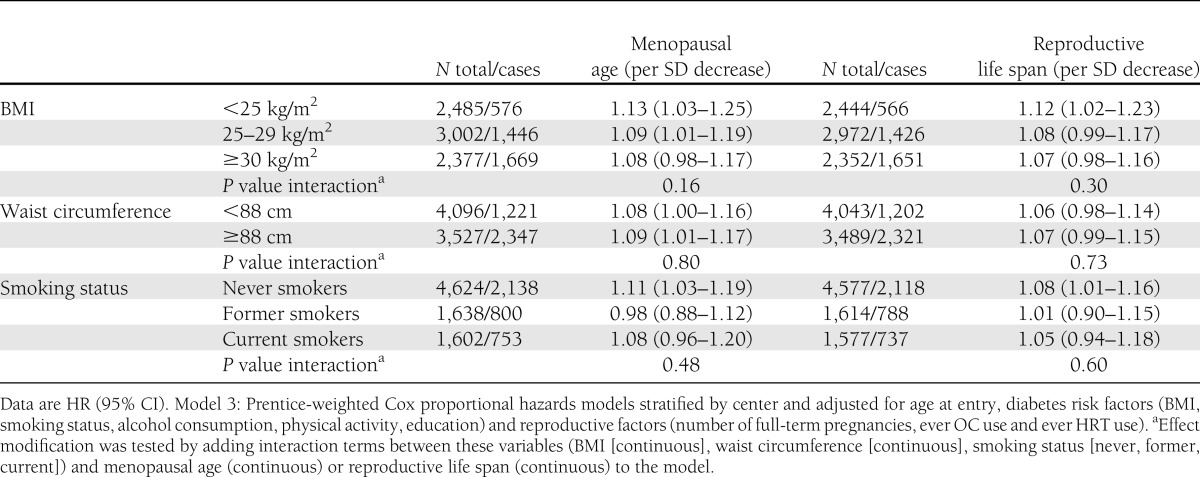
Interaction analysis showed that the associations of menopausal age and reproductive life span with type 2 diabetes did not differ by BMI, waist circumference, or smoking status (P values for interaction were 0.16, 0.80, and 0.48 for menopausal age and 0.30, 0.73, and 0.60 for reproductive life span, respectively). (See also Table 3.)
Table 3.
Multivariable adjusted hazard ratios of type 2 diabetes per SD decrease of menopausal age and reproductive life span, stratified by BMI, waist circumference, and smoking status: the EPIC-InterAct study
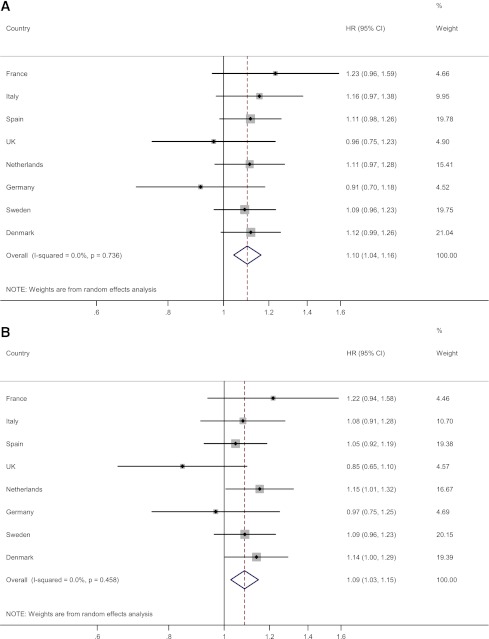
Country-specific and pooled HRs of type 2 diabetes are shown in Fig. 1. There was no evidence of heterogeneity in the associations between countries (I2 = 0.0%, P = 0.74, for menopausal age and I2 = 0.0%, P = 0.46, for reproductive life span), indicating that country-specific HRs were sufficiently similar to justify pooling across countries.
Figure 1.
Country-specific HRs of type 2 diabetes per SD decrease in menopausal age and reproductive life span: the EPIC-InterAct study. HRs and 95% CIs are derived from Prentice-weighted Cox proportional hazards models adjusted for center, age at entry, diabetes risk factors (BMI, smoking, alcohol consumption, physical activity, and education), and reproductive factors (number of full-term pregnancies, ever oral contraceptive use, and ever HRT use). A: HR of type 2 diabetes per SD decrease in menopausal age. B: HR of type 2 diabetes per SD decrease in reproductive life span. (A high-quality color representation of this figure is available in the online issue.)
CONCLUSIONS
In this prospective case-cohort study, we found that an earlier age at menopause was associated with a greater risk of type 2 diabetes. The hazard of type 2 diabetes was 32% higher in women who entered their menopause before 40 years of age compared with women having their menopause at 50–54 years. Similarly, a shorter reproductive life span was associated with a higher diabetes risk. All associations were robust to adjustment for a wide range of potential confounding factors, and effect estimates were of similar magnitude after excluding women with a hysterectomy and/or oophorectomy and women using HRT. No effect modification by BMI, waist circumference, or smoking was found.
The strengths of our study include its prospective design, the use of verified incident diabetes cases, and adjustment in the analyses for a comprehensive set of potential confounders. Data collection on reproductive factors was fully standardized across cohorts, except for the number of full-term pregnancies, which was collected slightly differently for one center. Nevertheless, our study also had some limitations. First of all, assessment of age at menarche and menopause was based on self-report, which is prone to recall bias, particularly in older women. However, previous studies have shown that the validity and reproducibility of self-reported age at menopause and menarche are fairly good (21–25). Because of the prospective design, any misclassification is most likely unrelated to the occurrence of diabetes, and such random misclassification if anything usually leads to an underestimation of risks. Second, the use of a clinical definition may have led to potential misclassification of individuals with undiagnosed diabetes. However, multiple sources were used for case ascertainment, and even if some underdiagnosis may have occurred, this would have tended to attenuate associations rather than to produce spurious ones. Third, we adjusted the analyses for a large number of confounders, but we cannot rule out the possibility of residual confounding. Also, potential effect modifiers were not measured at the actual onset of menopause but somewhere in between menopause and follow-up. This may have limited the interaction analyses and might explain the lack of interactions observed. Finally, despite the prospective design, the observed associations may partially reflect reverse causation. Women with type 1 diabetes enter menopause several years earlier than nondiabetic women (26), but thus far no data exist on the effect of early-onset type 2 diabetes on menopausal timing. Glycosylation of functional proteins may cause ovarian dysfunction, but type 1 diabetes could also be linked to menopausal age through distinct mechanisms involving autoimmunity.
Several studies have examined the impact of menopause on diabetes risk. Most studies, however, investigated the relationship with menopause status rather than age at menopause onset and did not find associations (27–29). Previous results regarding menopausal age are mixed (12–14). In a large study among women entering menopause clinics, no association between menopausal age and type 2 diabetes was found (13). In another cross-sectional study, diabetes was more prevalent among women with premature menopause, but this association was not statistically significant after multivariable adjustment (14). On the other hand, Malacara et al. (12) found a positive correlation between age at menopause and age at diabetes diagnosis. This is the first prospective study looking at menopausal age and type 2 diabetes risk. Associations with menopausal age and reproductive life span were of similar strength. Previous studies have linked an early age at menarche to a greater risk of type 2 diabetes (30,31), which may suggest that earlier timing of menopause per se, rather than a shorter interval between menarche and menopause, is the main determinant of diabetes risk. However, given the close correlation between menopausal age and reproductive life span (r = 0.95), it is difficult to truly distinguish between the relative contributions of these two factors.
Associations between menopausal age and risk of chronic diseases are usually attributed to a short or prolonged exposure to endogenous estrogens. In contrast to breast cancer, where the available evidence on reproductive factors, endogenous estrogen levels, and exogenous estrogen supplementation all points to an important role of estrogen exposure (32), results are more equivocal for diabetes. Experimental data support a protective role for estrogens in glucose metabolism. Mechanistic studies have demonstrated beneficial long-term effects of exogenous estrogens on insulin secretion and glucose homeostasis (33), and in postmenopausal women estrogen replacement has been associated with a lower incidence of type 2 diabetes (34,35). Observational data, however, argue against a simple protective effect of estrogens. In postmenopausal women, high endogenous estrogen levels have been associated with an increase rather than a decrease in diabetes risk (36,37). Moreover, an early start of estrogen exposure (i.e., an early age at menarche) appears to have an adverse effect on diabetes risk (30,31). Thus, apart from the dramatic reduction in endogenous estrogen, other menopause-related factors may play a role in explaining the observed increase in diabetes risk with early menopause. The menopausal transition is also characterized by a shift toward androgen predominance including a decrease in sex hormone–binding globulin levels (38,39). Increased androgenicity, in turn, has been linked to a higher risk of type 2 diabetes in postmenopausal women (36,37). Alternatively, early menopause may represent a marker of premature ageing. A recent meta-analysis of genome-wide association studies found 17 loci for age at menopause that have previously been related to DNA damage repair and replication important processes in determining longevity (40). More research is needed to unravel the mechanisms through which the timing of menopause influences metabolic disease risk.
The findings of the current study are of interest in light of the high prevalence of type 2 diabetes among postmenopausal women. The direct effect of early menopause may be relevant for the prevention of diabetes in women. For example, early menopause might be a factor to take into account when considering diabetes screening or direct preventive action. However, beforehand, more studies are needed to evaluate whether timing of menopause has any added value in diabetes prediction and prevention.
In conclusion, this is the first prospective study demonstrating that women with an early age at menopause are at higher risk of developing type 2 diabetes.
Supplementary Material
Acknowledgments
Funding for the InterAct project was provided by the European Union FP6 program (grant LSHM_CT_2006_037197). In addition, the InterAct investigators acknowledge funding from the following agencies: Y.T.v.d.S., verification of diabetes cases additionally funded by NL Agency Grant IGE05012 and an Incentive Grant from the Board of the University Medical Center Utrecht; K.-T.K., the Medical Research Council and Cancer Research UK; E.A., Health Research Fund of the Spanish Ministry of Health, Navarre Regional Government and CIBER Epidemiología y Salud Pública; M.-D.C., Health Research Fund of the Spanish Ministry of Health and Murcia Regional Government (no. 6236); F.L.C., Cancer Research UK; E.J.D., the Spanish Ministry of Health (ISCII RETICC RD06/0020); P.W.F., Swedish Research Council, Swedish Diabetes Association, and Swedish Heart-Lung Foundation; L.C.G., Swedish Research Council; R.K., German Cancer Aid and German Ministry of Research; T.J.K., Cancer Research UK; P.M.N., Swedish Research Council; K.O., Danish Cancer Society; S.P., Compagnia di San Paolo; J.R.Q., Asturias Regional Government; O.R., Västerboten County Council; B.T., German Cancer Aid; A.T., Danish Cancer Society; R.T., AIRE-ONLUS Ragusa, AVIS-Ragusa, and the Sicilian Regional Government; D.L.v.d.A., Dutch Ministry of Public Health, Welfare and Sports, Netherlands Cancer Registry, LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund, and Statistics Netherlands; and E.R., the Imperial College Biomedical Research Centre. P.W.F. also received funding from Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
None of the sponsors had any role in the study design, collection, analysis and interpretation of the data, or writing of the manuscript or the decision to submit the manuscript for publication.
J.S.B. designed the analysis; analyzed data; wrote the manuscript with close assistance from working team members; contributed to either EPIC or InterAct data collection, study management, or study coordination; contributed to data interpretation and critical reading of the manuscript; and saw and approved the final version of the manuscript. Y.T.v.d.S. and N.C.O.-M. designed the analysis and wrote the manuscript with close assistance from working team members; contributed to either EPIC or InterAct data collection, study management, or study coordination; contributed to data interpretation and critical reading of the manuscript; and saw and approved the final version of the manuscript. S.J.S., K.K.O., and K.-T.K. were working team members; assisted with writing the manuscript; contributed to either EPIC or InterAct data collection, study management, or study coordination; contributed to data interpretation and critical reading of the manuscript; and saw and approved the final version of the manuscript. E.A., P.A., H.B., M.-D.C., F.C.-C., F.L.C., B.d.L.-G., E.J.D., G.F., P.W.F., S.G., L.C.G., R.K., T.J.K., P.M.N., K.O., D.P., S.P., J.R.Q., O.R., C.S., M.-J.S., N.S., B.T., A.T., R.T., and D.L.v.d.A. contributed to either EPIC or InterAct data collection, study management, or study coordination; contributed to data interpretation and critical reading of the manuscript; and saw and approved the final version of the manuscript. E.J.M.F., C.L., N.G.F., and E.R. were working team members; assisted with writing the manuscript; contributed to either EPIC or InterAct data collection, study management, or study coordination; contributed to data interpretation and critical reading of the manuscript; and saw and approved the final version of the manuscript. N.J.W. was a working team member; assisted with writing the manuscript; is the principal investigator of InterAct; contributed to either EPIC or InterAct data collection, study management, or study coordination; contributed to data interpretation and critical reading of the manuscript; and saw and approved the final version of the manuscript. J.S.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the American Heart Association EPI-NPAM Scientific Sessions, San Diego, California, 13–16 March 2012.
The authors thank all EPIC participants and staff for their contribution to the study. The authors thank Nicola Kerrison (Medical Research Council Epidemiology Unit, Institute of Metabolic Science, Addenbrooke's Hospital) for managing the data for the InterAct project.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1020/-/DC1.
References
- 1.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause 2006;13:265–279 [DOI] [PubMed] [Google Scholar]
- 2.van Der Voort DJ, van Der Weijer PH, Barentsen R. Early menopause: increased fracture risk at older age. Osteoporos Int 2003;14:525–530 [DOI] [PubMed] [Google Scholar]
- 3.van der Klift M, de Laet CE, McCloskey EV, et al. Risk factors for incident vertebral fractures in men and women: the Rotterdam Study. J Bone Miner Res 2004;19:1172–1180 [DOI] [PubMed] [Google Scholar]
- 4.Monninkhof EM, van der Schouw YT, Peeters PH. Early age at menopause and breast cancer: are leaner women more protected? A prospective analysis of the Dutch DOM cohort. Breast Cancer Res Treat 1999;55:285–291 [DOI] [PubMed] [Google Scholar]
- 5.Trichopoulos D, MacMahon B, Cole P. Menopause and breast cancer risk. J Natl Cancer Inst 1972;48:605–613 [PubMed] [Google Scholar]
- 6.Dossus L, Allen N, Kaaks R, et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2010;127:442–451 [DOI] [PubMed] [Google Scholar]
- 7.Tsilidis KK, Allen NE, Key TJ, et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer 2011;105:1436–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev 1993;15:36–47 [DOI] [PubMed] [Google Scholar]
- 9.van der Graaf Y, de Kleijn MJ, van der Schouw YT. Menopause and cardiovascular disease. J Psychosom Obstet Gynaecol 1997;18:113–120 [DOI] [PubMed] [Google Scholar]
- 10.Kritz-Silverstein D, Barrett-Connor E, Wingard DL. Hysterectomy, oophorectomy, and heart disease risk factors in older women. Am J Public Health 1997;87:676–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pirimoglu ZM, Arslan C, Buyukbayrak EE, et al. Glucose tolerance of premenopausal women after menopause due to surgical removal of ovaries. Climacteric 2011;14:453–457 [DOI] [PubMed] [Google Scholar]
- 12.Malacara JM, Huerta R, Rivera B, Esparza S, Fajardo ME. Menopause in normal and uncomplicated NIDDM women: physical and emotional symptoms and hormone profile. Maturitas 1997;28:35–45 [DOI] [PubMed] [Google Scholar]
- 13.Di Donato P, Giulini NA, Bacchi Modena A, et al. Gruppo di Studio Progetto Menopausa Italia Risk factors for type 2 diabetes in women attending menopause clinics in Italy: a cross-sectional study. Climacteric 2005;8:287–293 [DOI] [PubMed] [Google Scholar]
- 14.Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod 2003;18:199–206 [DOI] [PubMed] [Google Scholar]
- 15.Langenberg C, Sharp S, Forouhi NG, et al. InterAct Consortium Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia 2011;54:2272–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med 2009;28:1982–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986;73:1–11 [Google Scholar]
- 18.Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med 1999;159:1061–1066 [DOI] [PubMed] [Google Scholar]
- 19.Kampert JB, Whittemore AS, Paffenbarger RS., Jr Combined effect of childbearing, menstrual events, and body size on age-specific breast cancer risk. Am J Epidemiol 1988;128:962–979 [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 21.den Tonkelaar I. Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas 1997;27:117–123 [DOI] [PubMed] [Google Scholar]
- 22.Colditz GA, Stampfer MJ, Willett WC, et al. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol 1987;126:319–325 [DOI] [PubMed] [Google Scholar]
- 23.Must A, Phillips SM, Naumova EN, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol 2002;155:672–679 [DOI] [PubMed] [Google Scholar]
- 24.Casey VA, Dwyer JT, Coleman KA, Krall EA, Gardner J, Valadian I. Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Ann Hum Biol 1991;18:155–166 [DOI] [PubMed] [Google Scholar]
- 25.Cairns BJ, Liu B, Clennell S, et al. Lifetime body size and reproductive factors: comparisons of data recorded prospectively with self reports in middle age. BMC Med Res Methodol 2011;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorman JS, Steenkiste AR, Foley TP, et al. Familial Autoimmune and Diabetes (FAD) Study Menopause in type 1 diabetic women: is it premature? Diabetes 2001;50:1857–1862 [DOI] [PubMed] [Google Scholar]
- 27.Mishra GD, Carrigan G, Brown WJ, Barnett AG, Dobson AJ. Short-term weight change and the incidence of diabetes in midlife: results from the Australian Longitudinal Study on Women’s Health. Diabetes Care 2007;30:1418–1424 [DOI] [PubMed] [Google Scholar]
- 28.Soriguer F, Morcillo S, Hernando V, et al. Type 2 diabetes mellitus and other cardiovascular risk factors are no more common during menopause: longitudinal study. Menopause 2009;16:817–821 [DOI] [PubMed] [Google Scholar]
- 29.Kim C, Edelstein SL, Crandall JP, et al. Diabetes Prevention Program Research Group Menopause and risk of diabetes in the Diabetes Prevention Program. Menopause 2011;18:857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He C, Zhang C, Hunter DJ, et al. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol 2010;171:334–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakshman R, Forouhi N, Luben R, et al. Association between age at menarche and risk of diabetes in adults: results from the EPIC-Norfolk cohort study. Diabetologia 2008;51:781–786 [DOI] [PubMed] [Google Scholar]
- 32.Persson I. Estrogens in the causation of breast, endometrial and ovarian cancers - evidence and hypotheses from epidemiological findings. J Steroid Biochem Mol Biol 2000;74:357–364 [DOI] [PubMed] [Google Scholar]
- 33.Godsland IF. Oestrogens and insulin secretion. Diabetologia 2005;48:2213–2220 [DOI] [PubMed] [Google Scholar]
- 34.Margolis KL, Bonds DE, Rodabough RJ, et al. Women’s Health Initiative Investigators Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 2004;47:1175–1187 [DOI] [PubMed] [Google Scholar]
- 35.Kanaya AM, Herrington D, Vittinghoff E, et al. Heart and Estrogen/progestin Replacement Study Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2003;138:1–9 [DOI] [PubMed] [Google Scholar]
- 36.Kalyani RR, Franco M, Dobs AS, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab 2009;94:4127–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia 2007;50:2076–2084 [DOI] [PubMed] [Google Scholar]
- 38.Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab 2000;85:2832–2838 [DOI] [PubMed] [Google Scholar]
- 39.Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas 1995;21:103–113 [DOI] [PubMed] [Google Scholar]
- 40.Stolk L, Perry JR, Chasman DI, et al. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet 2012;44:260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.